Published online Sep 26, 2019. doi: 10.4252/wjsc.v11.i9.618
Peer-review started: March 4, 2019
First decision: April 11, 2019
Revised: March 23, 2019
Accepted: July 16, 2019
Article in press: July 16, 2019
Published online: September 26, 2019
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the gastrointestinal tract associated with multifactorial conditions such as ulcerative colitis and Crohn’s disease. Although the underlying mechanisms of IBD remain unclear, growing evidence has shown that dysregulated immune system reactions in genetically susceptible individuals contribute to mucosal inflammation. However, conventional treatments have been effective in inducing remission of IBD but not in preventing the relapse of them. In this way, mesenchymal stromal cells (MSC) therapy has been recognized as a promising treatment for IBD due to their immunomodulatory properties, ability to differentiate into several tissues, and homing to inflammatory sites. Even so, literature is conflicted regarding the location and persistence of MSC in the body after transplantation. For this reason, recent studies have focused on the paracrine effect of the biofactors secreted by MSC, especially in relation to the immunomodulatory potential of soluble factors (cytokines, chemokines, and growth factors) and extracellular vehicles that are involved in cell communication and in the transfer of cellular material, such as proteins, lipids, and nucleic acids. Moreover, treatment with interferon-γ, tumor necrosis factor-α, and interleukin-1β causes MSC to express immunomodulatory molecules that mediate the suppression via cell-contact dependent mechanisms. Taken together, we present an overview of the role of bioactive factors and cell membrane proteins derived from MSC as a cell-free therapy that can improve IBD treatment.
Core tip: Recent experimental studies have suggested that both bioactive factors and surface proteins of mesenchymal stem cells demonstrate great therapeutic potential for overcoming the deficiencies of current therapies for inflammatory bowel diseases. Our goal in this review is to describe cell-free therapy based upon the therapeutic potential of mesenchymal stem cells, while avoiding the practical issues associated with the use of living cells.
- Citation: da Costa Gonçalves F, Paz AH. Cell membrane and bioactive factors derived from mesenchymal stromal cells: Cell-free based therapy for inflammatory bowel diseases. World J Stem Cells 2019; 11(9): 618-633
- URL: https://www.wjgnet.com/1948-0210/full/v11/i9/618.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i9.618
Inflammatory bowel diseases (IBD) comprise ulcerative colitis (UC) and Crohn’s disease (CD), both of which are chronically multifactorial inflammatory disorders of the gastrointestinal system. Although the pathogenesis of IBD remains unclear, mounting evidence suggests that abnormal immune regulation in genetically susceptible individuals and/or environmental factors contribute to mucosal inflammation[1-3]. Conventional treatments for IBD involve immunosuppressive drugs that lead to the remission of intestinal inflammation and related symptoms. However, there is no known medical/surgical cure for IBD[4,5]. Therefore, additional therapeutic strategies, such as cell-based therapy, are required for unresponsive patients.
In this way, mesenchymal stromal cells (MSC) are a promising strategy for treating inflammatory diseases, immune disorders, and tissue regeneration due to their immunomodulatory properties, ability to differentiate into several tissues, and homing to inflammatory sites, by which they control inflammation and the pro-duction of cytokines[6-8]. However, there are conflicts in the literature regarding the location and persistence of MSC in the body after transplantation. Cell-tracking studies have shown that most MSC are localized in the lungs after intravenous infusion and have a short-term survival span[9-12]. After 24 h of infusion, MSC tend to disappear from the lungs, suggesting that they probably transmit their effects to resident cells[9]. Based on this, MSC may interact with resident cells through the secretion of paracrine factors or cell-cell communication[13-15].
Recent studies have focused on the paracrine effect of the biofactors secreted by MSC, especially in relation to the immunomodulatory potential of soluble factors (cytokines, chemokines, and growth factors)[16-18]. Also, MSC alter their immune function in response to the inflammatory environment, especially by the stimulation of proinflammatory cytokines interferon-γ (IFN-γ) and the tumor necrosis factor (TNF)-α[19,20]. After activation, MSC upregulate the expression of interleukin (IL)-6, IL-10, indoleamine 2,3 dioxygenase (IDO), transforming growth factor (TGF), prostaglandin E2 (PGE-2), hepatocyte growth factor (HGF), nitric oxide, and heme oxygenase-1[20-23]. It is also known that MSC are capable of releasing extracellular vehicles (EVs) that are involved both in cell communication and in the transfer of cellular material, such as proteins, lipids, and nucleic acids[24-26]. Moreover, MSC express immunomodulatory molecules in their cell membrane such as ATPases, CD73, and Toll-like receptors (TLRs)[27-29], and, under inflammatory conditions, they express the programmed death ligand 1 (PD-L1) and the Fas ligand[22,30-34]. For this reason, both biofactors secretion and cell contact may be required for efficient MSC immunomodulation[14,35].
Therefore, the study of bioactive factors and cell membrane molecules of MSC becomes important in the search for new cell-free therapeutic methodologies that aim to reduce the complications associated with the administration of MSC but while preserving the immunological properties of these cells.
IBD are chronic inflammatory disorders of the gastrointestinal system associated with multifactorial conditions, such as UC and CD. Although the underlying mechanisms of UC and CD remain uncertain, growing evidence has shown that failure of the epithelial barrier and dysregulation of the immune system in genetically predisposed individuals contribute to mucosal inflammation[1-3]. These diseases are characterized by the dysfunction of mucosal T cells, alteration in the production and secretion of cytokines, and cellular inflammation affecting the digestive tract, especially the distal small intestine and colon mucosa[36]. While CD may affect any part of the gastroin-testinal segment and is characterized by an inflammatory process that recruits macrophages and forms granulomas, UC is generally limited to the colon and rectum, characterized by neutrocytic infiltrate with formation of cryptic abscesses and epithelial ulceration[37]. Patients with UC experience continuous inflammation confined to the mucosal layer. In CD, the inflammation is discontinuous and affects all intestine layers[2].
At the clinic, patients with IBD have recurrent episodes of abdominal pain, dia-rrhea, bloody stools, and weight loss. Moreover, they may present extra intestinal manifestations in the skin, joints, eyes, and less frequently in the abdominal organs, for example, the biliary tract[38]. Treatment of IBD is based on the severity, site, clinical manifestations, and complications of the disease in each case. Several cellular and molecular pathological pathways have been identified as targets for IBD treatment[39]. The exacerbated progression of IBD requires a scale ranging from anti-inflammatory treatment to biological agents, usually with limited success[40]. Furthermore, medical treatments are expensive and common drugs are toxic and ineffective for most patients, making surgical resection of the parts of the intestine necessary in many cases[2,41]. Regarding the progress achieved by intensive clinical-drug treatment, approximately 20% of patients with UC and 50% of patients with CD require surgical intervention within 10 years of diagnosis[41]. Accordingly, some studies have addressed MSC therapy as a promising approach for treating IBD[42].
MSC are multipotent cells capable of differentiating into mesodermal lines, par-ticularly osteoblastic, adipogenic, and chondrogenic strains[43,44]. There is controversy over the naming and definition of MSC in the scientific community. The term “mesenchymal stromal cell” is used in parallel with the terms “mesenchymal stem cell” and “multipotent mesenchymal stromal cell”[45]. MSC are indeed a heterogeneous population of cells characterized immunophenotypically by the expression of CD73, CD90, and CD105 and do not present the expression of CD45, CD34, CD11, CD14, CD19, CD79A, and human leukocyte antigen (HLA)-DR hematopoietic lineage markers[46]. Most of the knowledge about these cells was generated from bone marrow-derived MSC. However, the origin of MSC production has been expanded to other tissues, including muscle, adipose, and neonatal tissues[47,48]. Recently, Soontararak et al[49] demonstrated that MSC derived from induced pluripotent stem cells are equivalent to MSC derived from adipose tissue in terms of improving the intestinal healing in IBD model.
MSC exhibit great therapeutic potential in regenerative medicine owing to their ability to differentiate in vitro, homing (the process in which cells are able to migrate and graft to the tissues) to inflamed tissues after in vivo infusion, and the secretion of various bioactive molecules[8]. In addition, the immunomodulatory properties of MSC suggest that even MSC of the incompatible HLA may be suitable for a wide variety of new therapeutic applications, especially for cellular therapy of inflammatory and autoimmune diseases[50]. Traditionally, MSC are isolated from bone marrow, but other cellular sources may be of greater benefit due to the higher number of MSC or easier accessibility[13]. Moreover, MSC from different tissue sources share several phenotypic and functional features. Even so, there are subtle peculiarities in the expression and differentiation abilities of specific markers on cell surface[13,45].
Studies have compared the ability of MSC from different tissues to suppress peripheral blood cells, and adipose tissue-derived MSC have demonstrated a greater immunomodulatory effect than MSC from other sources[51,52]. Although adipose and bone marrow-derived MSC share several properties, there are variation in gene expression and growth factor secretion profiles[53]. In addition, various types of adipose tissue may have distinct properties. MSC isolated from abdominal and mammary adipose tissue, for example, present discrepancy in the expression of fibroblast growth factor and receptor, suggesting variability in angiogenic potential[54].
Neonatal tissues (cord blood, umbilical cord, placenta, amnion, and chorion) have been an alternative for MSC isolation. These tissues are usually discarded as a residual product after delivery and can be obtained in large quantities in an easy and noninvasive way[55]. Another relevant advantage of neonatal tissues is that they supply immature cells, which present a lower risk of mutations and superior cell activity, such as increased differentiation, homing, and grafting ability[56,57]. Previous studies have shown that neonatal sources exhibit superior proliferation and immunosuppressive and regenerative potential when compared to adult tissues, for example, bone marrow and adipose tissue[47,58].
MSC have demonstrated low levels of HLA or major histocompatibility complex (MHC) class I and insignificant levels of HLA class II. Moreover, they do not express co-stimulatory molecules such as CD40, CD40L, B7-1 (CD80), and B7-2 (CD86)[22,51,59-61]. Consequently, MSC present low immunogenicity and may “escape” the immune system due to their surface phenotypes that are not recognized by T cells. Absence of MHC II or T cell co-stimulatory molecules make the MSC immune-privileged cells and may explain the mechanism by which MSC are not recognized by T cells[62]. Interestingly, both syngeneic and allogeneic MSC are immunotolerable by the receptor[63].
MSC have presented a high immunosuppressive capacity and interaction with immune cells through several mechanisms. Studies have shown that MSC-mediated immunosuppression may occur through the secretion of soluble factors and the presence of MSC membrane protein[51,64]. Regarding soluble factors, important anti-inflammatory molecules, such as transforming TGF-β, PGE-2, and HGF, have been enrolled[21,23]. Through secretion of TGF-β and other factors, MSC promote the induction of regulatory cells, including T cells[65], macrophages[66], and B cells[67] and thus transmit their immunosuppressive effects to different types of cells that exert several mechanisms of immune suppression. MSC can also be induced to produce IDO enzyme, which has an effective ability to inhibit lymphocyte proliferation by metabolism of L-tryptophan to L-kynurenine. Thus, low levels of L-tryptophan and high levels of L-kynurenine impose a block of lymphocyte proliferation[68].
Moreover, MSC exert their immunomodulatory function through molecules in their cell membrane, such as ATPases and CD73 (ecto-5’-nucleotidase, Ecto5’NTase)[28,69]. ATPase converts ATP to ADP and then to AMP. CD73 further dephosphorylates AMP to adenosine, which exhibits immunosuppressive properties[69,70]. Also, the ability of MSC to modify the immune system response can be enhanced by treatment with proinflammatory cytokines, TNF-α and IFN-γ in particular[19-21]. Under inflammatory conditions, MSC express membrane proteins with an immunological regulatory function, such as PD-L1 and the Fas ligand, through which they direct to the target cells and prevent their activation/function[6,22,32-34].
Secreted bioactive molecules as well as the membrane proteins of MSC demonstrate an ability to mediate immunosuppression. Nevertheless, there are controversies in the literature about the interaction of MSC with immune cells[71-73]. For MSC treatments to reach their full potential, a broader understanding of how cells exert their immunosu-ppression must be developed. Then it will be possible to define the relevant pathways for improving MSC treatment for specific inflammation or immune disorders.
Factors secreted by MSC have modulated the expression profile of cytokines in macrophages, inducing the polarization of these cells to an anti-inflammatory phenotype (M2)[74,75]. However, González et al[63] reported a partial dependence of the cell-cell contact mechanism by the induction of secretion of immunosuppressive factors. In their study, different co-culture systems of adipose tissue-derived MSC and macrophages were assessed and high levels of IL-10 were only observed in the co-culture system that promoted cell-cell contact. Furthermore, macrophages that phagocyte MSC acquire regulatory properties[35]. In this sense, bioactive factors and/or cell-cell interaction promote distinct types of regulatory macrophages that can change their function in response to the proinflammatory signals of the micro-environment[76]. MSC may also affect populations of monocytes, precursors of macrophages and dendritic cells, through the secretion of HGF that induces monocytes to an immunomodulatory phenotype (CD14+CD16-) with IL-10 production[27].
However, some authors suggest that activation of T cells by MSC is independent of cell contact[77-79]. Several soluble factors have been associated with the immuno-modulatory ability of MSC to affect the activation or proliferation of T cells, such as the secretion of HGF, TGF-β, IDO, and PGE-2[21,23,68,71,73]. Saldanha-Araujo et al[80] demonstrated an additional mechanism by which MSC suppress T cell proliferation. This study demonstrated that, during the interaction between MSC and T cells, there is adenosine production by MSC, which reduce T cell proliferation by flagging the adenosine receptor on the membrane surface. Still, other authors suggest that cell-cell contact is essential for an intense immunosuppressive effect[78]. In fact, MSC express integrins, intercellular adhesion molecules, and vascular cell adhesion protein on their surface and can bind to T cells with high affinity[71,81]. Quaedackers et al[82] cultivated MSC with peripheral blood mononuclear cells and indicated that such interaction is a specific process, since the subpopulations of lymphocytes that interacted with MSC were distinct from cells that remained in suspension. The T cell fraction that adhered to MSC was regulatory T cells exhibiting an immunosuppressive phenotype. In inflammatory environment, the expression of the molecules PD-L1 and PD-L2 on the MSC surface enhance, restricting the function of T cells through PD-1 ligands[83]. The high levels of these inhibitory molecules on the MSC membrane confirm that one of the mechanisms of MSC immunosuppression is also by cell–cell contact-mediated responses.
MSC can also immunomodulate other cell types of the innate and the adaptive immune system, such as B lymphocytes, dendritic cells, and natural killer (NK) cells[72,84]. Luk et al[14] have demonstrated that MSC, under normal culture conditions, promote B cell survival and induce the formation of regulatory B cells, but negligible interference on B cell proliferation and immunoglobulin G production was observed. However, after pre-treatment with IFN-γ, MSC appear to inhibit B cell proliferation and reduce immunoglobulin G production, even though these primed cells lose the ability to induce the formation of regulatory B cells. Moreover, the authors evaluated distinct mechanisms as key factors for MSC-mediated immunomodulation. First, they demonstrated that the effects of MSC on B cells do not depend only on soluble factors, since no production of regulatory B cells or IL-10 was induced when MSC were cultured in a Transwell system with separated chambers. Second, they assessed that the presence of heat-inactivated MSC (dead cells not capable of secreting factors but phenotypically intact) was not sufficient to induce IL-10 producing B cells. These data suggest that B cell modulation by MSC is mediated by an active metabolic process and requires B cell and MSC contact.
Another mechanism of immunosuppression exerted by MSC has been observed in the inhibition of the differentiation and maturation of dendritic cells derived from CD14+ monocytes[85]. Co-culture with Transwell suggests that IL-6 and macrophage colony stimulating factor are partially involved in the inhibition of dendritic cell differentiation by MSC. MSC may also alter the cytokine secretion profile of dendritic cells[86]. Co-culture with MSC demonstrates decreased secretion of TNF-α by mature myeloid dendritic cells, while increasing IL-10 secretion by plasmacytoid dendritic cells[87]. Moreover, MSC are sensitive to lysis by activated NK cells but are resistant to naive NK cells[88]. Spaggiari et al[89] observed that MSC treated with IFN-γ are resistant to lysis by NK cells. In addition, they found that some inhibitory effects of MSC on NK cells demand cell-cell contact, while others are regulated by soluble factors, including PGE-2 and TGF-β1. Therefore, these data together suggest that the mechanism of immunoregulation of MSC depends on the types of cell populations, the inflammatory conditions, and the presence or absence of cell-cell contact.
Autologous and allogeneic MSC have been evaluated in clinical trials in two different modalities: Local injection of MSC to treat fistulizing CD and intravenous (IV) infusion of MSC to treat UC or luminal colitis[42,90-93]. Published work demonstrates that to date 117 IBD patients with refractory disease or intolerant to standard treatment have received one or more IV infusions of autologous or allogeneic MSC[94]. Currently, results of clinical trials are particularly encouraging in terms of safety and efficacy; however, due to different study designs regarding tested groups, short follow-up, and a lack of endoscopic data and unified primary outcomes, no final conclusions can be made. In fact, MSC demonstrated their ability to repair perianal fistulas in CD patients, refractory to conventional or biological therapy in several controlled trials[95,96]. Still, MSC need to demonstrate their clear efficacy on luminal CD and UC[97,98].
In this sense, experimental studies in animal models have contributed to a better understanding of cellular, molecular, and immunological mechanisms of IBD associated with cell therapy. Recent studies have demonstrated a clinical and histopathological improvement of colitis after an infusion of MSC, such as decreased inflammation and increased survival[99,100]. In addition, much has been researched regarding the homing of exogenous MSC infused by different pathways in response to an inflammatory insult. A comparative study between the IV and intraperitoneal (IP) routes in a trinitrobenzene sulfonic acid (TNBS)-induced colitis model concluded that, in systemic administration, MSC accumulated preferentially in the lungs, with no evidence of migration to the colon. On the other hand, MSC injected via IP were located in the inflamed colon[101]. Another study in an experimental model of sodium dextran sulfate (DSS)-induced colitis demonstrated that MSC migrate towards the lung, liver, and spleen, and even a small amount to the inflamed colon after 24 h of IV infusion. In contrast, IP and intracolonic routes showed more cell grafting in the colon and fewer cells trapped in the pulmonary alveoli[100]. However, a previous study conducted by our research group also evaluated the effect of MSC administered by different routes, IV and IP, in DSS-induced acute colitis. We demonstrated that infusion of MSC by IV route decreased intestinal inflammation, modulated serum cytokines, and induced apoptosis of T cells of the intestinal mucosa. Meanwhile, the same effect was not found in animals treated by IP route[102].
In this way, there are controversial studies about the location and persistence of MSC in the body after cell transplantation. The efficiency of cellular delivery is dependent on the route of administration[101,102]. IV infusion, for example, has been used as a cellular delivery route for preclinical studies[9,10,102] and for recent clinical trials[103] due to its wide distribution and easy access. However, cell-tracking studies have shown that most MSC are localized in the lungs and have a short-term survival in the body after IV infusion[9-12]. The entrapment of MSC in the lungs is occasioned by their size[24], which exceeds the width of the pulmonary micro-capillaries[25,26]. In patients, respiratory discomfort has been reported after transfusion of MSC[10]; and high and subsequent doses of cells are usually required to observe some effect in animal models[104]. Interestingly, in experiments of Lee et al[105], MSC retained in the lungs improved cardiac function after myocardial infarction by releasing the anti-inflammatory protein TSG-6[105].
After 24 h of infusion, MSC tend to disappear from the lungs, and their cellular debris are distributed to other sites, particularly the liver, suggesting that these cells pass their effects to the resident immune cells[106]. Then, the resident cells possibly mediate the immunomodulatory effects induced by transplanted MSC[9]. Moreover, immune system cells are likely to play a role in the removal of MSC. Activated NK cells, for example, have been shown to be able to lyse autologous MSC in vitro[89]. Also, apoptosis of infused cells may trigger an immunomodulatory response. Lu et al[35] demonstrated that macrophages adapt a new immunoregulatory function after the phagocytosis of dead MSC[35,107]. Based on this information, MSC probably interact with resident cells through distinct mechanisms, either by the secretion of paracrine factors or through a direct cell-cell interaction[13-15,108]. Intestinal organoids derived from stem cells also provide a system to mimic ex vivo interactions between the lamina propria and epithelium intestinal by cell contact–dependent/independent mechanisms[109].
Thus, the application of MSC in vivo requires, in addition to the biological knowledge of the cells, a deep understanding of the mechanisms involved in IBD. For this, the immunological properties of the MSC type, the infusion route, and the inflammatory and immunological conditions of the disease should be considered. Therefore, MSC therapy may exert its therapeutic effects mainly by secreting soluble immunomodulating bioactive factors and/or by cell-cell contact and consequently by interaction with immune cells, establishing a favorable environment for regeneration.
Infused MSC have been demonstrated to promote the intestinal repair processes in both humans[42] and animal models[105,110]. Nevertheless, the engraftment and the homing mechanism of MSC are not well elucidated and depend on intricate interactions between signaling pathways. Short survival after infusion and poor biodistribution of MSC have proven to be significant technical challenges to overcome before this therapy can be used for clinical purposes. An alteration in the MSC treatment that avoids these issues, but maintains the immunomodulatory properties of MSC, would enhance this therapy. Also, though positive effects of MSC infusion can last longer than the half-life of the cells themselves, they reduce over time. It is thus plausible that successive MSC administrations are necessary as maintenance therapy[94].
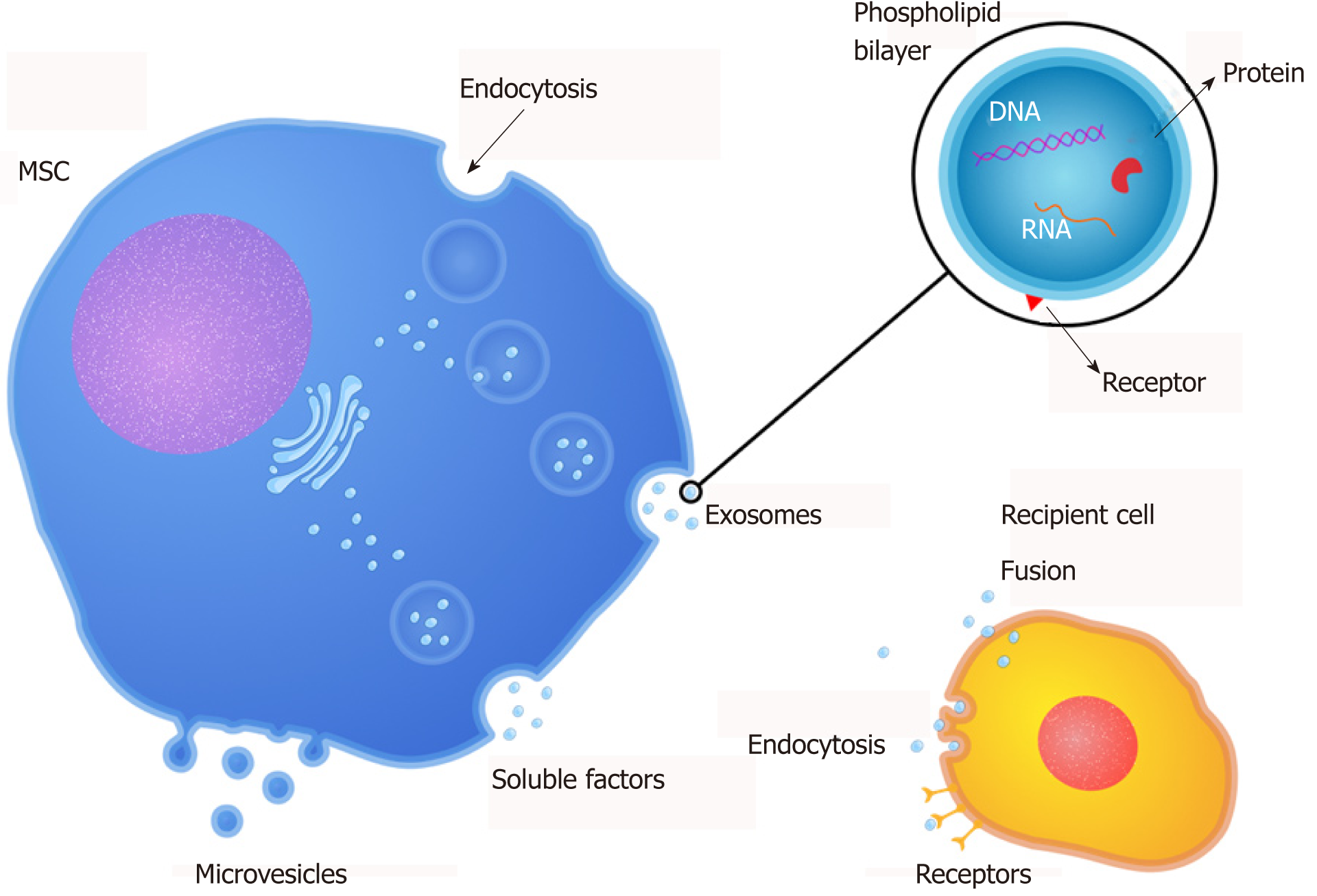
Recent studies have reported that the regenerative potential of MSC therapy has been, at least in part, mediated by paracrine actions[15,111,112]. Thus, studies about MSC-secreted factors have demonstrated that these factors can induce tissue repair in conditions involving tissue/organ damage[111]. Furthermore, they are referred to in the literature as “secretome” (soluble factors), and extracellular vesicles and can be found in the MSC culture supernatant; thus, the supernatant of MSC is a denominated conditioned medium (CM). CM is commonly prepared by confluent MSC cultures incubated in serum-free media for 24 h without any concentration, selection, or purification of cell products. Therefore, the components of CM are in fact both free soluble factors (cytokines, chemokines, and growth factors) and EVs, which may mediate the therapeutic potential of MSC[16,113] (Figure 1).
Few studies have used the MSC-CM as a therapeutic strategy for IBD (Table 1). The paracrine effect of MSC-CM has been observed in experimental models of colitis induced chemically by TNBS and DSS[15,114,115]. Heidari et al[116] and Pouya et al[117] have shown the effects of MSC-CM in DSS-induced colitis by increasing anti-inflammatory responses associated with an increase of regulatory T cells percentage and IL-10 production. Watanabe et al[15] demonstrated that MSC-CM was effective for the inductive stage of TNBS-induced colitis and for the recovery stage of DSS-induced colitis independent of the systemic delivery route. Similarly, Robinson et al[115] (2014) and (2015)[118] showed that MSC and CM treatments prevented damage to the enteric nervous system and alleviated gut dysfunction caused by TNBS-induced colitis. In our previous study, we investigated the paracrine role of MSC in DSS-induced colitis of colon organ culture. Our results have shown that colonic inflammation is alleviated by MSC-CM, and this effect is independent of cell contact[119]. Also, CM has been evaluated in several other conditions and diseases, such as myocardial infarction[120], bone defect[121], hepatic insufficiency[122], and spinal cord injury[123]. Liu et al[124] suggest that MSC-CM administration has the potential to suppress proliferation of artery smooth muscle cells in an experimental model of pulmonary hypertension. Another study reported that the application of MSC-CM in diabetic rats prevented renal disease, primarily by attenuating the expression of TGF-β1[125]. Therefore, it may be observed that MSC-CM contains several factors that intervene in different pathophysiological manifestations, such as inflammation, proliferation, angiogenesis, and tissue remodeling[15]. Taken together, these results indicate that MSC-secreted factors are able to preserve the intestinal barrier in IBD independent of cell transplantation.
| Bioactive factors | MSC origin | IBD model | Pathway | Results | Ref. |
| CM | Rat bone-marrow | DSS and TNBS-induced colitis in rats | IP, IV, and enema | Effective for the inductive phase of TNBS-induced colitis and for recovery phase of DSS-induced colitis. | Watanabe et al[15], 2014 |
| CM | Human bone-marrow | TNBS-induced colitis in pigs | Enema | Prevent loss of myenteric neurons and damage of nerve process. | Robinson et al[115], 2014 |
| EVs | Rat bone-marrow | TNBS-induced colitis in rats | IV | Inhibit NF-κBp65 pathways, modulate anti-oxidant/oxidant balance, and apoptosis. | Yang et al[132], 2015 |
| Exosomes | Human umbilical cord | DSS-induced colitis in mice | IV | Increase IL-10 and decrease TNF-α, IL-1β, IL-6, iNOS, and IL-17. | Mao et al[114], 2017 |
| Extract | Human umbilical cord | DSS-induced colitis in mice | IP | Inhibit inflammatory cytokines and alter macrophage functional phenotype from M1 to M2 | Song et al[107], 2017 |
| CM | Mouse adipose tissue | DSS-induced colitis in mice | IP | Increase Treg, IL-10, and TGF-β, and decreased IL-17. | Heidari et al[116], 2018 |
| CM | Mouse adipose tissue | DSS-induced colitis in mice | IP | Increase Treg, IL-10, and TGF-β, and decreased IL-17. | Pouya et al[117], 2018 |
| CM | Human umbilical cord | DSS-induced colitis in mice | Culture medium (organ culture) | Decrease IL-6 and increase Ki-67. | DA Costa Gonçalves et al[119], 2018 |
| Exosomes | Human umbilical cord | DSS-induced colitis in mice | IV | Downregulated ubiquitin inhibiting NF-κB and mTOR activation. | Wu et al[133], 2018 |
| EVs | Mouse bone-marrow overexpressing miR-146a | TNBS-induced colitis in rats | IV | Suppress the activation of NF-kB pathway, decrease TNF-α, IL-6, and IL-1β. | Wu et al[131], 2019 |
MSC also have been displayed to release EVs, which can be implicated in cell-cell interaction by carrying biologically active lipids, proteins, and nucleic acids in and on their membrane (Table 1)[26,28]. EVs are cell-derived membranous structures released by cells. They can be originated from multivesicular bodies (exosomes) or directly from the cell membrane (microvesicles - MVs). Both exosomes and MVs are comprised of two regions: The membrane and the natural internal cargo. The outer membrane, composed of lipid layer and proteins, packages bioactive molecules and protects the internal cargo, consisting of lipids, proteins, DNA, mRNA, micro-RNA, and other components from the parental cell. The cargo carried by EVs dictated their function[126,127]. The MSC treatment may be mediated by free soluble factors and components contained in EVs, which composition is cell-origin specific[24,25,114]. Kim et al[128] profiled MSC-derived MVs proteome and identified 730 proteins engaged in processes associated with self-renewal and/or differentiation of MSC. Also, they pointed out that the micro-RNAs (miRNAs) profile of MVs presents a pattern shared with their cells of origin. Nevertheless, selected miRNAs were evident only in the released MVs but absent in the MSC[128]. Accordingly, another study has extracted RNA from MSC and their MVs for 365 known mature miRNAs, and they observed that 41 miRNAs were co-expressed in MVs and cells, others were cumulated within MVs and absent in the cells after MVs secretion, and still others were maintained within the cells and not liberated in MVs[129].
Thus, several studies have been focused on MSC-derived EVs as a treatment for IBD (Table 1). Wong et al[130] identified in circulating exosomes of DSS-induced colitis mouse 56 differentially expressed proteins that are involved in macrophage activation. Wu et al[131] demonstrated the role of miR146-a in attenuating experimental colitis since EVs derived from MSC overexpressing miR146-a significantly inhibited TNF receptor–associated factor 6 and IL-1 receptor associated kinase 1. Yang et al[132] demonstrated that EVs protect against TNBS-induce colitis by attenuating oxidative stress and apoptosis. Mao et al[114] demonstrated that exosomes released from human umbilical cord-derived MSC homed to colon tissue of IBD mice and relieved the severity of the DSS-induced colitis by altering the expression of inflammatory genes and decreasing the infiltration of macrophages. Interestingly, ubiquitination may play an important role in the anti-inflammatory effect observed in IBD animals treated with MSC-derived exosomes. Ubiquitin is upregulated in IBD mice and promotes activation of the nuclear factor kappa B pathway by increasing the ubiquitination and degradation of inhibitor of kappa B alpha and regulating inflammation through mTOR signaling. However, the expression of ubiquitin was inhibited in the colonic mucosa and spleen after the MSC-derived exosomes treatment[133].
In this way, the secreted bioactive factors could play an essential role in repairing damaged colon from experimental colitis to regenerative medicine. In relation to cellular therapy, bioactive factors are easier to prepare and store. Then, it is a therapy devoid of cells, and possible risks of rejection between donor and recipient can be minimized. Lastly, pulmonary capillaries are not a barrier for this therapy in IV transplantation, and CM can reach sites beyond the lung[111].
There is inconclusive evidence that bioactive anti-inflammatory factors alone are responsible for the immunomodulatory effects of transplanted MSC. The short-term survival of MSC in the body after infusion raises the questions of whether MSC have sufficient time to be activated by inflammatory conditions and then secreted factors[9]. Hoogduijn et al[106] found that MSC infusion initiates a mild and immediate systemic inflammatory response, which may be the activator of posterior immunosuppression. In this study, the inflammatory response was found in the lung and characterized by an increased expression of pro-inflammatory monocyte and cytokines. Also, MSC infusion provides inflammatory conditions for their activation and that at least part of the immunomodulatory response mediated by MSC is independent of activation by anti-inflammatory soluble factors. Instead, passive interactions with host cells probably mediate these effects[14]. de Witte et al[134] demonstrated that infused MSC are internalized by monocytes and induce phenotypical and functional changes in these cells, which subsequently migrate from the lungs to other body sites and modulate immune cells response. Other studies also indicated that MSC induced an immunosuppressive phenotype on macrophages after phagocytosis and that the macrophage depletion reduced the therapeutic effect of MSC[35,135].
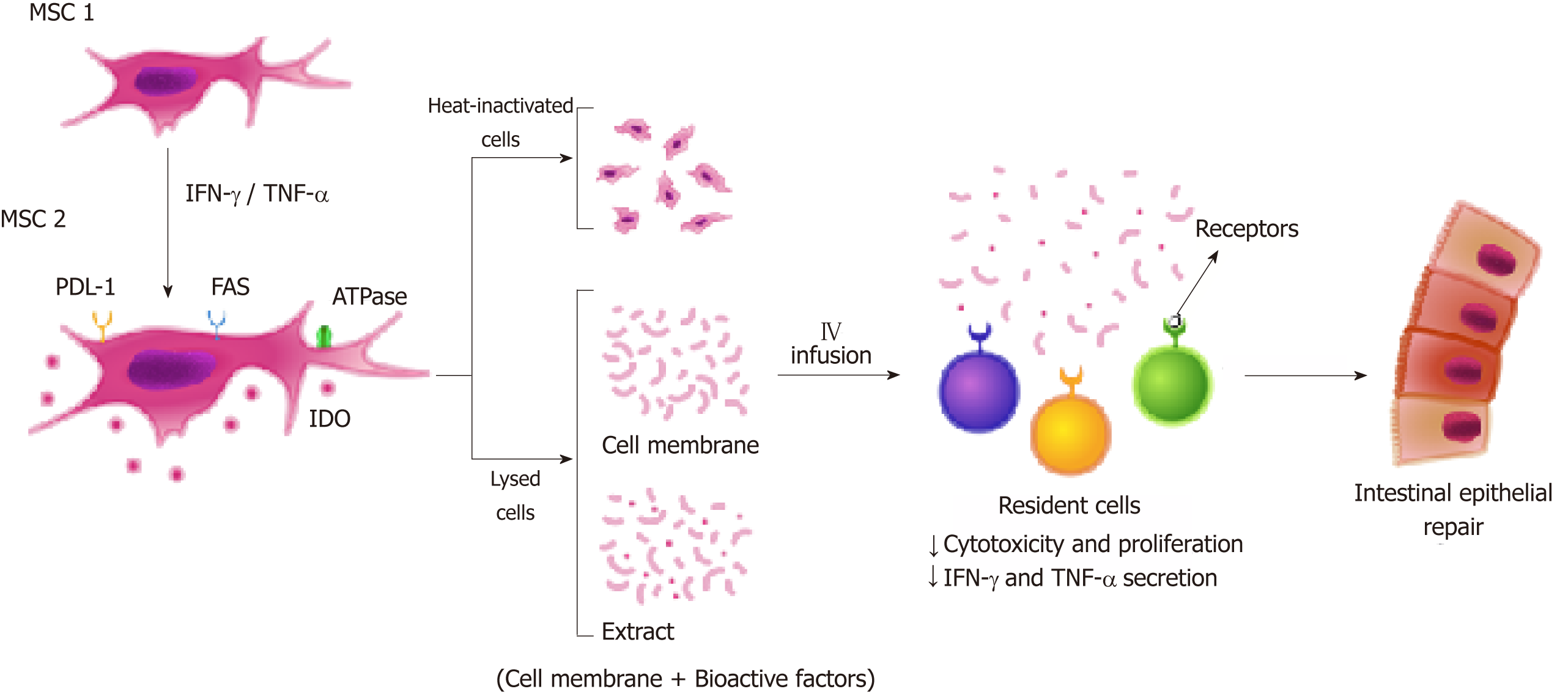
Based on this analysis, some studies have shown that MSC exert their effects through intermediary cells by contact with the cell membrane[14,134,136,137]. MSC express immunomodulatory molecules on their membrane, such as CD90 (Thy-1 membrane glycoprotein) that is involved in MSC differentiation pathways, ATPases, and CD73, which dephosphorylate ATP into AMP and AMP into adenosine, respectively[136]. Interestingly, a recent study has determined that heat-inactivated MSC preserve their immunomodulatory capacity after IV infusion in a lipopolysaccharide-induced model, suggesting that cell-membrane-dependent interactions with immune cells are triggering factors of the immunological regulatory effects[14]. Song et al[107] systemically administered MSC extract obtained by cell lysis in experimental colitis, and their results demonstrated that the MSC extract polarized the macrophage functional phenotyping from M1 to M2. This new therapeutic approach could overcome the low homing efficiency of MSC in patients with IBD.
It is known that MSC-mediated immunosuppression is induced by inflammatory cytokines. Treatment with IFN-γ, TNF-α, and IL-1β causes MSC to express immunomodulatory molecules that mediate the suppression via cell-contact dependent mechanisms, including TLRs, PD-L1/PD-1 pathway, and FAS-L/FAS interaction[73,138-140] (Figure 2). Duijvestein et al[141] showed that MSC stimulated with IFN-γ enhance their immunosuppressive capacities, resulting in diminished mucosal damage in experimental colitis. Kang et al[142] found that IFN-γ primed MSC secrete tryptophanyl-tRNA synthetase and alleviate the experimental colitis by inducing apoptosis of immune cells. In our previous study, we have shown that MSC stimulated with IFN-γ decreased lymphocyte population in colonic organ culture of DSS-treated mice, but no effect of CM treatment was observed. That could be explained by the fact that T cell immunosuppression has a partial dependence on the cell-cell contact mechanism[119]. Furthermore, Fan et al[143] showed that IL-1β primed MSC have enhanced immunosuppressive capacities and migration ability, and Cheng et al[144] demonstrated that IL-25 primed MSC inhibited Th17 immune response and induced regulatory T cell phenotyping in DSS-induced colitis[143,144].
In another previous study conducted by our research group, we developed a cell-free therapy based on small particles from the membranes of MSC stimulated and unstimulated with IFN-γ. These particles contain the membrane-bound proteins of MSC, several of which have an immunomodulatory function and may also overcome the low homing efficiency of MSC. We have demonstrated that membrane particles keep immune regulatory properties of MSC and are potentially capable of passing the lung barrier and exerting their effects at sites beyond the lungs[136]. Most importantly, we also found that the immunomodulation induced by membrane particles stimu-lated and unstimulated with IFN- γ is different. According to this information, the surface phenotype of MSC (protein molecules present on their membrane) is determinants for the therapeutic effect of MSC in patients with IBD.
Taken together, these findings highlight that bioactive factors, cell surface proteins, and metabolic pathways derived from MSC offer unique opportunities for the development of promising cell-free therapies for IBD that associate the potential of MSC with the reduction of the practical complexities arising from the use of living cells. Recent experimental studies have suggested that not only the secretion of bioactive factors but also the surface proteins of MSC have great therapeutic potential that can overcome the deficiencies of current therapy for IBD and could open new frontiers in gastrointestinal medicine.
The authors thank the graphic designer, Lucas Dreher, for creating the figures.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Netherlands
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hassouna AAA, Huang YC S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 992] [Cited by in F6Publishing: 956] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 2. | Uniken Venema WT, Voskuil MD, Dijkstra G, Weersma RK, Festen EA. The genetic background of inflammatory bowel disease: from correlation to causality. J Pathol. 2017;241:146-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 3. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2894] [Cited by in F6Publishing: 3122] [Article Influence: 183.6] [Reference Citation Analysis (9)] |
| 4. | Bao X, Feng Z, Yao J, Li T, Yin Y. Roles of Dietary Amino Acids and Their Metabolites in Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017;2017:6869259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 6. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 971] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 7. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 8. | Hao L, Sun H, Wang J, Wang T, Wang M, Zou Z. Mesenchymal stromal cells for cell therapy: besides supporting hematopoiesis. Int J Hematol. 2012;95:34-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 526] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 794] [Cited by in F6Publishing: 850] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 11. | Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 12. | Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee W, Choi S. The therapeutic effect of human adult stem cells derived from adipose tissue in endotoxemic rat model. Int J Med Sci. 2013;10:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Luk F, de Witte SF, Korevaar SS, Roemeling-van Rhijn M, Franquesa M, Strini T, van den Engel S, Gargesha M, Roy D, Dor FJ, Horwitz EM, de Bruin RW, Betjes MG, Baan CC, Hoogduijn MJ. Inactivated Mesenchymal Stem Cells Maintain Immunomodulatory Capacity. Stem Cells Dev. 2016;25:1342-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014;49:270-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Abreu SC, Weiss DJ, Rocco PR. Extracellular vesicles derived from mesenchymal stromal cells: a therapeutic option in respiratory diseases? Stem Cell Res Ther. 2016;7:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Crivelli B, Chlapanidas T, Perteghella S, Lucarelli E, Pascucci L, Brini AT, Ferrero I, Marazzi M, Pessina A, Torre ML; Italian Mesenchymal Stem Cell Group (GISM). Mesenchymal stem/stromal cell extracellular vesicles: From active principle to next generation drug delivery system. J Control Release. 2017;262:104-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Fierabracci A, Del Fattore A, Luciano R, Muraca M, Teti A, Muraca M. Recent advances in mesenchymal stem cell immunomodulation: the role of microvesicles. Cell Transplant. 2015;24:133-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 977] [Cited by in F6Publishing: 985] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 20. | Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 461] [Cited by in F6Publishing: 466] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 517] [Cited by in F6Publishing: 505] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 22. | Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1179] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 23. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1552] [Cited by in F6Publishing: 1478] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 24. | Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, Bruno S, Amoroso A, Giovarelli M, Porta M, Perin PC, Tetta C, Camussi G, Zanone MM. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014;57:1664-1673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. 2014;33:1055-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 26. | Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 451] [Cited by in F6Publishing: 475] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 27. | Chen PM, Liu KJ, Hsu PJ, Wei CF, Bai CH, Ho LJ, Sytwu HK, Yen BL. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J Leukoc Biol. 2014;96:295-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Sayed M, Drummond CA, Evans KL, Haller ST, Liu J, Xie Z, Tian J. Effects of Na/K-ATPase and its ligands on bone marrow stromal cell differentiation. Stem Cell Res. 2014;13:12-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Vega-Letter AM, Kurte M, Fernández-O'Ryan C, Gauthier-Abeliuk M, Fuenzalida P, Moya-Uribe I, Altamirano C, Figueroa F, Irarrázabal C, Carrión F. Differential TLR activation of murine mesenchymal stem cells generates distinct immunomodulatory effects in EAE. Stem Cell Res Ther. 2016;7:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 265] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 31. | Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 498] [Cited by in F6Publishing: 476] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 32. | Hoogduijn MJ. Are mesenchymal stromal cells immune cells? Arthritis Res Ther. 2015;17:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 34. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 509] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 35. | Lu W, Fu C, Song L, Yao Y, Zhang X, Chen Z, Li Y, Ma G, Shen C. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int J Cardiol. 2013;165:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1309] [Cited by in F6Publishing: 1307] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 37. | Singh UP, Singh NP, Singh B, Mishra MK, Nagarkatti M, Nagarkatti PS, Singh SR. Stem cells as potential therapeutic targets for inflammatory bowel disease. Front Biosci (Schol Ed). 2010;2:993-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 362] [Cited by in F6Publishing: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 39. | Lichtenstein GR, Abreu MT, Cohen R, Tremaine W; American Gastroenterological Association. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 354] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 40. | Sandborn WJ. The future of inflammatory bowel disease therapy: where do we go from here? Dig Dis. 2012;30 Suppl 3:140-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 536] [Cited by in F6Publishing: 584] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 42. | Grégoire C, Lechanteur C, Briquet A, Baudoux É, Baron F, Louis E, Beguin Y. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:205-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [PubMed] [Cited in This Article: ] |
| 44. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15372] [Cited by in F6Publishing: 14786] [Article Influence: 591.4] [Reference Citation Analysis (0)] |
| 45. | Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 577] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 46. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11055] [Cited by in F6Publishing: 11806] [Article Influence: 694.5] [Reference Citation Analysis (1)] |
| 47. | Araújo AB, Salton GD, Furlan JM, Schneider N, Angeli MH, Laureano ÁM, Silla L, Passos EP, Paz AH. Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy. 2017;19:577-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1715] [Cited by in F6Publishing: 1652] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 49. | Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, Dow S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl Med. 2018;7:456-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 50. | Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 51. | Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 2014;4:5-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 29] [Reference Citation Analysis (0)] |
| 52. | Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 53. | Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle. 2012;11:377-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 54. | Hanson SE, Kim J, Hematti P. Comparative analysis of adipose-derived mesenchymal stem cells isolated from abdominal and breast tissue. Aesthet Surg J. 2013;33:888-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1018] [Cited by in F6Publishing: 1115] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 56. | Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010;2:81-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Martinelli D, Pereira RC, Mogni M, Benelli R, Mastrogiacomo M, Coviello D, Cancedda R, Gentili C. A humanized system to expand in vitro amniotic fluid-derived stem cells intended for clinical application. Cytotherapy. 2016;18:438-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 59. | Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120-4126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 963] [Cited by in F6Publishing: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 60. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1141] [Cited by in F6Publishing: 1067] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 61. | Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 872] [Cited by in F6Publishing: 989] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 62. | Jorgensen C, Djouad F, Apparailly F, Noël D. Engineering mesenchymal stem cells for immunotherapy. Gene Ther. 2003;10:928-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 63. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 64. | Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 279] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 65. | Engela AU, Hoogduijn MJ, Boer K, Litjens NH, Betjes MG, Weimar W, Baan CC. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol. 2013;173:343-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 66. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 430] [Cited by in F6Publishing: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 67. | Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, Li H, Zhou M, Huang F, Fan Z, Sun J, Liu Q, Ke M, Li X, Zhang Q, Xiang AP. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29:636-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 68. | Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 668] [Cited by in F6Publishing: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 69. | Chen X, Shao H, Zhi Y, Xiao Q, Su C, Dong L, Liu X, Li X, Zhang X. CD73 Pathway Contributes to the Immunosuppressive Ability of Mesenchymal Stem Cells in Intraocular Autoimmune Responses. Stem Cells Dev. 2016;25:337-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 686] [Cited by in F6Publishing: 803] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 71. | Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 72. | Mundra V, Gerling IC, Mahato RI. Mesenchymal stem cell-based therapy. Mol Pharm. 2013;10:77-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33:136-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 74. | Asami T, Ishii M, Fujii H, Namkoong H, Tasaka S, Matsushita K, Ishii K, Yagi K, Fujiwara H, Funatsu Y, Hasegawa N, Betsuyaku T. Modulation of murine macrophage TLR7/8-mediated cytokine expression by mesenchymal stem cell-conditioned medium. Mediators Inflamm. 2013;2013:264260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 75. | Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 427] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 76. | Chiossone L, Conte R, Spaggiari GM, Serra M, Romei C, Bellora F, Becchetti F, Andaloro A, Moretta L, Bottino C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 2016;34:1909-1921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 77. | Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, Baan C, Dahlke MH. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10:1496-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 78. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2455] [Cited by in F6Publishing: 2304] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 79. | Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 80. | Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, Zago MA, Panepucci RA. Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 2011;7:66-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 369] [Cited by in F6Publishing: 356] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 82. | Quaedackers ME, Baan CC, Weimar W, Hoogduijn MJ. Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. Eur J Immunol. 2009;39:3436-3446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 290] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 84. | Reis M, Mavin E, Nicholson L, Green K, Dickinson AM, Wang XN. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front Immunol. 2018;9:2538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 85. | Ivanova-Todorova E, Bochev I, Mourdjeva M, Dimitrov R, Bukarev D, Kyurkchiev S, Tivchev P, Altunkova I, Kyurkchiev DS. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 86. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 577] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 87. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3271] [Cited by in F6Publishing: 3170] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 88. | Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 683] [Cited by in F6Publishing: 657] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 89. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 773] [Cited by in F6Publishing: 756] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 90. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 91. | Mayer L, Pandak WM, Melmed GY, Hanauer SB, Johnson K, Payne D, Faleck H, Hariri RJ, Fischkoff SA. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant crohn's disease: a phase 1 study. Inflamm Bowel Dis. 2013;19:754-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 92. | Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, Phillips M, Herrmann RP. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014;12:64-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 93. | Melmed GY, Pandak WM, Casey K, Abraham B, Valentine J, Schwartz D, Awais D, Bassan I, Lichtiger S, Sands B, Hanauer S, Richards R, Oikonomou I, Parekh N, Targan S, Johnson K, Hariri R, Fischkoff S. Human Placenta-derived Cells (PDA-001) for the Treatment of Moderate-to-severe Crohn's Disease: A Phase 1b/2a Study. Inflamm Bowel Dis. 2015;21:1809-1816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Ciccocioppo R, Corazza GR. Mesenchymal stromal cells: an opportunity to treat chronic inflammatory intestinal conditions. Cytotherapy. 2018;20:1223-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ, Verspaget HW, Fibbe WE, van der Meulen-de Jong AE, Hommes DW. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2015;149:918-27.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 96. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 634] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 97. | Dhere T, Copland I, Garcia M, Chiang KY, Chinnadurai R, Prasad M, Galipeau J, Kugathasan S. The safety of autologous and metabolically fit bone marrow mesenchymal stromal cells in medically refractory Crohn's disease - a phase 1 trial with three doses. Aliment Pharmacol Ther. 2016;44:471-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 98. | Liang J, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cell transplantation in seven patients with refractory inflammatory bowel disease. Gut. 2012;61:468-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | da Costa Gonçalves F, Grings M, Nunes NS, Pinto FO, Garcez TN, Visioli F, Leipnitz G, Paz AH. Antioxidant properties of mesenchymal stem cells against oxidative stress in a murine model of colitis. Biotechnol Lett. 2017;39:613-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Wang M, Liang C, Hu H, Zhou L, Xu B, Wang X, Han Y, Nie Y, Jia S, Liang J, Wu K. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci Rep. 2016;6:30696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 101. | Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Souza SA, Gutfilen B, Fonseca LM, Elia C, Madi K, Schanaider A, Rossi MI, Souza HS. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 102. | Gonçalves Fda C, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP, Cirne-Lima EO, Meurer L, Paz AH. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol. 2014;20:18228-18239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 51] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 103. | Luk F, de Witte SF, Bramer WM, Baan CC, Hoogduijn MJ. Efficacy of immunotherapy with mesenchymal stem cells in man: a systematic review. Expert Rev Clin Immunol. 2015;11:617-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int. 2013;2013:732742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 289] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 105. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1329] [Cited by in F6Publishing: 1353] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 106. | Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W, Baan CC. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825-2835. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 107. | Song JY, Kang HJ, Hong JS, Kim CJ, Shim JY, Lee CW, Choi J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7:9412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 108. | Huang YC, Parolini O, Deng L. The potential role of microvesicles in mesenchymal stem cell-based therapy. Stem Cells Dev. 2013;22:841-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Dotti I, Salas A. Potential Use of Human Stem Cell-Derived Intestinal Organoids to Study Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2018;24:2501-2509. [PubMed] [Cited in This Article: ] |
| 110. | Song WJ, Li Q, Ryu MO, Ahn JO, Ha Bhang D, Chan Jung Y, Youn HY. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci Rep. 2017;7:5187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 111. | Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 112. | Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M, Hashemi SM. Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 2019;234:9910-9926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 113. | Wang J, Cen P, Chen J, Fan L, Li J, Cao H, Li L. Role of mesenchymal stem cells, their derived factors, and extracellular vesicles in liver failure. Stem Cell Res Ther. 2017;8:137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X, Xu W. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Relieve Inflammatory Bowel Disease in Mice. Biomed Res Int. 2017;2017:5356760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 115. | Robinson AM, Sakkal S, Park A, Jovanovska V, Payne N, Carbone SE, Miller S, Bornstein JC, Bernard C, Boyd R, Nurgali K. Mesenchymal stem cells and conditioned medium avert enteric neuropathy and colon dysfunction in guinea pig TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1115-G1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 116. | Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR, Hashemi SM. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol. 2018;233:8754-8766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Pouya S, Heidari M, Baghaei K, Asadzadeh Aghdaei H, Moradi A, Namaki S, Zali MR, Hashemi SM. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 118. | Robinson AM, Miller S, Payne N, Boyd R, Sakkal S, Nurgali K. Neuroprotective Potential of Mesenchymal Stem Cell-Based Therapy in Acute Stages of TNBS-Induced Colitis in Guinea-Pigs. PLoS One. 2015;10:e0139023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | DA Costa Gonçalves F, Serafini MA, Mello HF, Pfaffenseller B, Araújo AB, Visioli F, Paz AH. Bioactive factors secreted from mesenchymal stromal cells protect the intestines from experimental colitis in a three-dimensional culture. Cytotherapy. 2018;20:1459-1471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, Guo Y, Xia X, Wang Y, Wang H, Wang WE, Zeng C. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. 2013;8:e59020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 121. | Inukai T, Katagiri W, Yoshimi R, Osugi M, Kawai T, Hibi H, Ueda M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem Biophys Res Commun. 2013;430:763-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 122. | Zagoura DS, Roubelakis MG, Bitsika V, Trohatou O, Pappa KI, Kapelouzou A, Antsaklis A, Anagnou NP. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. 2012;61:894-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 123. | Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, Brook G, Schoenen J, Franzen R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS One. 2013;8:e69515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 124. | Liu J, Han Z, Han Z, He Z. Mesenchymal stem cell-conditioned media suppresses inflammation-associated overproliferation of pulmonary artery smooth muscle cells in a rat model of pulmonary hypertension. Exp Ther Med. 2016;11:467-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Park JH, Hwang I, Hwang SH, Han H, Ha H. Human umbilical cord blood-derived mesenchymal stem cells prevent diabetic renal injury through paracrine action. Diabetes Res Clin Pract. 2012;98:465-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 126. | Merino AM, Hoogduijn MJ, Borras FE, Franquesa M. Therapeutic potential of extracellular vesicles. Front Immunol. 2014;5:658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11:150-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 128. | Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 129. | Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 496] [Cited by in F6Publishing: 471] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 130. | Wong WY, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP, Tai WC. Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 2016;16:1131-1145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 131. | Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, Dong Y, Liu Y, Nan Z, Wang Y, Yu T, Liu X. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 132. | Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 133. | Wu Y, Qiu W, Xu X, Kang J, Wang J, Wen Y, Tang X, Yan Y, Qian H, Zhang X, Xu W, Mao F. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquitination. Am J Transl Res. 2018;10:2026-2036. [PubMed] [Cited in This Article: ] |
| 134. | de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36:602-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 135. | Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 136. | Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, López-Iglesias C, Baan CC, Merino A, Hoogduijn MJ. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci Rep. 2017;7:12100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 137. | Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, Baan CC, Hoogduijn MJ, Franquesa M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front Immunol. 2017;8:1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 138. | Cao W, Cao K, Cao J, Wang Y, Shi Y. Mesenchymal stem cells and adaptive immune responses. Immunol Lett. 2015;168:147-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 139. | Shi Y, Hu G, Su J, Li W, Chen Q, Shou P, Xu C, Chen X, Huang Y, Zhu Z, Huang X, Han X, Xie N, Ren G. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 2010;20:510-518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 140. | Sivanathan KN, Gronthos S, Rojas-Canales D, Thierry B, Coates PT. Interferon-gamma modification of mesenchymal stem cells: implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation. Stem Cell Rev. 2014;10:351-375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 141. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 142. | Kang I, Lee BC, Lee JY, Kim JJ, Lee SE, Shin N, Choi SW, Kang KS. Interferon-γ-mediated secretion of tryptophanyl-tRNA synthetases has a role in protection of human umbilical cord blood-derived mesenchymal stem cells against experimental colitis. BMB Rep. 2019;52:318-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 143. | Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 155] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 144. | Cheng W, Su J, Hu Y, Huang Q, Shi H, Wang L, Ren J. Interleukin-25 primed mesenchymal stem cells achieve better therapeutic effects on dextran sulfate sodium-induced colitis via inhibiting Th17 immune response and inducing T regulatory cell phenotype. Am J Transl Res. 2017;9:4149-4160. [PubMed] [Cited in This Article: ] |