Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1385
Revised: March 4, 2003
Accepted: March 15, 2003
Published online: July 15, 2003
Diverticular disease of the colon is a common disease worldwide. Although the disease is asymptomatic in about 70%-80% of patients, it represents, at least in Western countries, one of the most important gastrointestinal diseases in terms of direct and indirect health costs. Pathogenesis of the disease is still unknown. However, it is the result of complex interactions between colonic structure, intestinal motility, diet and genetic factors. Whilst efficacious preventive strategies remain to be identified, fibre supplementation in the diet is recommended. Why symptoms develop is still unclear. Results of recent experimental studies on irritable bowel syndrome speculated that low grade inflammation of colonic mucosa, induced by changes in bacterial microflora, could affect the enteric nervous system, which is crucial for normal gut function, thus favouring symptom development. This hypothesis could be extrapolated also for diverticular disease, since bacterial overgrowth is present, at least in a subgroup of patients. These perspectives on symptom development are reviewed and new therapeutic approaches are hypothesized.
- Citation: Colecchia A, Sandri L, Capodicasa S, Vestito A, Mazzella G, Staniscia T, Roda E, Festi D. Diverticular disease of the colon: New perspectives in symptom development and treatment. World J Gastroenterol 2003; 9(7): 1385-1389
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1385.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1385
Diverticular disease (DD) of the colon is very common especially in the elderly[1]. Although DD is present worldwide, a higher incidence has been reported in developed countries, compared to underdeveloped countries[2]. DD has important socioeconomic implications on the health system, due not only to its worldwide distribution, but also to the lack of knowledge concerning its natural history and the risk factors involved in development of symptoms. As a consequence, it is difficult to define efficacious preventive strategies.
According to a recent report by the American Gastroente-rological Association on the burden of digestive diseases in the United States[3], DD represents, in terms of direct and indirect costs, the 5th most important gastrointestinal disease, with a mortality-rate of 2.5 per 100000 per year.
DD is a manifestation of an acquired deformity of the colon wall and is characterized by the development of pseudo-diverticula, i.e., protrusions of the mucosa and submucosa through the muscular wall. These protrusions occur in weak areas of the wall where blood vessels penetrate due to the high pressure inside the colon[4].
No uniform definition of DD exists as yet. However, according to a recent Consensus Development Conference[5], colonic DD can be defined as a condition involving primarily the sigmoid region of the colon. This condition may be either asymptomatic, and is referred to as "diverticulosis", or associated with symptoms, and termed "diverticular disease", which may, in turn, be either complicated or non-complicated. The term diverticulitis is used to indicate inflammation of the bowel wall.
A brief review is made herein of the epidemiology, pathogenesis and treatment of DD, and in accordance with recent experimental results, some hypotheses on pathogenesis of symptom development are advanced, which may be usefully taken into consideration in defining more effective preventive strategies.
Epidemiological studies have demonstrated that the prevalence rates differ considerably from one country to another. Indeed, the disease is very common in Western developed countries, with a much higher frequency (30%-40%) than that in Eastern and developing countries (1%-4%)[2,6-8].
Furthermore, in Western countries, about 90% of the patients have diverticula in the sigmoid segment, while in Asian populations the caecum and the right colon are most frequently involved[9-12].
Recent studies, however, indicate an increase in the prevalence rate of DD also in Eastern populations, possibly due to increasing globalization and the fact that lifestyle has become increasingly similar in various parts of the world[11-13].
No definitive data have emerged, so far, on the prevalence rate of DD, mainly because the majority of patients are asymptomatic, and are hence difficult to identify. The most important risk factor appears to be aging, the prevalence rate increases with age and varies from < 10% in subjects < 40 years old to an estimated 50%-66% in patients > 85 years[1,14,15].
Some studies have reported a slightly higher frequency in females, however, no sex-related predominance has been demonstrated[16,17].
The pathogenetic mechanisms of DD are still poorly understood, however it is generally recognized that these are probably related to complex interactions between colon structure, intestinal motility, diet as well as genetic features[18].
DD has been correlated with "a low residue diet"[2], and furthermore, the prevalence of diverticulosis is higher with a reduced dietary intake of raw fibres[19] and lower in vegetarians[20]. These data are supported by studies both in animals[21,22] and humans[23-28]. However, although the fibre deficiency hypothesis has been widely quoted, conflicting evidence and much controversy still exist[29,30]. The exact mechanism involved remains to be defined, even if prolonged colonic transit time and decreased stool volume in subjects on a low residue diet, seem to induce an increase in intraluminal pressure, which in turn, predisposes to diverticular herniation[31,32]. Furthermore, the lower faecal bile acid output in patients with DD suggests a pathogenetic role of these compounds which stimulate colon motor activity and as a result reduce colonic transit time[33]. Albeit, these hypotheses have not been confirmed by controlled clinical studies comparing healthy subjects with DD patients[1].
As far as colonic structure is concerned, early surgical and autopsy studies demonstrated an association between muscular hypertrophy of the colon and presence of DD[34,35], thus suggesting that increased muscle bulk plays a role in enhancing intraluminal pressure.
Furthermore, electron microscopy evidence of a two-fold increase in elastin content in the taenia coli[36] suggests a further pathogenetic mechanism. The elastin content in the taenia results in contraction and bunching of the circular muscle, giving the appearance of a hypertrophic muscle with narrowing of the bowel lumen.
The increased prevalence of DD with aging could be due to a progressive, age-related accumulation of elastin in the taenia coli[36,37]. In fact, in the elderly, the bowel wall is invariably increased in thickness with reduced elasticity[1] and thus, intraluminal pressure increases and, according to Laplace's law, formation of diverticula is more likely.
The tendency to elastin accumulation in the taenia coli could be resulted from a low residue diet that extends the bowel intermittently and incompletely, thus favouring prolin (an elastin precursor) uptake[1].
Whilst several pathogenetic hypotheses have been advanced to explain the development of colon diverticula, the fact remains that the pathologic aspects of the disease are resulted from lifelong exposure to a low residue diet and a complex interaction between colonic structure, intestinal motility and genetic factors[18].
Current classifications of DD are based on localization, distribution, symptoms, clinical presentation and pathology[1,5,38-40]. Two different types of classification have been proposed: a clinical classification and the Hinchey classification[41], which is used to describe the stages of perforated DD. For the purposes of the present article, the clinical classification is taken into consideration here (Table 1).
| Clinical classification (modified from ref. 5) |
| · Symptomatic uncomplicated disease |
| · Recurrent symptomatic disease |
| · Complicated disease (haemorrhage, abscess, phlegmon, perforation, purulent and faecal peritonitis, stricture, fistula, small-bowel obstruction due to post-inflammatory adhesions) |
| Modified Hinchey classification (modified from refs. 41, 71) |
| · Stage I: pericolic abscess |
| · Stage IIa: distant abscess amenable to percutaneous drainage |
| · Stage IIb: complex abscess associated with/without fistula |
| · Stage III: generalized purulent peritonitis |
| · Stage IV: faecal peritonitis |
However, the hallmark of painful DD is abdominal pain in the absence of any indications of inflammation. Pain is usually colicky, but may also be steady. It is exacerbated by eating, and is typically relieved by flatus or bowel movements. Associated symptoms vary considerably: diarrhoea, constipation, flatulence, heartburn, nausea and vomiting, palpable abdominal mass, abdominal distension[1,5,38].
The natural history of DD remains to be elucidated and the few prospective studies carried out so far[9,42-44] indicate that 80%-85% of patients with DD remain asymptomatic. Of the 15%-20% of patients presenting abdominal pain, approximately 75% have a painful DD whilst the remainder have diverticulitis, as well as complications of diverticulitis and haemorrhage[1]. Furthermore, about 1%-2% will require hospitalisation and 0.5% will require surgery[1,45,46]. Unfortunately, due to the lack of prospective studies, factors predicting the development of symptoms remain to be identified. However, it has been suggested[47] that evaluation of the colonic motility index (pressure amplitude exceeding 120 mmHg), together with a brief history of left lower quadrant pain, a short segment of involved colon and a relatively younger age (about 50 years), may be useful in recognizing a group of patients at risk of developing symptoms.
Moreover, investigations[48,49] have suggested that lack of physical activity is independently associated with an increased risk of symptomatic DD, while smoking, caffeine and alcohol intake are not associated with a substantially increased risk of asymptomatic disease. Furthermore, a significant inverse association has been found between insoluble dietary fibre intake (especially fruit and vegetables, e.g. cellulose fibre) and the risk of subsequently developing symptomatic DD[24]. In contrast, a study on the efficacy of fibre supplementation in symptomatic patients with DD did not lead to an improvement in symptoms[50].
Since few data exist on risk factors, preventive measures for the development of diverticula are only speculative, and can be aimed only at preventing development of symptoms. Despite controversial data, fibre supplementation is recommended[1,38].
The causes of symptom development, in some patients, are still unclear. Since it has been observed that DD patients who have a history of diverticulitis have more episodes of recurrent abdominal pain and impaired bowel function[6,51,52], a possible role of previous episodes of intestinal inflammation may be hypothesised. This finding is not unlike that which has been recently demonstrated in other gastrointestinal diseases such as infectious enteritis and inflammatory bowel disease[53,54] and, as also speculated[55] in irritable bowel syndrome (IBS). In these patients, the presence of a chronic, low-grade intestinal inflammation would induce a sensory-motor dysfunction, leading to symptom development and/or persistence[53-56].
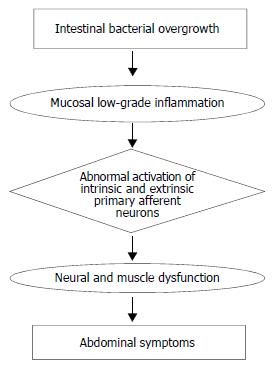
Changes in intestinal microflora could be one of the putative mechanisms responsible for low grade inflammation, at least in IBS[55]. In patients with DD, bacterial overgrowth may be present[57]. This bacterial overgrowth aided by the faecal stasis inside the diverticula, could contribute to chronic low-grade inflammation which sensitises both intrinsic primary efferent and extrinsic primary afferent neurons. These alterations could lead to smooth muscle hypertrophy, and increased sensitivity to abdominal distension[56,58], and finally, to symptom development. This hypothesis is based mainly on experimental studies, investigation in man, being limited at present. However, an increased level of the neuropeptide substance P, which may be related to impaired visceral sensation, has been demonstrated in patients with DD with abdominal pain but without inflammation[59]. This finding is not unlike that observed by Di Sebastiano et al[60] who documented a role of neuroproliferation within the appendix, associated with an increased concentration of substance P and vasoactive intestinal polypeptide, in the pathophysiology of right iliac fossa pain in the absence of inflammation. Moreover, in patients with diverticulitis, abnormal nerves with axonal sprouting have been observed[61], suggesting previous injury. These findings would appear to be compatible with post-inflammatory neural and muscle dysfunction, probably induced also by intestinal bacterial overgrowth, which would contribute to symptom development.
Further studies, both experimental and in man, are obviously needed to confirm the pathogenetic role (which is summarized in Figure 1) of intestinal infection and low grade inflammation, in the development of symptoms in patients with DD.
There is a general consensus that conservative treatment is indicated in cases with newly onset uncomplicated diverticulitis[5,38,62,63]. The rationale for this strategy is that about 50%-70% of patients treated for a first episode of acute diverticulitis will recover and have no further clinical problems. Furthermore, only about 20% of these patients will develop symptoms whilst those with recurrent symptoms have a 60% risk of developing disease complications[5,44].
In patients with uncomplicated diverticulosis, a diet with abundant fruit and vegetables is recommended since it seems that this protective effect reduces symptom development and prevents major complications as demonstrated in uncontrolled studies. Nevertheless, recent guidelines[38] advise the use of a high fibre diet, which should be prescribed also for the well-known potential health benefits.
Anticholinergic and antispasmodic agents may be effective in some cases of uncomplicated DD. However, their use remains to be confirmed by controlled studies.
Although the role of antibiotics in uncomplicated DD is still debated[38], recent clinical studies[64-66] have demonstrated that cyclic administration of rifaximin (Normix®, Alfa Wassermann S.p.A., Alanno Scalo, Chieti, Italy) (a broad-spectrum poorly absorbable antibiotic) is more effective in reducing symptoms than fibre supplementation alone (Table 2).
| First author (year, ref) | Pts (n) | Treatment (type) | Duration (months) | Reduction in symptoms(%) | P |
| Papi (1992, 64) | 217 | R (400 mg daily) + glucomannan (2 g daily) vs glucomannan | 12 | 63.9 vs 47.6 | < 0.001 |
| Papi (1995, 65) | 168 | R (400 mg daily) + glucomannan (2 g daily) vs placebo +glucomannan | 12 | 68.9 vs 38.5 | < 0.001 |
| Latella (2003, 66) | 968 | R (400 mg daily) + glucomannan (4 g daily) vs glucomannan | 12 | 56.5 vs 29.2 | < 0.001 |
Latella et al[66] performed a large, multicentre, prospective, randomized study, enrolling 968 outpatients with symptomatic diverticulosis. Among them, 595 patients received fibre supplement (glucomannan 4 g/d) plus rifaximin 400 mg bid for 7 d, per month, and 373 patients received glucomannan alone. After 12 mo, a significant reduction in the occurrence rate of symptoms was documented in the group treated with rifaximin and fibre. 56.5% of the patients were asymptomatic as compared to 29.2% of the fibre group. Moreover, the incidence of major complications was lower in the rifaximin plus fibre group vs the group treated with fibre alone. The mechanism of of rifaximin in reducing the frequency of symptoms and the rate of complications of DD is only speculative. It has been suggested that rifaximin reduces the metabolic activity of intestinal bacterial flora, the degradation of dietary fibres, and the production of gas. The latter effect is important since an increased production of intestinal gas and of gas-related symptoms such as pain and bloating have recently been documented, in patients with IBS[67]. Furthermore, treatment with non-absorbable antibiotics was shown to reduce symptoms frequency and intensity in these patients. Similar results were obtained by others[68] who documented an association between small intestinal overgrowth and functional intestinal disorders. Moreover, eradication of bacterial overgrowth seems to be related to a reduction in intestinal symptoms.
In conclusion, if low grade mucosal inflammation is confirmed in DD patients, and if such inflammation is provoked and maintained by changes in bacterial microflora as in IBD (change IBD with IBS), then these impairments likely play a role in the pathogenesis and symptom development of both diseases, and thus, new preventive approaches could be identified. According to this hypothesis, cyclic administrations of antibiotics, and in particular of non-absorbable antibiotics such as rifaximin, could reverse the process, i.e., intestinal bacterial overgrowth which is held to trigger the cascade of events which starts from intestinal low grade inflammation to reach symptom generation. In fact, rifaximin, which is highly effective against anaerobic bacteria[69], is effective also in intestinal bacterial overgrowth[70,71].
It is important, at this stage, to identify and characterize those DD patients with intestinal bacterial overgrowth and, then, to perform controlled clinical trials to evaluate the effects of antibiotic administration on symptom and complication frequency, i.e., on the natural history of this disease.
Edited by Xu XQ and Wang XL
| 1. | Farrell RJ, Farrell JJ, Morrin MM. Diverticular disease in the elderly. Gastroenterol Clin North Am. 2001;30:475-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Painter NS, Burkitt DP. Diverticular disease of the colon: a deficiency disease of Western civilization. Br Med J. 1971;2:450-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 571] [Cited by in F6Publishing: 508] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1062] [Cited by in F6Publishing: 966] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | Truelove SC. Movements of the large intestine. Physiol Rev. 1966;46:457-512. [PubMed] [Cited in This Article: ] |
| 5. | Köhler L, Sauerland S, Neugebauer E. Diagnosis and treatment of diverticular disease: results of a consensus development conference. The Scientific Committee of the European Association for Endoscopic Surgery. Surg Endosc. 1999;13:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 317] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Cheskin LJ, Lamport RD. Diverticular disease. Epidemiology and pharmacological treatment. Drugs Aging. 1995;6:55-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | KIM EH. HIATUS HERNIA AND DIVERTICULUM OF THE COLON. THEIR LOW INCIDENCE IN KOREA. N Engl J Med. 1964;271:764-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Vajrabukka T, Saksornchai K, Jimakorn P. Diverticular disease of the colon in a far-eastern community. Dis Colon Rectum. 1980;23:151-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:53-69. [PubMed] [Cited in This Article: ] |
| 10. | Perry PM, Morson BC. Right-sided diverticulosis of the colon. Br J Surg. 1971;58:902-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Chia JG, Wilde CC, Ngoi SS, Goh PM, Ong CL. Trends of diverticular disease of the large bowel in a newly developed country. Dis Colon Rectum. 1991;34:498-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Lee YS. Diverticular disease of the large bowel in Singapore. An autopsy survey. Dis Colon Rectum. 1986;29:330-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 145] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ihekwaba FN. Diverticular disease of the colon in black Africa. J R Coll Surg Edinb. 1992;37:107-109. [PubMed] [Cited in This Article: ] |
| 14. | Young-Fadok TM, Roberts PL, Spencer MP, Wolff BG. Colonic diverticular disease. Curr Probl Surg. 2000;37:457-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Larsson PA. [Diverticulitis is increasing among the elderly. Significant cause of morbidity and mortality]. Lakartidningen. 1997;94:3837-340, 3842. [PubMed] [Cited in This Article: ] |
| 16. | Eide TJ, Stalsberg H. Diverticular disease of the large intestine in Northern Norway. Gut. 1979;20:609-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | KOEHLER R. THE INCIDENCE OF COLONIC DIVERTICULOSIS IN FINLAND AND SWEDEN. Acta Chir Scand. 1963;126:148-155. [PubMed] [Cited in This Article: ] |
| 18. | Simpson J, Scholefield JH, Spiller RC. Pathogenesis of colonic diverticula. Br J Surg. 2002;89:546-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 115] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Heller SN, Hackler LR. Changes in the crude fiber content of the American diet. Am J Clin Nutr. 1978;31:1510-1514. [PubMed] [Cited in This Article: ] |
| 20. | Gear JS, Ware A, Fursdon P, Mann JI, Nolan DJ, Brodribb AJ, Vessey MP. Symptomless diverticular disease and intake of dietary fibre. Lancet. 1979;1:511-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 191] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Hodgson WJ. An interim report on the production of colonic diverticula in the rabbit. Gut. 1972;13:802-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Fisher N, Berry CS, Fearn T, Gregory JA, Hardy J. Cereal dietary fiber consumption and diverticular disease: a lifespan study in rats. Am J Clin Nutr. 1985;42:788-804. [PubMed] [Cited in This Article: ] |
| 23. | Brodribb AJ, Humphreys DM. Diverticular disease: three studies. Part I--Relation to other disorders and fibre intake. Br Med J. 1976;1:424-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 92] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Aldoori WH, Giovannucci EL, Rimm EB, Wing AL, Trichopoulos DV, Willett WC. A prospective study of diet and the risk of symptomatic diverticular disease in men. Am J Clin Nutr. 1994;60:757-764. [PubMed] [Cited in This Article: ] |
| 25. | Brodribb AJ. Treatment of symptomatic diverticular disease with a high-fibre diet. Lancet. 1977;1:664-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 151] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Painter NS, Burkitt DP. Diverticular disease of the colon, a 20th century problem. Clin Gastroenterol. 1975;4:3-21. [PubMed] [Cited in This Article: ] |
| 27. | Leahy AL, Ellis RM, Quill DS, Peel AL. High fibre diet in symptomatic diverticular disease of the colon. Ann R Coll Surg Engl. 1985;67:173-174. [PubMed] [Cited in This Article: ] |
| 28. | Miettinen TA, Tarpila S. Fecal beta-sitosterol in patients with diverticular disease of the colon and in vegetarians. Scand J Gastroenterol. 1978;13:573-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Davey WW. Diet and diverticulitis. Br Med J. 1968;1:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Mendeloff AI. A critique of "fiber deficiency". Am J Dig Dis. 1976;21:109-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | PAINTER NS, TRUELOVE SC. THE INTRALUMINAL PRESSURE PATTERNS IN DIVERTICULOSIS OF THE COLON. I. RESTING PATTERNS OF PRESSURE. II. THE EFFECT OF MORPHINE. Gut. 1964;5:201-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 173] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | PAINTER NS, TRUELOVE SC. THE INTRALUMINAL PRESSURE PATTERNS IN DIVERTICULOSIS OF THE COLON.3. THE EFFECT OF PROSTIGMINE. IV. THE EFFECT OF PETHIDINE AND PROBANTHINE. Gut. 1964;5:365-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 97] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Tarpila S, Miettinen TA, Metsäranta L. Effects of bran on serum cholesterol, faecal mass, fat, bile acids and neutral sterols, and biliary lipids in patients with diverticular disease of the colon. Gut. 1978;19:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | ARFWIDSSON S, KNOCK NG, LEHMANN L, WINBERG T. PATHOGENESIS OF MULTIPLE DIVERTICULA OF THE SOGMOID COLON IN DIVERTICULAR DISEASE. Acta Chir Scand Suppl. 1964;63:SUPPL 342: 1-34268. [PubMed] [Cited in This Article: ] |
| 35. | Slack WW. Bowel muscle in diverticular disease. Gut. 1966;7:668-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 61] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Whiteway J, Morson BC. Elastosis in diverticular disease of the sigmoid colon. Gut. 1985;26:258-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 171] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Smith AN. Colonic muscle in diverticular disease. Clin Gastroenterol. 1986;15:917-935. [PubMed] [Cited in This Article: ] |
| 38. | Stollman NH, Raskin JB. Diagnosis and management of diverticular disease of the colon in adults. Ad Hoc Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1999;94:3110-3121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 39. | Torsoli A, Inoue M, Manousos O, Smith A, Van Steensel CJ. Diverticular disease of the colon: data relevant to management. Gastroenterol Int. 1991;4:3-20. [Cited in This Article: ] |
| 40. | Wong WD, Wexner SD, Lowry A, Vernava A, Burnstein M, Denstman F, Fazio V, Kerner B, Moore R, Oliver G. Practice parameters for the treatment of sigmoid diverticulitis--supporting documentation. The Standards Task Force. The American Society of Colon and Rectal Surgeons. Dis Colon Rectum. 2000;43:290-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 41. | Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg. 1978;12:85-109. [PubMed] [Cited in This Article: ] |
| 42. | Haglund U, Hellberg R, Johnsén C, Hultén L. Complicated diverticular disease of the sigmoid colon. An analysis of short and long term outcome in 392 patients. Ann Chir Gynaecol. 1979;68:41-46. [PubMed] [Cited in This Article: ] |
| 43. | Ambrosetti P, Robert JH, Witzig JA, Mirescu D, Mathey P, Borst F, Rohner A. Acute left colonic diverticulitis in young patients. J Am Coll Surg. 1994;179:156-160. [PubMed] [Cited in This Article: ] |
| 44. | Farmakis N, Tudor RG, Keighley MR. The 5-year natural history of complicated diverticular disease. Br J Surg. 1994;81:733-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Almy TP, Howell DA. Medical progress. Diverticular disease of the colon. N Engl J Med. 1980;302:324-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 243] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Simmang CL, Shires GT. Diverticular disease of the colon. In: Feldman M, Sleisenger MH, Scharschmidt BF, Eds, sleisinger & fordtran's gastrointestinal and liver disease, 6th Edition, Philadelphia, WB. Saunders Company 1998; 1788-1798. [Cited in This Article: ] |
| 47. | Cortesini C, Pantalone D. Usefulness of colonic motility study in identifying patients at risk for complicated diverticular disease. Dis Colon Rectum. 1991;34:339-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Aldoori WH, Giovannucci EL, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Wing AL, Trichopoulos DV, Willett WC. Prospective study of physical activity and the risk of symptomatic diverticular disease in men. Gut. 1995;36:276-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 137] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Aldoori WH, Giovannucci EL, Rimm EB, Wing AL, Trichopoulos DV, Willett WC. A prospective study of alcohol, smoking, caffeine, and the risk of symptomatic diverticular disease in men. Ann Epidemiol. 1995;5:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 88] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Ornstein MH, Littlewood ER, Baird IM, Fowler J, North WR, Cox AG. Are fibre supplements really necessary in diverticular disease of the colon A controlled clinical trial. Br Med J (. Clin Res Ed). 1981;282:1353-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Simpson J, Neal KR, Scholefield JH, Spiller RC. Relation between inflammatory and non inflammatory pain in diverticular disease. Gut. 2000;46:A80. [DOI] [Cited in This Article: ] |
| 52. | Simpson J, Neal KR, Scholefield JH, Spiller RC. Symptomatology following acute diverticulitis. Neurogastroenterol Mot. 2001;13:43. [Cited in This Article: ] |
| 53. | Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 454] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 54. | Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 206] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome. Gut. 2002;51 Suppl 1:i41-i44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 185] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 56. | Collins SM, Vallance B, Barbara G, Borgaonkar M. Putative inflammatory and immunological mechanisms in functional bowel disorders. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:429-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Ventrucci M, Ferrieri A, Bergami R, Roda E. Evaluation of the effect of rifaximin in colon diverticular disease by means of lactulose hydrogen breath test. Curr Med Res Opin. 1994;13:202-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051-1057. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Watanabe T, Kubota Y, Muto T. Substance P containing nerve fibers in rectal mucosa of ulcerative colitis. Dis Colon Rectum. 1997;40:718-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Di Sebastiano P, Fink T, di Mola FF, Weihe E, Innocenti P, Friess H, Büchler MW. Neuroimmune appendicitis. Lancet. 1999;354:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Brewer DB, Thompson H, Haynes IG, Alexander-Williams J. Axonal damage in Crohn's disease is frequent, but non-specific. J Pathol. 1990;161:301-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Larson DM, Masters SS, Spiro HM. Medical and surgical therapy in diverticular disease: a comparative study. Gastroenterology. 1976;71:734-737. [PubMed] [Cited in This Article: ] |
| 63. | Ferzoco LB, Raptopoulos V, Silen W. Acute diverticulitis. N Engl J Med. 1998;338:1521-1526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 249] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 64. | Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin on symptoms of uncomplicated diverticular disease of the colon. A pilot multicentre open trial. Diverticular Disease Study Group. Ital J Gastroenterol. 1992;24:452-456. [PubMed] [Cited in This Article: ] |
| 65. | Papi C, Ciaco A, Koch M, Capurso L. Efficacy of rifaximin in the treatment of symptomatic diverticular disease of the colon. A multicentre double-blind placebo-controlled trial. Aliment Pharmacol Ther. 1995;9:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 103] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 66. | Latella G, Pimpo MT, Sottili S, Zippi M, Viscido A, Chiaramonte M, Frieri G. Rifaximin improves symptoms of acquired uncomplicated diverticular disease of the colon. Int J Colorectal Dis. 2003;18:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Di Stefano M, Strocchi A, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Non-absorbable antibiotics for managing intestinal gas production and gas-related symptoms. Aliment Pharmacol Ther. 2000;14:1001-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503-3506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 469] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 69. | Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs. 1995;49:467-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Sher ME, Agachan F, Bortul M, Nogueras JJ, Weiss EG, Wexner SD. Laparoscopic surgery for diverticulitis. Surg Endosc. 1997;11:264-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |