Published online Jan 15, 2003. doi: 10.3748/wjg.v9.i1.35
Revised: April 29, 2002
Accepted: June 1, 2002
Published online: January 15, 2003
AIM: To detect the expression of PTEN encoding product in normal mucosa, intestinal metaplasia (IM), dysplasia and carcinoma of the stomach, and to investigate its clinical implication in tumorigenesis and progression of gastric carcinoma.
METHODS: Formalin-fixed paraffin embedded specimens from 184 cases of gastric carcinoma, their adjacent normal mucosa, IM and dysplasia were evaluated for PTEN protein expression by SABC immunohistochemistry. PTEN expression was compared with tumor stage, lymph node metastasis, Lauren’s and WHO’s histological classification of gastric carcinoma. Expression of VEGF was also detected in 60 cases of gastric carcinoma and its correlation with PTEN was concerned.
RESULTS: The positive rates of PTEN protein were 100% (102/102), 98.5% (65/66), 66.7% (4/6) and 47.8% (88/184) in normal mucosa, IM, dysplasia and carcinoma of the stomach, respectively. The positive rates in dysplasia and carcinoma were lower than in normal mucosa and IM (P < 0.01). Advanced gastric cancers expressed less frequent PTEN than early gastric cancer (42.9% vs 67.6%, P < 0.01). The positive rate of PTEN protein was lower in gastric cancer with than without lymph node metastasis (40.3% vs 63.3%, P < 0.01). PTEN was less expressed in diffuse-type than in intestinal-type gastric cancer (41.5% vs 57.8%, P < 0.05). Signet ring cell carcinoma showed the expression of PTEN at the lowest level (25.0%, 7/28); less than well and moderately differentiated ones (P < 0.01). Expression of PTEN was not correlated with expression of VEGF (P > 0.05).
CONCLUSION: Loss or reduced expression of PTEN protein occures commonly in tumorigenesis and progression of gastric carcinoma. It is suggested that PTEN can be an objective marker for pathologically biological behaviors of gastric carcinoma.
- Citation: Yang L, Kuang LG, Zheng HC, Li JY, Wu DY, Zhang SM, Xin Y, Chen Y, Yang S. PTEN encoding product: A marker for tumorigenesis and progression of gastric carcinoma. World J Gastroenterol 2003; 9(1): 35-39
- URL: https://www.wjgnet.com/1007-9327/full/v9/i1/35.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i1.35
PTEN/MMAC1/TEP1 gene (phosphatase and tensin homology deleted from chromosome ten/mutated in multiple advanced cancer 1/TGF-β-regulated and epithelial cell enriched phosphatase 1) was the firstly defined tumor suppressor which product acted as phosphatase and shared extensive homology with cytoskeletal protein, mapping to human chromosome 10q23.3. PTEN encoding product could not only dephosphorylate the phoshatidylinositol-3, 4, 5-triphosphate (PIP3), but also be involved in cytoskeletal reconstruction and cellular mobility[1-6]. Recently, many studies showed there were several putative mechanisms relating to tumor suppression as follows: inhibiting cell invasion and metastasis by dephosphorating focal adhesion kinase (FAK); inhibiting cell apoptosis and increasing cell growth by dephosphorating PIP3; restraining cell differentiation by inhibiting mitogen-activated protein kinase (MAPK) signal pathway[7-9]. Mutation or abnormal expression of PTEN protein occurred commonly in multiple tumors and significantly correlates with tumorigenesis and progression of different malignancies[10-20]. It was reportedly suggested that deletion or mutation of PTEN could enhance the expression of vascular epithelial growth factor (VEGF) and stimulate the proliferation of microvessels in tumor tissues, which in turn closely correlated with tumor invasion and metastasis[21-28].
Gastric carcinoma was one of the commonest malignancies in the world, and even the most frequent in China[29]. Although the achievement of early diagnosis and treatment have somewhat improved the patients’ outcome, gastric cancer still remains the major killer among Chinese because the mechanisms of its tumorigenesis and progression were unclear[30]. In this study, we detected the expression of PTEN proteins in gastric cancer and its adjacent noncancerous mucosa, compared PTEN protein expression with its pathologically biological behaviors, and discussed the relationship between the expression of PTEN and VEGF in order to explore the role of PTEN gene product in tumorigenesis and progression of gastric cancer, and to provide scientific foundation for evaluating prognosis of gastric carcinoma.
One hundred and eighty-four cases of surgically removed specimens of gastric carcinoma were collected from Cancer Institute, China Medical University. This study included 102 cases of adjacent normal mucosa, 63 cases of adjacent IM and 6 cases of adjacent dysplasia. According to clinical staging, 37 cases were early, and 147 cases advanced. According to metastasis, 124 cases were accompanied with lymph node metastasis, 6 with liver metastasis (4 of them with lymph node metastasis) and 2 with ovary metastasis. All gastric specimens were classified according to the Lauren’s and WHO’s histological classification criteria.
All specimens were fixed in 4% formaldehyde solution, embedded in paraffin and incised into 5 μm sections. The rabbit anti-human polyclonal antibody against PTEN (ready to use) and mouse anti-human monoclonal antibody against VEGF (ready to use) were purchased from Maixim Biotech. SABC complex kit was from Boster Biotech. For negative control, sections were incubated with PBS (0.01 mol/L, pH7.4) instead of the primary antibodies.
Clearly brown staining was restricted to cytoplasm, which was considered as positive for PTEN or VEGF. Slides were scored semi-quantitatively based on staining intensity and distribution. Two pathologists assessed the positive rate according to the percent of positive cells in all counted cells from 5 randomly selected representative fields. The degree of staining was graded in the light of proportion of positive cells as follows: negative (-), positive rate < 5%; weakly positive (+); 5%-25%; moderately positive (++): 25%-50%; strongly positive (+++~++++): > 50%.
Statistical evaluation was performed by chi-square test to differentiate the rates between two groups. P-value less than 0.05 was considered as statistically significant. SPSS 10.0 software was employed to analyze all data.
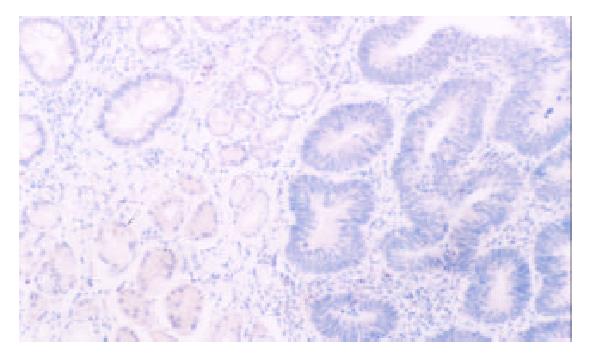
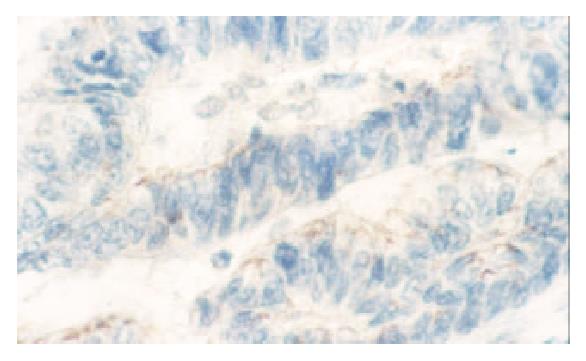
PTEN was expressed in normal mucosa, intestinal metaplasia, dysplasia and carcinoma of the stomach at the rate of 100% (102/102), 98.5% (65/66), 66.7% (4/6), 47.8% (88/184), respectively. Dysplasia and carcinoma expressed less frequent than normal mucosa or intestinal metaplasia (P < 0.01) (Table 1, Figure 1, Figure 2).
Positive rate of PTEN in advanced gastric carcinoma (AGC) was 42.9% (63/147), lower than in early one (EGC)(67.6%, 25/37) (P < 0.01). In 124 cases with lymph node metastasis, 50 expressed PTEN protein (40.3%), whose positive rate of PTEN was higher than those without lymph node metastasis (63.3%, 38/60) (P < 0.01). 41.5 percent of 118 diffuse-type gastric cancers expressed PTEN, less than that of intestinal-type ones (51.8%, 37/64). Signet ring cell carcinoma expressed PTEN protein at the lowest level (25.0%, 7/28), more than well and moderately differentiated adenocarcinoma (61.8%, 21/34) (P < 0.01) (Table 2).
| n | PTEN expression | % | ||
| +~++++ | - | |||
| Clinicopathological staging | ||||
| Early | 37 | 25 | 12 | 67.6 |
| Advanced | 147 | 63 | 84 | 42.9a |
| Lymph node metastasis | ||||
| + | 124 | 50 | 74 | 40.3b |
| - | 60 | 38 | 22 | 63.3 |
| Lauren’s classification | ||||
| Intestinal type | 64 | 37 | 27 | 57.8 |
| Diffused type | 118 | 49 | 96 | 41.5c |
| Mixed type | 2 | 2 | 0 | 100.0 |
| WHO’s histological classification | ||||
| Papillary adenocarcinoama | 20 | 10 | 10 | 50.0 |
| Well-differentiaed adenocarcinoma | 9 | 5 | 4 | 55.6 |
| Moderated-differentiated adenocarcinoma | 25 | 16 | 9 | 64.0 |
| Poorly-differentiated adenocarcinoma | 85 | 39 | 64 | 45.9 |
| Undifferentiated adenocarcinoma | 5 | 3 | 2 | 60.0 |
| Signet ring-cell carcinoma(SRC) | 28 | 7 | 21 | 25.0d |
| Mucinous adenocarcinoma | 10 | 6 | 4 | 60.0 |
| Carcinoid | 1 | 1 | 0 | - |
| Squamous cell carcinoma | 1 | 1 | 0 | - |
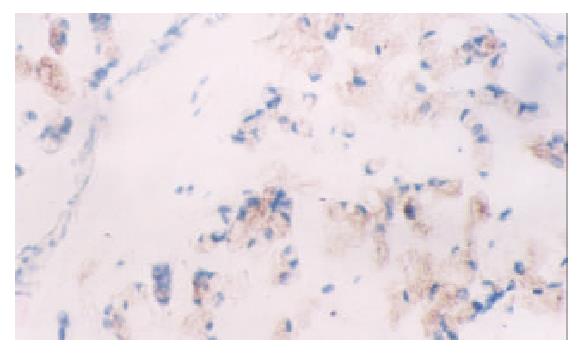
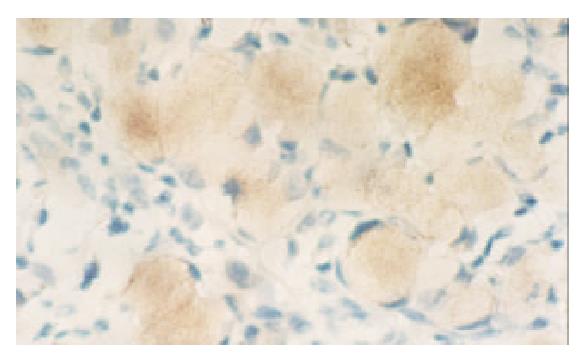
None of the gastric normal mucosa showed expression of VEGF, while 75.0 percent of gastric carcinoma expressed it (45/60) (P < 0.05) (Figure 3, Figure 4). The PTEN-positive cases expressed VEGF at the rate of 78.1% (25/32), whereas PTEN-negative ones did it at the rate of 71.4% (20/28). Both rates were not significantly different by statistical analysis (P > 0.05).
Deletion or down-regulation of tumor suppressing genes plays an important role in the multiple steps of tumorigenesis and progression of gastric carcinoma. Previous studies on the relationship between alteration of tumor suppressor genes and the development of gastric carcinoma focused on p53[31,32], p16[33], p27[34], p33 (ING1)[35], RB[36], DCC[37] etc. However, few reports were involved in the newly discovered tumor suppressing gene-PTEN in tumorigenesis and progression of gastric carcinoma.
As a tumor-suppressing gene, PTEN makes great contribution to cellular differentiation, reproduciton and apoptosis, as well as cellular adhesion and mobility. Some studies showed down-regulation of PTEN protein expression due to genetic changes like mutation, loss of heterozygosity, hypermethylation in gastric cancer, prostrate cancer and breast cancer[2,14,16,19,38]. Our results showed that decreased expression of PTEN during the courses of normal mucosa→intestinal metaplasia→dysplasia→carcinoma. Gastric dysplasia or carcinoma expressed less PTEN than normal mucosa or intestinal metaplasia (P < 0.01), revealing that genetic changes of PTEN gene may play an important role in malignant transition of epithelial cells of gastric mucosa.
Low expression of PTEN gene product was involved in clinicopathological stage and metastasis of stomach neoplasms. We found that 42.9 percent of AGC expressed PTEN, less than EGC (P < 0.01). Positive rate of PTEN was lower in gastric cancer with than without lymph node metastasis (40.3% vs 63.3%, P < 0.01). One of the six liver metastases showed negative expression of PTEN in primary or liver metastasis, while the other five cases with liver metastasis showed reduced expression of PTEN protein. These results were similar to other kinds of tumors[39-46]. It is suggested that deletion or reduced expression of PTEN protein probably facilitate the metastatic ability of gastric cancer cells. Hwang et al[47] found that PTEN could enhance mobility and metastasis of tumor cells by regulating matrix metalloproteinases (MMPs) and VEGF. There was another report that PTEN dephosphorated FAK so as to be involved in cellular adhesion[7]. Deletion or reduced expression of PTEN could result in decreasing cellular adhesion, increasing synthesis of MMPs and VEGF, which subsequently contributed to invasion and angiogenesis of cancer cells. These biological effects possibly underlay prelude of invasion and metastasis of tumor. Our results revealed that reduced expression of PTEN was implicated in progression of gastric cancer probably by decreasing cellular adhesion, increasing cellular mobility and angiogenesis and could act as an objective marker to reflect the biological behaviors of gastric cancer.
In addition, signet ring cell carcinoma showed the lowest expression of PTEN among histological classifications, less than well and moderately differentiated adenocarcinoma (P < 0.01), suggesting that decreased expression of PTEN was closely associated with carcinogenesis of signet ring cell carcinoma. Diffuse-type cancer showed less expression of PTEN at the rate of 41.5% than intestinal-type one. (P < 0.05). In this sense, it supported that there were different tumorigenetic pathways between diffuse and intestinal-type gastric carcinoma. Diffuse-type gastric cancer, main part of which was signet ring cell carcinoma, showed diffusely invasive growth pattern. It is possible that down-regulation of PTEN could affect the function of cellular skeleton, mobility and adhesion of cancer cells.
Some reports suggested that decreased expression of PTEN encoding product could down-regulate PI3K/AKT pathway, leading to increasing synthesis of VEGF induced by hypoxia inducing factor-1 (HIF-1)[48-50]. Our study showed that 75.0 percent of gastric carcinomas expressed VEGF (45/60), significantly more than normal mucosa (0/5) (P < 0.05), indicating that VEGF was up-regulated in gastric cancer. But PTEN was down-regulated in gastric cancer. Both PTEN and VEGF showed negative correlation, which was not statistically significant (P > 0.05). The relationship between expression of both PTEN and VEGF in tumorigenesis and progression of gastric cancer need proving by amplifying the sample.
In all, loss or reduced expression of PTEN protein occurred commonly in gastric carcinogenesis. Altered expression of PTEN contributed to progression of gastric cancer by increasing cell adhesion, angiogenesis, cell mobility and so on. It was suggested that PTEN could be a useful marker for pathologically biological behaviors of gastric carcinoma. However, the role of PTEN gene and its encoding protein in tumorigenesis and progression of gastric cancer need further investigation.
Edited by Ma JY
| 1. | Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2004] [Cited by in RCA: 2034] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 2. | Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3525] [Cited by in RCA: 3577] [Article Influence: 127.8] [Reference Citation Analysis (0)] |
| 3. | Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124-2129. [PubMed] |
| 4. | Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240-4245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1536] [Cited by in RCA: 1493] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 5. | Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587-15591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 475] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96:6199-6204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 598] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 7. | Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 362] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 8. | Besson A, Robbins SM, Yong VW. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 342] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589-593. [PubMed] |
| 11. | Rubin MA, Gerstein A, Reid K, Bostwick DG, Cheng L, Parsons R, Papadopoulos N. 10q23.3 loss of heterozygosity is higher in lymph node-positive (pT2-3,N+) versus lymph node-negative (pT2-3,N0) prostate cancer. Hum Pathol. 2000;31:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Depowski PL, Rosenthal SI, Ross JS. Loss of expression of the PTEN gene protein product is associated with poor outcome in breast cancer. Mod Pathol. 2001;14:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3-carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Garcia JM, Silva JM, Dominguez G, Gonzalez R, Navarro A, Carretero L, Provencio M, España P, Bonilla F. Allelic loss of the PTEN region (10q23) in breast carcinomas of poor pathophenotype. Breast Cancer Res Treat. 1999;57:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Dillon DA, Howe CL, Bosari S, Costa J. The molecular biology of breast cancer: accelerating clinical applications. Crit Rev Oncog. 1998;9:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Lin WM, Forgacs E, Warshal DP, Yeh IT, Martin JS, Ashfaq R, Muller CY. Loss of heterozygosity and mutational analysis of the PTEN/MMAC1 gene in synchronous endometrial and ovarian carcinomas. Clin Cancer Res. 1998;4:2577-2583. [PubMed] |
| 17. | Shao X, Tandon R, Samara G, Kanki H, Yano H, Close LG, Parsons R, Sato T. Mutational analysis of the PTEN gene in head and neck squamous cell carcinoma. Int J Cancer. 1998;77:684-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Hosoya Y, Gemma A, Seike M, Kurimoto F, Uematsu K, Hibino S, Yoshimura A, Shibuya M, Kudoh S. Alteration of the PTEN/MMAC1 gene locus in primary lung cancer with distant metastasis. Lung Cancer. 1999;25:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Celebi JT, Shendrik I, Silvers DN, Peacocke M. Identification of PTEN mutations in metastatic melanoma specimens. J Med Genet. 2000;37:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 2000;60:1800-1804. [PubMed] |
| 21. | Huang J, Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760-10766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Harmey JH, Bouchier-Hayes D. Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: implications for anti-angiogenic therapy. Bioessays. 2002;24:280-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724-8729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Dias S, Choy M, Alitalo K, Rafii S. Vascular endothelial growth factor (VEGF)-C signaling through FLT-4 (VEGFR-3) mediates leukemic cell proliferation, survival, and resistance to chemotherapy. Blood. 2002;99:2179-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219-221. [PubMed] |
| 27. | Umeda N, Ozaki H, Hayashi H, Kondo H, Uchida H, Oshima K. Non-paralleled increase of hepatocyte growth factor and vascular endothelial growth factor in the eyes with angiogenic and nonangiogenic fibroproliferation. Ophthalmic Res. 2002;34:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL. Characterization of protein kinase C beta isoform's action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA. 2002;99:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1727] [Cited by in RCA: 1706] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 30. | Chen XY, van Der Hulst RW, Shi Y, Xiao SD, Tytgat GN, Ten Kate FJ. Comparison of precancerous conditions: atrophy and intestinal metaplasia in Helicobacter pylori gastritis among Chinese and Dutch patients. J Clin Pathol. 2001;54:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Günther T, Schneider-Stock R, Häckel C, Kasper HU, Pross M, Hackelsberger A, Lippert H, Roessner A. Mdm2 gene amplification in gastric cancer correlation with expression of Mdm2 protein and p53 alterations. Mod Pathol. 2000;13:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Liu XP, Tsushimi K, Tsushimi M, Oga A, Kawauchi S, Furuya T, Sasaki K. Expression of p53 protein as a prognostic indicator of reduced survival time in diffuse-type gastric carcinoma. Pathol Int. 2001;51:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Jang TJ, Kim DI, Shin YM, Chang HK, Yang CH. p16(INK4a) Promoter hypermethylation of non-tumorous tissue adjacent to gastric cancer is correlated with glandular atrophy and chronic inflammation. Int J Cancer. 2001;93:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Migaldi M, Zunarelli E, Sgambato A, Leocata P, Ventura L, De Gaetani C. P27Kip1 expression and survival in NO gastric carcinoma. Pathol Res Pract. 2001;197:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Oki E, Maehara Y, Tokunaga E, Kakeji Y, Sugimachi K. Reduced expression of p33(ING1) and the relationship with p53 expression in human gastric cancer. Cancer Lett. 1999;147:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Lee WA, Woo DK, Kim YI, Kim WH. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathol Res Pract. 1999;195:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Yoshida Y, Itoh F, Endo T, Hinoda Y, Imai K. Decreased DCC mRNA expression in human gastric cancers is clinicopathologically significant. Int J Cancer. 1998;79:634-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Lee JI, Soria JC, Hassan KA, El-Naggar AK, Tang X, Liu DD, Hong WK, Mao L. Loss of PTEN expression as a prognostic marker for tongue cancer. Arch Otolaryngol Head Neck Surg. 2001;127:1441-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Verma RS, Manikal M, Conte RA, Godec CJ. Chromosomal basis of adenocarcinoma of the prostate. Cancer Invest. 1999;17:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291-4296. [PubMed] |
| 42. | Rustia A, Wierzbicki V, Marrocco L, Tossini A, Zamponi C, Lista F. Is exon 5 of the PTEN/MMAC1 gene a prognostic marker in anaplastic glioma. Neurosurg Rev. 2001;24:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Nozaki M, Tada M, Kobayashi H, Zhang CL, Sawamura Y, Abe H, Ishii N, Van Meir EG. Roles of the functional loss of p53 and other genes in astrocytoma tumorigenesis and progression. Neuro Oncol. 1999;1:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Minaguchi T, Yoshikawa H, Oda K, Ishino T, Yasugi T, Onda T, Nakagawa S, Matsumoto K, Kawana K, Taketani Y. PTEN mutation located only outside exons 5, 6, and 7 is an independent predictor of favorable survival in endometrial carcinomas. Clin Cancer Res. 2001;7:2636-2642. [PubMed] |
| 45. | Tada K, Shiraishi S, Kamiryo T, Nakamura H, Hirano H, Kuratsu J, Kochi M, Saya H, Ushio Y. Analysis of loss of heterozygosity on chromosome 10 in patients with malignant astrocytic tumors: correlation with patient age and survival. J Neurosurg. 2001;95:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Mills GB, Lu Y, Kohn EC. Linking molecular therapeutics to molecular diagnostics: inhibition of the FRAP/RAFT/TOR component of the PI3K pathway preferentially blocks PTEN mutant cells in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:10031-10033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Hwang PH, Yi HK, Kim DS, Nam SY, Kim JS, Lee DY. Suppression of tumorigenicity and metastasis in B16F10 cells by PTEN/MMAC1/TEP1 gene. Cancer Lett. 2001;172:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995-4004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 965] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 49. | Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541-1545. [PubMed] |