Published online Oct 15, 2000. doi: 10.3748/wjg.v6.i5.766
Revised: May 8, 2000
Accepted: May 15, 2000
Published online: October 15, 2000
- Citation: Xia ZW, Inoue Y, Ohse M, Shinka T, Kuhara T. A study on α-ketoadipic aciduria by gas chromatographic-mass spectrometry. World J Gastroenterol 2000; 6(5): 766-769
- URL: https://www.wjgnet.com/1007-9327/full/v6/i5/766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v6.i5.766
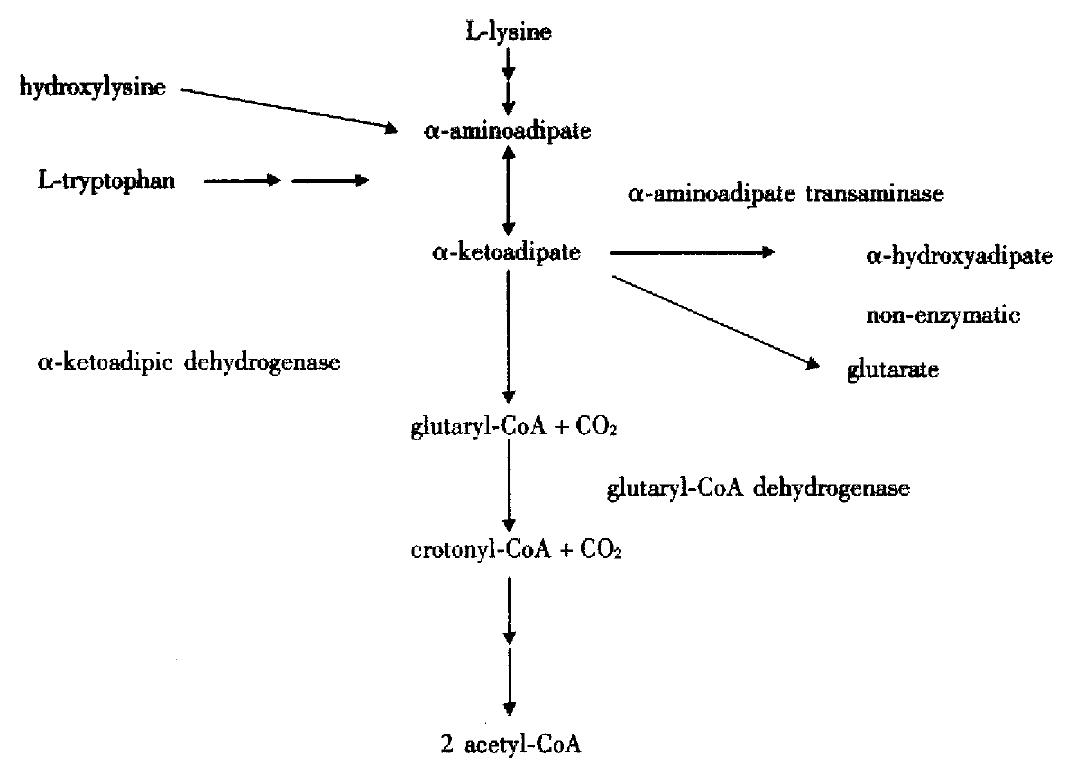
α-ketoadipate (α-KA), an intermediate in the catabolism of L-lysine, hydroxylysine, and L-tryptophan, undergoes oxidative decarboxylation to form glutaryl-CoA and then dehydrogenates to form crotonyl-CoA, the latter undergoes further degradation and enters in TCA cycle, as shown in Figure 1. α-ketoadipic aciduria (Mckusick 245130) is a rare inborn error in the metabolism of α-KA to glutaryl-CoA and is characterized by the increased excretion of α-KA, α-aminoadipate (α-AA) and α-hydrocyadipate (α-HAA). Since Przyrembel et al[1] firstdescribed it in 1975, only 13 cases of α-ketoadipic aciduria have been reported over the past 25 years, including 7 symptomatic, and 6 asympotmaticones even in the symptomatic siblings with α-ketoadipic aciduria[1-10]. The clinical manifestations of this metabolic disorder showed heterogeneity. However, no follow-up study on either symptomatic or asymptomatic case has been available so far. We followed up two cases of α-ketoadipic aciduria clinically and metabolically using organic solvent extraction, new urease-pretreatment and gas chromatography-mass spectrometry (GC/MS).
Two male children were studied. Pregnancy and delivery were uneventful. Their clinical data were collected after diagnosis of α-ketoadipic aciduria.
Creatinine-d3 (methyl-d3) was purchased from Nippon Sanso Ltd., Tokyo, Japan, and 2-aminoadipate, 2-oxoadipate, 2, 2-dimethylsuccinate (DMS) and urease type C3 were obtained from Sigma Chemical Co. St. Louis, MO, USA. All chemicals were of analytical grade.
Twenty nmol of DMS was used as the analyte of internal standard for preparing standard curves of α-KA and glutarate, 50 nmol of DMS was also chosen as the analyte of internal standard for making standard curves of α-AA and α-HAA. Comparing with peak area of internal standard, quantitation of urinary α-KA, α-HAA, α-AA and glutarate was performed using standard curves.
Urine samples from two cases were collected at different detecting time. All samples were frozen at -20 °C until analysis. Creatinine was determined and urine volumes equivalent to 1 μmol creatinine were prepared prior to GC/MS analysis by organic solvent extraction[11] or urease-pretreatment[12]
Samples were analyzed using GC/MS-computer systems of QP-5000 (Shimadzu, Japan) and HP-6890 (Hewlett Packard, USA) as well as the new diagnostic method described previously[12,14,15]. The concentrations of these compounds were normalized to urinary creatinine and expressed as mmol per mol creatinine.
Loading tests of tryptophan and lysine (each was 100 mg/kg of body weight) were made in Case 1 at the age of 1 year and 4 months.
The two cases were followed up clinically for a period of 15 years (8 months-15 years in Case 1) and 5 years (1 d-5 years in Case 2), respectively. Case 1 was slightly delayed in growth after birth and CT on his head showed mild cortical atrophic change at the age of 9 months. At 1 year and 4 months, the analysis of first urine sample revealed high levels of α-KA and α-AA using GC/MS. Loading test was performed. The values of α-KA, α-HAA, glutarate and α-AA reached 16-fold, 4-fold, 9-fold and 4.5-fold, respectively after lysine was taken orally. The concentrations of these four compounds increased 7-fold, 2-fold, 3-fold and 4-fold, respectively after tryptophan was also administered orally. Treatment was carried out with a low-lysine diet (70mg/kg daily) and a low-tryptophan diet (17 mg/kg daily) after diagnosis. His CT was within normal range and his mild cortical atrophic change disappeared at the age of 4 years. The dietary treatment was discontinued due to normal development. His growth is normal at present. Case 2 developed cyanosis, clonic seizures and hypoglycemia 1 d after birth, then grew normally without low protein restriction.
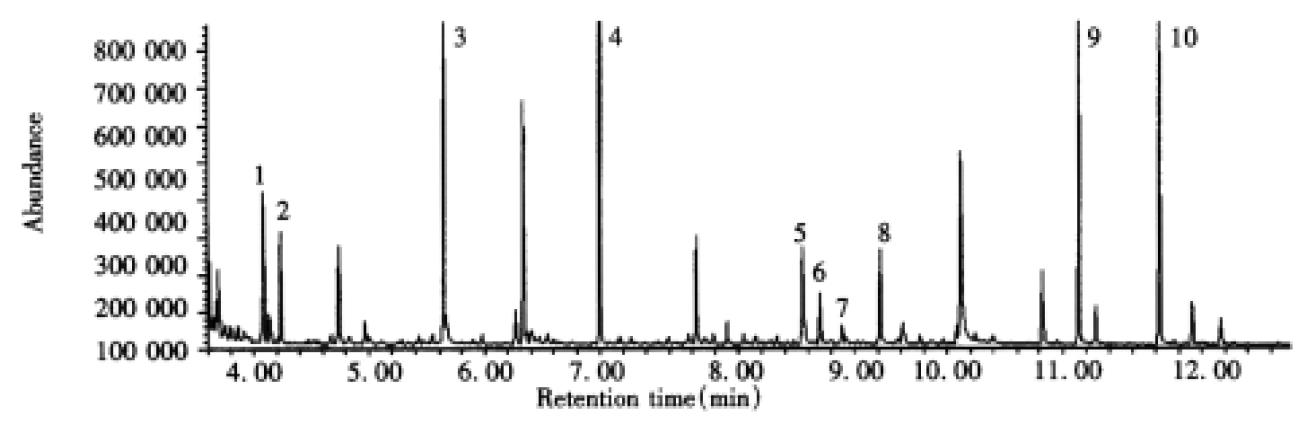
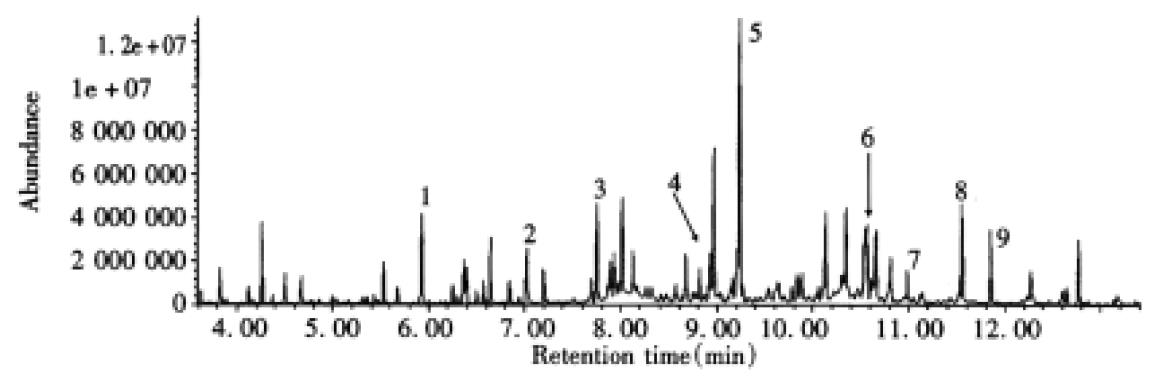
Excretion of abnormal urinary metabolites profile compatible with α-ketoadipic aciduria in two cases was continuously observed using organic solvent extraction, urease-pretreatment and GC/MS techniques (Table 1). Total ion current (TIC) chromatogram of TMS derivatives of organic acid in urine from Case 1 is shown in Figure 2. Ten major compounds were identified. Peak 4, 6 and 7 represent glutarate, α-HAA and α-KA, respectively. Figure 3 shows the TIC of TMS derivatives of metabolites in urine from the same patient using urease-pretreatment and 9 major compounds were confirmed. Peak 2, 4, and 5 demonstrated glutarate, α-HAA and α-AA, respectively. Abnormal metabolites profile of α-KA, α-HAA, α-AA and glutarate were also detected in the urine of Case 2 with those techniques. Compared to the case with α-ketoadipic aciduria, three abnormal peaks, including α-KA, α-HAA and glutarate after organic solvent extraction and α-AA, α-HAA and glutarate using urease-pretreatment, did not appear in the urine from healthy age-matched control at same retention time. The value of α-AA is much higher than that of α-KA, and glutarate was also detected and found increased in the urine of two cases. The concentrations of α-KA, α-HAA and glutarate ranged between 9-49 mmol/mol creatinine, 12-55 mmol/mol creatinine and 9-216 mmol/mol creatinine, respectively. The amounts of α-AA were 92-450 mmol/mol creatinine in analysis of urinary amino acid (Table 1).
| Cases | Detecting age | α-KA | α-AA | α-HAA | Glutarate | Total value of metabolites |
| 1 | 1.4 yrs | 33 (ND) | 223 (2-25) | 28 (ND) | 29 (0.04) | 313 |
| 4.2 yrs | 33 (ND) | 266 (0-51) | 17 (ND) | 24 (0.04) | 340 | |
| 15 yrs | 33 (ND) | 92 (0-4) | 21 (ND) | 53 (ND) | 199 | |
| 2 | 13 d | 31 (ND) | 200 (1-11) | 12 (ND) | 9 (0.03) | 252 |
| 29 d | 49 (ND) | 450 (5-197) | 17 (ND) | 54 (0.03) | 570 | |
| 5 yrs | 9 (ND) | 92 (0-4) | 55 (ND) | 216 (ND) | 372 | |
| Average | 31.3 | 220.5 | 25 | 64.2 |
In analyzing organic acid, GC/MS has been proven by several institutions to be the most efficient method for chemical diagnosis of inborn error of metabolism.
In 1991, Shoemaker et al reported that urinary organic acids, amino acids and sugar could be analyzed simultaneously by GC/MS after excessive urea in the urine was degraded with urease and removed. This method, however, takes several hours, needs skillful technicians, and is not so practical. Therefore, Shoemaker’s procedure was simplified for use in multiple sample analysis[12]. As a result, rapid practical and simultaneous analysis of amino acids and organic acids became possible. Further improved-procedure was adopted, which is a stable isotope dilution method using not only d3-creatinine but also stable-isotope-labeled amino acids as internal standards. It only takes 1 hour for pretreatment of one sample and is a highly comprehensive diagnostic tool for a wide range of metabolic disorders[13]. However, for detecting α-KA, the approach of organic solvent extraction is more sensitive than that of urease-pretreatment in this study.
α-ketoadipic aciduria was first described by Przyrembel et al[1-3,6-9] in 1975 since then 7 cases have been reported using GC/MS or other analytical methods, and some subjects with α-ketoadipic aciduria were found by re-examination[3]. In addition to symptomatic cases, 6 asymptomatic cases have also been reported[2-5,10]. Up to now no follow-up study on this case with or without symptoms has been described by employing organic solvent extraction, urease-pretreatment and GC/MS techniques.
Almost all probands were identified when prominent spots of α-AA were noted on amino acid chromatography of urine, with subsequent investigations demonstrating α-aminoadipic acidemia and increased urine concentrations of α-KA and α-HAA. Different clinical symptoms were also described according to 7 affected individuals. The major manifestations include psychomotor retardation (5 cases)[1,3,6-8], men talretardation (3 cases)[1-3], hypotonia (3 cases)[1,3,7] and seizures (2 cases)[6,8]. The data indicate that the individuals with α-ketoadipic aciduria presented with nonspecific symptoms, but the central nervous system may be especially vulnerable. In this study, two cases showed symptoms at onset, including an 8-month-old boy with growth reta rdation (Case 1) and a boy with seizures (Case 2) as described above symptoms. In the two cases, the values of α-KA, α-AA, α-HAA and glutarate were always high at the different detecting time using present method. Meanwhile, loading tests of lysine and tryptophan were performed at 1 year and 4 months in Case 1. The concentrations of α-KA, α-AA, α-HAA and glut arate increased after lysine or tryptophan was taken orally. So the diagnosis of α-ketoadipic aciduria was confirmed. Three abnormal peaks were still identified in recent detection as α-KA, α-HAA and glutarate after organic solvent extraction, and the three abnormal peaks of α-AA, α-HAA and glutarate also appeared by urease-pretreatment compared with healthy age-matched control.
In normal condition, α-KA and α-HAA could not be detected and only a trace of α-AA and glutarate exist in the urine, and when the patients are in the interim period, organic acid and amino acid in serum and urine are normal[7]. In this study, abnormal excretion of these compounds was found and the value of α-AA was much higher than that of α-KA in the urine of two cases, but we still considered and diagnosed them as α-ketoadipic aciduria. Based on this α-aminoadipic aciduria is caused by the deficiency of mitochondrial α-aminoadipic acid aminotransferase, which leads to elevated urinary excretion of α-AA without α-KA.
A small amount of glutarate was also detected in urine in the two cases, but the level was much lower than that found in glutaric aciduria type I, and 3-hy droxyglutarate was not detected, so it is almost certainly because of spontaneou s decarboxylation of α-KA or artifact[6,7].
The accumulated metabolites suggest a block in α-ketoadipic acid dehydrogenase, and intact mutant fibroblasts are almost totally unable to oxidize α-amino[1-14C]adipate and α-keto[1-14C] adipate to 14CO2, but a defect in α-ketoadipic acid dehydrogenase has not been demonstrated directly. If α-ketoadipic and α-ketoglutaric acid dehydrogenase are indeed the same, it is not clear a defect can produce so mild a phenotype. This may indicate that the two enzymes are different. Vallat et al[16,17] recently reported that significant increment of α-AA occurred in the plasma and urine of 8 vigabatrin (VGB) treated children suggesting that VGB strongly inhibit α-aminoadipic acid transminase, α-ketoadipic acid dehydrogenase, or glutaryl-CoA dehydrogenase. However, more knowledge about underlying the mechanism is required.
Although protein restriction was reported to improve clinical symptoms like Case 1 and Case 2 in which no seizures occurred without treatment. Our results and results previously reported[3,8], suggest that the clinical course of α-ketoadipic aciduria is incongruity and the condition is not apparently deleterious. Some authors investigated normal siblings of the patients, who excreted excessive amounts of α-KA and α-AA, and thought mental retardation may result from other causes[2,3]. Others researchers consider that the analysis of mass spectrometry for this disorder would not be useful because α-ketoadipic aciduria is a nondeleterious inherited metabolic defect. But up to now, the relationship between the biochemical abnormality and clinical manifestations in α-ketoadipic aciduria is still unclear.
Inheritance as an autosomal recessive trait is inferred from the pedigrees. There is no evidence so far that heterozygous carriers can be distinguished from control subjects. The incidence is not known. For these reasons, it is necessary to follow up this case with α-ketoadipic aciduria using GC/MS in order to clarify the mechanism of the clinical heterogeneity of this defect.
We are indebted to Dr. Isamu Matsumoto (Professor Emeritus, Kanazawa Medical University) for his continuing interest and encouragement.
Edited by Ma JY
| 1. | Przyrembel H, Bachmann D, Lombeck I, Becker K, Wendel U, Wadman SK, Bremer HJ. Alpha-ketoadipic aciduria, a new inborn error of lysine metabolism; biochemical studies. Clin Chim Acta. 1975;58:257-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Wilson RW, Wilson CM, Gates SC, Higgins JV. Alpha-ketoadipic aciduria: a description of a new metabolic error in lysine-tryptophan degradation. Pediatr Res. 1975;9:522-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Fischer MH, Brown RR. Tryptophan and lysine metabolism in alpha-aminoadipic aciduria. Am J Med Genet. 1980;5:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Wilcken B, Smith A, Brown DA. Urine screening for aminoacidopathies: is it beneficial? Results of a long-term follow-up of cases detected bny screening one millon babies. J Pediatr. 1980;97:492-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Scriver CR, Beaudet AL, Sly WS, Valle D. The metabolic mol bases of inherited diseases. In: Goodman SI, Frerman FE, eds. Organic acidemias due to defects in lysine oxidation; 2 ketoadipic acidemia and glutaric acidemia. 7th ed. MacGraw Hill. 1995;1451-1460. [Cited in This Article: ] |
| 6. | Duran M, Beemer FA, Wadman SK, Wendel U, Janssen B. A patient with alpha-ketoadipic and alpha-aminoadipic aciduria. J Inherit Metab Dis. 1984;7:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Vianey liaud C, Divry P, Cotte J. αAminoadipic and αketoadipic aciduria; detection of a new case by a screening program using two dimensional thin layer chromatography of amino acids. J Inher Metab Dis. 1985;8:133-134. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Jakobs C, de Grauw AJ. A fatal case of 2-keto-, 2-hydroxy- and 2-aminoadipic aciduria: relation of organic aciduria to phenotype? J Inherit Metab Dis. 1992;15:279-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Takechi T, Okada T, Wakiguchi H, Morita H, Kurashige T, Sugahara K, Kodama H. Identification of N-acetyl-alpha-aminoadipic acid in the urine of a patient with alpha-aminoadipic and alpha-ketoadipic aciduria. J Inherit Metab Dis. 1993;16:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Peng H, Shinka T, Inoue Y, Mitsubuchi H, Ishimatsu J, Yoshino M, Kuhara T. Asymptomatic alpha-ketoadipic aciduria detected during a pilot study of neonatal urine screening. Acta Paediatr. 1999;88:911-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 11. | Matsumoto M, Kuhara T, Inoue Y, Shinka T, Matsumoto I, Kajita M. Mass spectrometric identification of 2-hydroxydodecanedioic acid and its homologues in urine from patients with hopantenate therapy during clinical episode. Biomed Environ Mass Spectrom. 1990;19:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Matsumoto I, Kuhara T. A new chemical diagnostic method for in-born errors of metabolism by mass spectrometry. MS Reviews. 1996;15:43-57. [Cited in This Article: ] |
| 13. | Kuhara T, Shinka T, Inoue Y, Ohse M, Zhen-wei X, Yoshida I, Inokuchi T, Yamaguchi S, Takayanagi M, Matsumoto I. Pilot study of gas chromatographic-mass spectrometric screening of newborn urine for inborn errors of metabolism after treatment with urease. J Chromatogr B Biomed Sci Appl. 1999;731:141-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991;562:125-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 110] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Schulman MF, Abramson FP. Plasma amino acid analysis by isotope ratio gas chromatography mass spectrometry computer techniques. Biomed Mass Spectrom. 1975;2:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Candito M, Richelme C, Parvy P, Dageville C, Appert A, Bekri S, Rabier D, Chambon P, Mariani R, Kamoun P. Abnormal alpha-aminoadipic acid excretion in a newborn with a defect in platelet aggregation and antenatal cerebral haemorrhage. J Inherit Metab Dis. 1995;18:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Vallat C, Rivier F, Bellet H, Magnan de Bornier B, Mion H, Echenne B. Treatment with vigabatrin may mimic alpha-aminoadipic aciduria. Epilepsia. 1996;37:803-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |