Published online Jun 15, 1998. doi: 10.3748/wjg.v4.i3.249
Revised: May 10, 1998
Accepted: May 20, 1998
Published online: June 15, 1998
AIM: To detect antibodies against Helicobacter pylori spiral and coccoid antigens in human sera.
METHODS: Blood samples were collected from 278 patients with gastric diseases. A 3-day-old culture of H. pylori on chocolate blood agar was used to providespiral form. ‘Synchronous’ coccoids were cultured in (BHY) (brain heart infusion supplemented with 10% horse serum and 0.4% yeast extract) medium in a chemostat. Antigens from spiral and coccoid form were prepared using acid glycine extraction. Enzyme-linked immunosorbent assay (ELISA) was performed to detect serum IgG antibodies against spiral and coccoid forms of H. pylori.
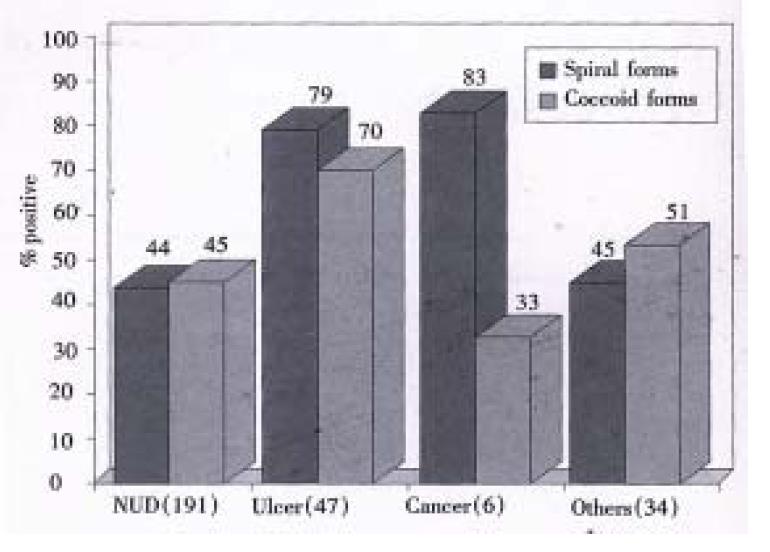
RESULTS: Seroprevalence of H. pylori infection was higher in patients with gastric ulcer (79%) and gastric cancer (83%) than those with non-ulcer dyspepsia (NUD) (44%) and other diseases (45%) (P < 0.05). IgG antibodies against spiral and coccoid antigens were detected in 50.7% (141/278) and 49.6% (138/278), respectively.
CONCLUSION: The spiral and coccoid forms of H. pylori coexist in patients infected with the bacterium.
- Citation: Hua JS, Khin MM, Zheng PY, Yeoh KG, Ng HC, Ho B. Serum IgG response to differentiated antigens of Helicobacter pylori. World J Gastroenterol 1998; 4(3): 249-251
- URL: https://www.wjgnet.com/1007-9327/full/v4/i3/249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i3.249
It was reported that Helicobacter pylori can convert to coccoid form from spiral form in vitro after prolonged incubation[1] or under antibiotic stress[2]. By histological examination, Chan et al[3] and Janas et al[4] observed two morphological forms of the bacteria in H. pylori-positive gastric specimens. The dimorphism of H. pylori has stimulated researchers to investigate whether coccoid form of H. pylori is viable and pathogenic[1,5,6]. Coccoid form like its spiral form has similar proteins except that a high-molecular-mass antigenic fraction (> 94 kDa), absent in spiral forms, was detected during coccoid conversion[6] and that a smaller number of immunoreactive bands were recognized in the coccoid antigens compared with the spiral antigens[5]. In this study, we used ELISA technique to detect immune response of IgG to spiral and coccoid antigens.
Blood samples were collected from 278 patients with gastric disorders at the National University Hospital, Singapore. Informed consent was obtained from all the patients. After collection, blood specimens were allowed to clot at room temperature for 36 min-60 min. The sera were removed from the clot and any remaining insoluble material removed by centrifugation at 2000 × g for 10min at 4 °C. Sera were stored at -20 °C until use.
A local H. pylori strain V2 isolated from a patient with non-ulcer dyspepsia was used. A 3-day-old culture of H. pylori on chocolate blood agar was used to provide spiral form. ‘Synchronous’ coccoids were cultured in BHY (brain heart infusion supplemented with 10% horse serum and 0.4% yeast extract) medium in a chemostat. Antigens from spiral and coccoid form were prepared according to a modified method of Goodwin et al[7] as described by Vijayakumari et al[5].
ELISA was performed according to the method described previously[8]. Briefly, flat-bottomed microtitre plates (Nunc) were coated with acid glycine extract antigens of H. pylori. The sera tested were diluted at 1:100. Each diluted serum was examined in triplicate. Positive control serum was diluted at 1:100, 1:200, 1:400, 1:800, 1:1600 and 1:3200 and negative control serum at 1:100. Horse radish peroxidase-labelled rabbit anti-human IgG (Dako) was used as conjugates. The substrate used was ophenylenediamine dihydrochloride (OPD, Sigma). The optical density at wavelength 490 and 620 nm reference filter was read immediately using an ELISA reader (Ceres 900 Bio-Tek Instruments, Inc). The cut-off value for the ELISA was derived according to Khin and Ho[8].
Seroprevalence of H. pylori infection was higher in patients with gastric ulcer (79%) and gastric cancer (83%) than those with non-ulcer dyspepsia (NUD) (44%) and other diseases (45%) (P < 0.05). Furthermore, of the 278 sera, IgG antibodies against NCTC 11637 spiral and coccoid antigens were detected in 141 (50.7%) cases and 138 (49.6%) cases.
In 191 NUD patients, 84 (43.9%) and 86 (45.0%) were detected with IgG antibodies to spiral and coccoid antigens, respectively. There was no significant difference in seroreactivity to spiral and coccoid antigens in patients with NUD (P > 0.05) (Figure 1).
Of the 47 peptic ulcer patients, 37 (78.7% and 33 (70.2%) showed antibodies IgG against spiral and coccoid antigens (P > 0.05). Among the peptic ulcer patients, 69.2% (9/13) and 76.9% (10/13) of the gastric ulcer patients while 82.1% (23/28) and 67.9% (19/28) were positive for spiral and coccoid antigens, respectively.Serum IgG antibodies against spiral antigens were detected in 83.3% (5/6) patients with both gastric and duodenal ulcer, while 66.7% (4/6) of these were seropositive for IgG antibodies against coccoid antigens (Figure 1).
Of the 6 cases of gastric cancer antibodies IgG 5 (83.3%) cases were positive with IgG antibodies to spiral antigens and 2 (33%) cases were positive with IgG antibodies to coccoid antigens. No statistical difference was found in IgG antibodies against spiral and coccoid for antigens in this group (P > 0.05) (Figure 1).
Of the 34 cases of other diseases, there was 45.4% (15/33) and 51.5% (17/33) had positive seroreactivity against H. pylori spiral and coccoid antigens respectively. There was no significant difference (P > 0.05) (Figure 1).
Most adult patients colonized with H. pylori elicit a measurable systemic antibody response which comprises predominantly IgG. In this study, the systemic immune response of 278 sera was examined by ELISA. IgG to H. pylori spiral and coccoid antigens was found to be 50.7% and 49.6%, respectively. The serological response to both spiral and coccoid antigens indicates the possibility of coexistence of two forms of H. pylori in these patients which leads to the production of similar IgG responses in patients. It has been shown earlier that there were differences in protein profiles of spiral and coccoid antigens[6]. in vitro, coccoid form could be detected 6 h after exposure to 10 ng/L of amoxycillin[2]. Janas et al[4] reported the presence of two different morphological forms of H. pylori in gastric antrum specimens. Could these two morphological forms of spiral and coccoid of H. pylori be the complete cell cycle in vivo The morphological changes could have been triggered under physical or chemical stress which is unfavourable to H. pylori. Spiral form may then convert into coccoid form. When the environment becomes favourable, the bacteria may revert from coccoid form to spiral form in vivo. However, what could have led to the revision is still unknown. Some in vitro studies indicated that coccoid form of H. pylori might be viable[1,9-11]. Further more, Vijayakumari et al[5] showed that the adherence patterns of coccoid form of H. pylori on kato III in vitro were similar to those observed with spiral form in gastric biopsy specimens in vivo. It was reported that the vital cytotoxic proteins of spiral forms were also conserved in the coccoids[6]. Therefore, like its counterpart, coccoid form could be an infective, transmissible, immunogenic and pathogenic form of H. pylori which participates in the pathogenesis of gastroduodenal diseases.
The coccoid form was present above damaged epithelial cells[4] and is not easy to be detected by the techniques now being used. Patients with morphological conversion of H. pylori from spiral to coccoid form after treatment may be neglected and considered as eradication of H. pylori. The possible cell cycle of H. pylori may have clinical relevance. Xia et al[12] reported that recurrence of H. pylori infection was probably caused by recrudescence in the patients studied. One possible reason of relapse could be morphological reversion of H. pylori form coccoid from to spiral form after treatment.
This study demonstrated the presence of antibodies to different antigens of H. pylori in patients. Coccoid form like its counterpart spiral form may have clinical relevance in the outcome of gastric diseases.
| 1. | Hua J, Ho B. Is the coccoid form of Helicobacter pylori viable? Microbios. 1996;87:103-112. [PubMed] [Cited in This Article: ] |
| 2. | Berry V, Jennings K, Woodnutt G. Bactericidal and morphological effects of amoxicillin on Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:1859-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Chan WY, Hui PK, Leung KM, Thomas TM. Modes of Helicobacter colonization and gastric epithelial damage. Histopathology. 1992;21:521-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Jans B, Czkwianianc E, Bak Romaniszyn L, Bartel H, Tosik D, Ptaneta-Malecka I. Electro microscopic study of association between coccoid forms of Helicobacter pylori and gastric epithelial cells. Am J Gastroenterol. 1995;90:1829-1833. [Cited in This Article: ] |
| 5. | Vijayakumari S, Khin MM, Jiang B, Ho B. The pathogenic role of the coccoid form of Helicobacter pylori. Cytobios. 1995;82:251-260. [PubMed] [Cited in This Article: ] |
| 6. | Benaissa M, Babin P, Quellard N, Pezennec L, Cenatiempo Y, Fauchère JL. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect Immun. 1996;64:2331-2335. [PubMed] [Cited in This Article: ] |
| 7. | Goodwin CS, Blincow E, Peterson G, Sanderson C, Cheng W, Marshall B, Warren JR, McCulloch R. Enzyme-linked immunosorbent assay for Campylobacter pyloridis: correlation with presence of C. pyloridis in the gastric mucosa. J Infect Dis. 1987;155:488-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 161] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Khin MM, Ho B. Immunological detection of Helicobacter pylori pregnant woman. Biomedical Letters. 1994;52:71-78. [Cited in This Article: ] |
| 9. | Mai U, Geis G, Leying H, Ruhl G, Opferkuch S. Dimorphism of Campylobacter pylori. In: Megraud F, Lamouliatee H, eds. Gastroduodenal pathology and Campylobacter pylori. Amsterdam:. Elsevier Science. 1989;29-33. [Cited in This Article: ] |
| 10. | Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993;111:483-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 151] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Sörberg M, Nilsson M, Hanberger H, Nilsson LE. Morphologic conversion of Helicobacter pylori from bacillary to coccoid form. Eur J Clin Microbiol Infect Dis. 1996;15:216-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Xia HX, Windle HJ, Marshall DG, Smyth CJ, Keane CT, O'Morain CA. Recrudescence of Helicobacter pylori after apparently successful eradication: novel application of randomly amplified polymorphic DNA fingerprinting. Gut. 1995;37:30-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |