Copyright
©The Author(s) 2025.
World J Gastroenterol. Jul 14, 2025; 31(26): 108375
Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.108375
Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.108375
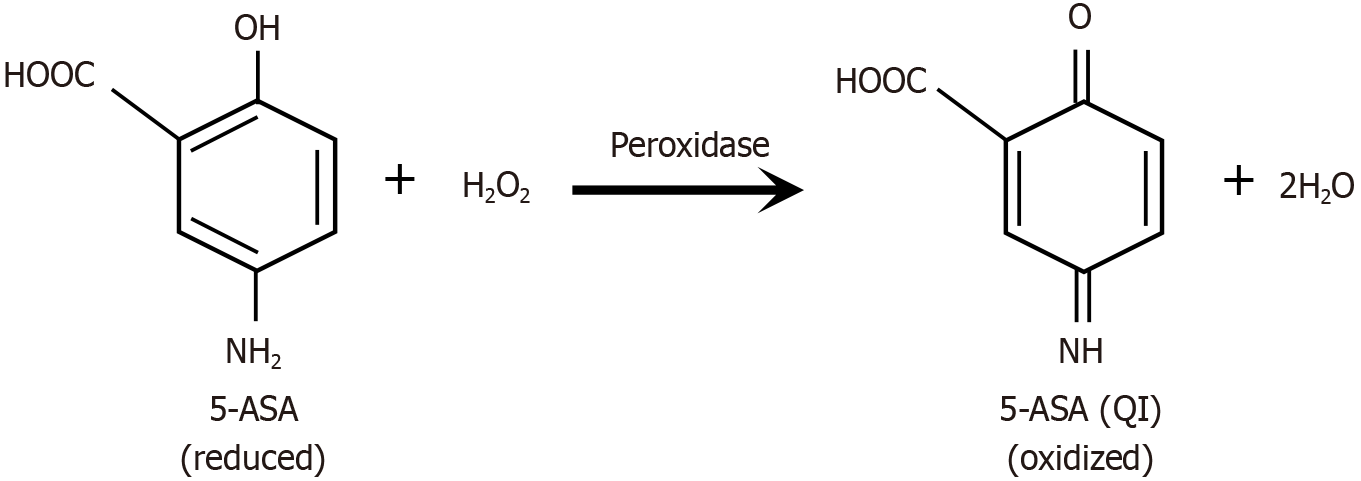
Figure 1 The redox-mediated reaction between 5-aminosalicylic acid, functioning as a reducing agent, and hydrogen peroxide, an oxidizing agent, involves the transfer of electrons.
In this process, a single molecule of reduced 5-aminosalicylic acid (5-ASA) donates two electrons, accompanied by two protons, to reduce one molecule of hydrogen peroxide into two molecules of water. In the process, 5-ASA is oxidized to the corresponding 5-ASA quinone imine. The reaction is spontaneous but enhanced by peroxidase enzymes found in the inflammatory field or colonic bacteria. 5-ASA: 5-aminosalicylic acid; H2O2: Hydrogen peroxide; H2O: Water; QI: Quinone imine.
- Citation: Pravda J. Ulcerative colitis: Timeline to a cure. World J Gastroenterol 2025; 31(26): 108375
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/108375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.108375