Copyright
©The Author(s) 2024.
World J Gastroenterol. Jan 28, 2024; 30(4): 290-307
Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.290
Published online Jan 28, 2024. doi: 10.3748/wjg.v30.i4.290
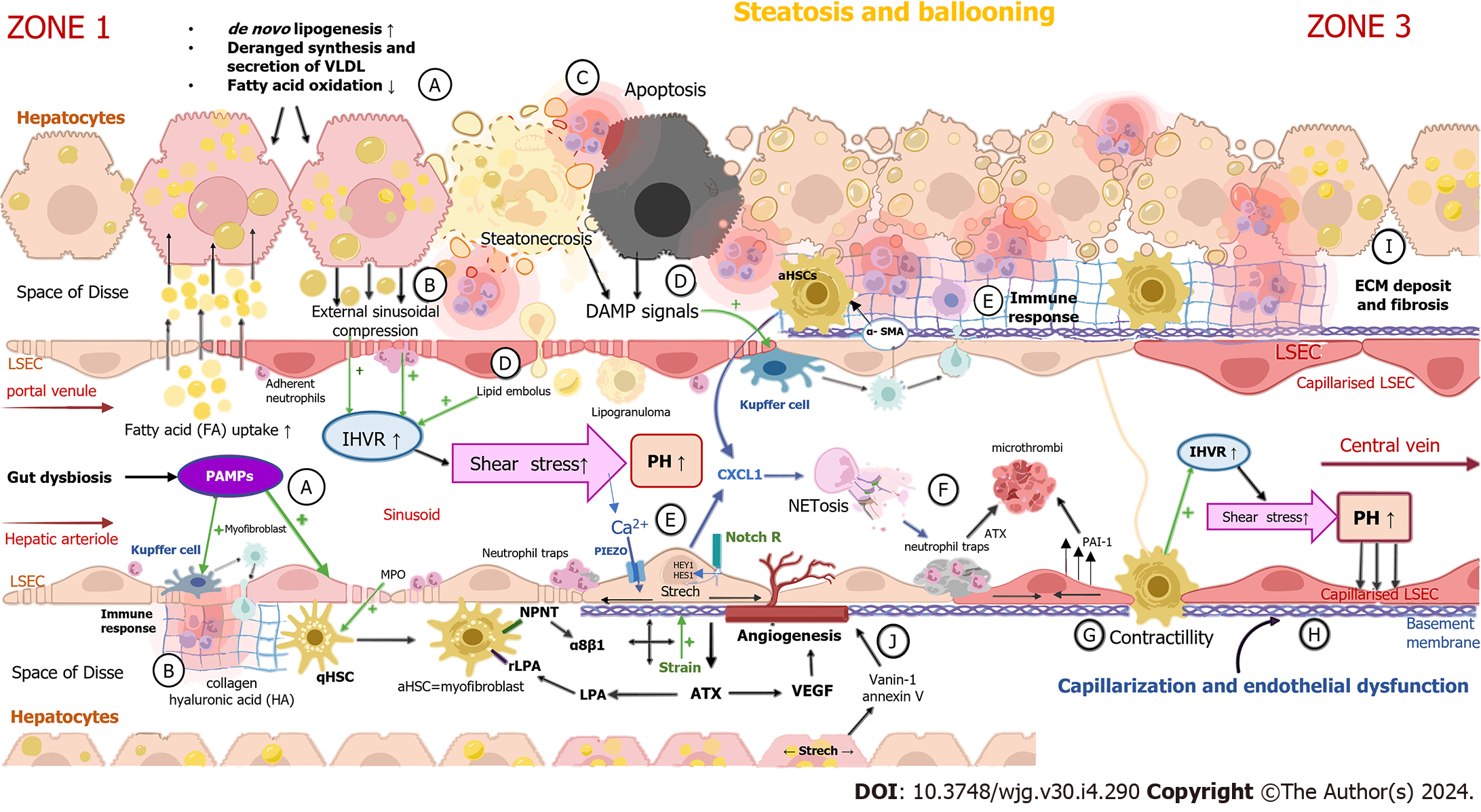
Figure 1 Development of portal hypertension in non-alcoholic fatty liver disease.
A: Ballooning of hepatocytes are caused by excessive lipid uptake. Pathogen-associated molecular patterns (PAMPs), along with other products of intestinal dysbiosis, arrive at the liver via the portal blood flow and act on liver sinusoidal endothelial cells (LSECs) and Kupffer cells (KCs) from the luminal side of the sinusoid; B: Excessive lipid accumulation in hepatocytes and PAMPs trigger inflammation by cytokine secretion and immune cell infiltration; C: Immune cell infiltration leads to steatonecrosis, apoptosis and hepatic stellate cells (HSCs) activation; D: Hepatocytes dying by steatonecrosis and apoptosis release damage-associated molecular pattern molecules, causing the activation of KCs and subsequently HSCs. The destruction of lipid-laden hepatocytes incites lipid embolus liberation in the sinusoidal lumen. Lipid droplets participate in lipogranuloma formation, which interferes with sinusoidal blood flow and results in elevated intrahepatic vascular resistance (IHVR); E: Activated HSCs transdifferentiate to proliferative, contractile and collagen-producing myofibroblasts which secrete vascular endothelial growth factor (VEGF) and inflammatory chemokines such as neutrophil chemotactic chemokines and synthesize α-smooth muscle actin. Notch-dependent neutrophil chemotaxis is also activated by the stretching of LSECs caused by the enlargement of hepatocytes and the liver; F: Stretch-activated LSECs promote the creation of neutrophil extracellular traps (NETs), web-like structures composed primarily of DNA-histone complexes originating from neutrophils. NETs contribute to the creation of microthrombi; G: The activation of HSCs is a key event mediating the elevation of IHVR by contracting around the sinusoid. IHVR is also elevated by extrasinusoidal compression caused by swollen steatotic hepatocytes and increased shear stress produced by intraluminal obstacles such as NETs; H: Sinusoid capillarization and the endothelial dysfunction of LSECs are important events that promote the activation of HSCs and KCs, initiating liver fibrosis and inflammation promotion; I: Myofibroblasts and mechanical forces lead to collagen and hyaluronic acid deposition in the space of Disse, causing an excessive increase in extracellular matrix (ECM) stiffness. The cross-linking of ECM proteins and collagen leads to the formation of perisinusoidal fibrosis; J: Angiogenesis occurs as liver fibrosis progresses. Stretched lipid-laden hepatocytes, HSCs, portal myofibroblasts and macrophages stimulate angiogenesis by producing a greater amount of VEGF and other similar mediators as a response to shear stress, hypoxia and inflammation. qHSC: Quiescent hepatic stellate cell; aHSCs: Activated hepatic stellate cells; DAMPs: Damage-associated molecular pattern molecules; αSMA: α-smooth muscle actin; FA: Fatty acid; HA: Hyaluronic acid; CXCL: Chemokine (C-X-C motif) ligand 1; α-SMA: Alpha-smooth muscle actin; MPO: Myeloperoxidase; PAI-1: Plasminogen activator inhibitor-1; ATX: Autotaxin; LPA: Lysophosphatidic acid.
- Citation: Madir A, Grgurevic I, Tsochatzis EA, Pinzani M. Portal hypertension in patients with nonalcoholic fatty liver disease: Current knowledge and challenges. World J Gastroenterol 2024; 30(4): 290-307
- URL: https://www.wjgnet.com/1007-9327/full/v30/i4/290.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i4.290