Published online Sep 15, 1997. doi: 10.3748/wjg.v3.i3.153
Revised: February 19, 1997
Accepted: March 10, 1997
Published online: September 15, 1997
AIM: To elucidate the effect of angiogenesis inhibitor, Linomide, on tumor growth and metastasis in nude mice implanted with human gastric cancer.
METHODS: A metastatic model of gastric cancer was established using orthotopic implantation of histologically intact tumor tissues into the gastric wall of nude mice. Linomide (0, 80, 160 mg·kg-1) was given p.o. every day after the implantation, and the mice were sacrificed after 10 wk to detect tumor size and metastasis. The microvessel counts were measured by immunohistochemical staining using a monoclonal antibody against Human Factor VIII related antigen.
RESULTS: Linomide treatment significantly decreased the size of the implanted tumors (control group: 1.36 ± 0.81 cm3vs Linomide treated group: 0.84 ± 0.51 cm3 and 0.62 ± 0.35 cm3, P < 0.05 and 0.01, respectively). Additionally, an antimetastatic effect of Linomide was clearly demonstrated in a dose dependent manner: mice given 80 mg·kg-1 Linomide developed liver metastasis in 4 of 10 cases, mice given 160 mg/kg developed metastasis in only 1 of 10 mice, while it developed in 19 of 28 mice of the control group (P < 0.05 and 0.01, respectively). The number of metastatic foci was also significantly less in the treated group. Furthermore, the microvessel counts in tumors of treated mice was reduced by 33%-42% as compared with the control tumors (P < 0.01).
CONCLUSION: Linomide has a strong inhibitory activity against in vivo tumor growth and metastasis of gastric cancer, effectively suppressing the growth of the primary tumor, preventing liver metastasis, and attenuating the rate of neovascularization.
- Citation: Tao HQ, Lin YZ, Yin HR, Gu QL, Zhu ZG, Yao M. Effects of Linomide on growth and metastasis of implanted human gastric cancer in nude mice. World J Gastroenterol 1997; 3(3): 153-155
- URL: https://www.wjgnet.com/1007-9327/full/v3/i3/153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i3.153
Although most gastric cancer is surgically resectable, tumor relapse and metastasis does develop in some patients. Therefore, many studies have attempted to address the control of relapse and metastasis in patients with these tumors. Neovascularization is not only the basis of tumor growth, but also the part of the complicated process of tumor metastasis, and is crucial to the development of hepatic metastasis. Since a small focus of tumor cells cannot grow at a secondary site without the induction of angiogenesis, it is expected that inhibition of angiogenesis should provide a potent form of therapy to prevent tumor recurrence and metastasis of gastric cancer.
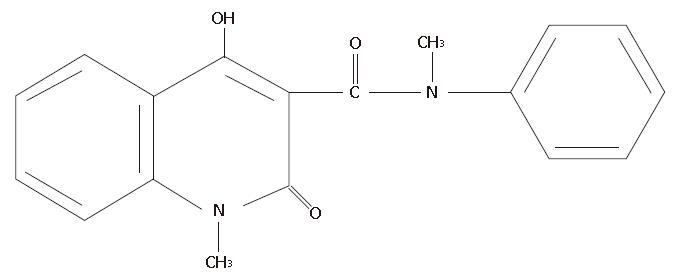
Angiogenesis inhibitors have been reported to have inhibitory activity against both tumor growth and metastasis, and some have achieved good results in animal experiments. Specifically. Linomide, a quinoline-3-carboxamide (Figure 1), has been demonstrated to have inhibitory effects on the growth and metastasis of several rodent tumor models, and its antitumor mechanism may involve antiangiogenic effects[1-3].
Implanting human tumor cells orthotopically into the corresponding organs of nude mice results in local tumor growth, and a much higher rate of metastasis. Human gastric cancer cells injected into the stomach wall of nude mice produced tumors that eventually metastasize to the liver, demonstrating that orthotopic implantation can enhance the metastatic potential of these tumor cells[4]. Recently, a new model of human gastric cancer was developed that avoids the disruption of tumor integrity, by using the orthotopic implantation of intact tumors[5]. Such a model should better reflect the original properties of human cancer, and could be of great value in the development of new drugs and new therapy strategies. In the present study, the inhibitory effect of Linomide on the local tumor growth and hepatic metastasis and the changing of microvessel density of tumors was examined in a nude mouse model of metastatic human stomach cancer using the orthotopic implantation of histologically intact tissues.
The Linomide was a kind gift of Kabi Pharmacia Therapeutics (Helsingborg, Sweden). It was dissolved in an isotonic solution, and was administered to the mice in their drinking water. These concentrations did not affect total daily water intake of the mice. Control animals were given ordinary drinking water only.
Male BALB/c nu/nu mice were obtained from the Shanghai Cancer Institute. In this study, only animals which were 6 to 8 wk old and weighed 20 to 22 g were used.
Human gastric adenocarcinoma cell line, SGC-7901, was kindly provided by the Shanghai Cancer Institute. Mice were inoculated s.c. in the flank with 1 × 106 SGC-7901 cells, and the xenograft was maintained by serial transplantation in nude mice. Small pieces of tissue were resected aseptically during the exponential growth phase from these tumors and then implanted into nude mice. Mice were anesthetized, and a small midline incision was made to carefully expose the stomach wall. A part of the serosal membrane, about 3 mm in diameter, in the middle of the greater curvature of the glandular stomach was removed at the site where the tumor pieces were to be implanted. A tumor piece of 5 mm in diameter was then fixed on each injured site of the serosal surface with a 7.0 Dexon transmural suture. The stomach was then returned to the peritoneal cavity, and the abdominal wall and skin were closed with 5. 0 Dexon sutures. The animals were kept in a specific-pathogen-free environment.
Animals were given Linomide at a dose of 80 or 160 mg/kg daily via the drinking water and the same volume of normal saline was given to other mice as control. Mice were sacrificed 10 wk after implantation, or earlier if they developed signs of distress. Autopsy was performed immediately, and the tumors growing on the gastric wall were removed, and its width (a) and length (b) were measured. Tumor volumes in cm3 were calculated by using a standard formula: ab2× 0.5236. The ratio of tumor inhibition were calculated as:
[(Volume of control - Volume of experiment)/Volume of control] × 100%.
The liver was examined by routine histology to detect metastases.
Intratumoral microvessels were identified by immunostaining using anti-human FVIII related antigen monoclonal antibody, and microvessel density (MVD) was assessed as the count of endothelial deposits/mm2 in the areas that were considered to be most active for neovascularization, as described previously[6].
Numerical values are expressed as the x- ± s. Student′s t test and the χ2 test were used for statistical analysis. Differences were considered significant if P < 0.05.
All tumor pieces implanted in the stomach showed local orthotopic growth. Treatment of Linomide significantly decreased the tumor volume as compared to the control group, and the inhibitory effect on tumor growth was related to the dose of Linomide. The ratio of inhibition of 80 and 160 mg/kg Linomide were 38.2% and 54.4% respectively. This result indicates that the growth of gastric carcinoma can be inhibited by Linomide (Table 1).
Similar to human patients, the nude mouse model of metastatic human gastric cancer using orthotopic implantation of histologically intact tissue also saw extensive metastases in regional lymph nodes, peritoneum, liver, spleen and other tissues. To appraise the inhibitory effect of Linomide in gastric cancer metastasis, we examined the incidence of hepatic metastasis and the number of metastatic foci in our mouse model. We found that Linomide inhibited hepatic metastasis in a dose dependent manner. The number of metastatic foci in the liver was 2.5 ± 0.8 in the 80 mg·kg-1 group and 2 in the 160 mg·kg-1 group. In contrast, 4.2 ± 1.4 metastatic foci were found in the control group (Table 2).
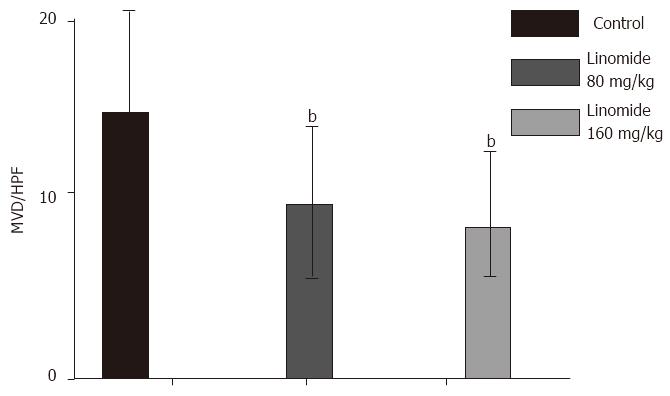
The capillary density in tumor of Linomide (80 and 160 mg/kg) treated mice was reduced by 33%-42% as compared with the control group. These data suggest that Linomide attenuated the rate of neovascularization, but did not completely block the initial activation of angiogenesis, nor the capability of each capillary to grow (Figure 2).
Recently, the mechanism of tumor angiogenic and angiogeneic inhibitors have become ‘hot spots’ in the field of tumor research. Since tumor growth is reported to generally depend on angiogensis, angiogenic inhibitors should have an inhibitory effect on in vivo tumor growth. The angiogeneic inhibitor, Linomide, has been reported to have an inhibitory effect on both tumor growth and metastasis in rodent prostatic cancer and melanoma. However, its effect has not been examined in human tumors. In this study, we investigated its inhibitory effect on the local growth and hepatic metastasis of human gastric cancer in a nude mouse model, constructed using the orthotopic implantation of histologically intact tissues. Although it was previously reported that Linomide had an inhibitory effect of tumor growth, because the tumors were inoculated s.c., the model may not reflect the natural environment of tumor growth. The host organ microenvironment can profoundly influence the growth of tumor cells. The model in the present study was more appropriate than heterotopic implantation models. In this study, we found that Linomide can inhibit gastric tumor growth, the ratio of inhibition of 80 and 160 mg·kg-1 were 38.2% and 54.4%, respectively. The result is consistent with the hypothesis that the rapidly proliferating tumor is more angiogenesis dependent.
Kalland[3] had reported that continuous treatment with Linomide in mice reduced the rate of pulmonary metastasis by 85%. In the present study, we found that it inhibited hepatic metastasis in a dose-dependent manner. Hepatic metastasis was observed in only 1 or 10 (10%) of the mice treated with 160 mg/kg, and was decreased significantly in mice treated with either 80 or 160 mg/kg of Linomide as compared with the control group. This result demonstrated that Linomide has an inhibitory effect on the metastasis of human tumors.
Tumor cell metastasis occurs through a complicated process that involves five main steps: (1) angiogenesis, (2) adhesion to endothelial cell basement membrane, (3) local proteolytic destruction of the basement membrane, (4) migration into secondary site, and (5) proliferation at the secondary sites. Tumor growth and metastasis require the development of new vessels. Mature capillaries have a thickened basement membrane, but growing capillaries have fragmented basement membrane. Because growing capillaries are leaky, these new vessels also increase the opportunity for tumor cells to enter the circulation. This study found that the microvessel density of Linomide treatment group was decreased by 33%-42% as compared with control group, indicating that efficacy for hepatic metastasis by Linomide may depend on the inhibition of angiogenesis at both the first and the final step of the metastatic process. Tumor cells rarely enter circulation because of the inhibition of capillary growth by Linomide. At the same time, tumor cells, when arriving at the target organs, cannot grow without the induction of angiogenesis. The tumor cells that arrived at the liver could not grow to a detectable mass since angiogenesis was inhibited by Linomide.
Vukanovic et al[1] thought that Linomide could inhibit tumor associated macrophage/monocyte number and their ability to secrete TNF-α. However, the precise mechanism of Linomide on human tumor neovascularization should be investigated further. Maeda et al[7] have reported that tumor recurrence and metastasis are closely correlated with microvessel count in gastric carcinoma. Our study indicated that hepatic metastasis of human gastric cancer was prevented by inhibiting tumor angiogenesis. Thus, hepatic metastasis may be prevented by angiogenesis inhibitors such as Linomide through reducing the opportunities for tumor cells to enter the circulation and inhibiting the growth of tumor cells arriving in the liver.
In summary, the angiogenesis inhibitor Linomide seems to be a potent tumor growth and metastatic inhibition agent, and might serve as a clinical treatment with further studies.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Hu S
| 1. | Vukanovic J, Isaacs JT. Linomide inhibits angiogenesis, growth, metastasis, and macrophage infiltration within rat prostatic cancers. Cancer Res. 1995;55:1499-1504. [PubMed] [Cited in This Article: ] |
| 2. | Ichikawa T, Lamb JC, Christensson PI, Hartley-Asp B, Isaacs JT. The antitumor effects of the quinoline-3-carboxamide linomide on Dunning R-3327 rat prostatic cancers. Cancer Res. 1992;52:3022-3028. [PubMed] [Cited in This Article: ] |
| 3. | Kalland T. Effects of the immunomodulator LS 2616 on growth and metastasis of the murine B16-F10 melanoma. Cancer Res. 1986;46:3018-3022. [PubMed] [Cited in This Article: ] |
| 4. | Yamashita T. Manifestation of metastatic potential in human gastric cancer implanted into the stomach wall of nude mice. Jpn J Cancer Res. 1988;79:945-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204-1208. [PubMed] [Cited in This Article: ] |
| 6. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4014] [Cited by in F6Publishing: 4027] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 7. | Maeda K, Chung YS, Takatsuka S, Ogawa Y, Onoda N, Sawada T, Kato Y, Nitta A, Arimoto Y, Kondo Y. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995;72:319-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 142] [Article Influence: 4.9] [Reference Citation Analysis (0)] |