Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.357
Peer-review started: September 23, 2022
First decision: October 30, 2022
Revised: November 12, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 14, 2023
The biliary tract has been considered for several decades a passive system just leading the hepatic bile to the intestine. Nowadays several researches demonstrated an important role of biliary epithelia (i.e. cholangiocytes) in bile formation. The study of biliary processes therefore maintains a continuous interest since the possible important implications regarding chronic cholestatic human diseases, such as primary biliary cholangitis or primary sclerosing cholangitis. Bile acids (BAs), produced by the liver, are the most represented organic molecules in bile. The physiologic importance of BAs was initially attributed to their behavior as natural detergents but several studies now demonstrate they are also important signaling molecules. In this minireview the effect of BAs on the biliary epithelia are reported focusing in particular on secondary (deriving by bacterial manipulation of primary molecules) ones. This class of BAs is demonstrated to have relevant biological effects, ranging from toxic to therapeutic ones. In this family ursodeoxycholic and lithocholic acid present the most interesting features. The molecular mechanisms linking ursodeoxycholic acid to its beneficial effects on the biliary tract are discussed in details as well as data on the processes leading to lithocholic damage. These findings suggest that expansion of research in the field of BAs/cholangiocytes interaction may increase our understanding of cholestatic diseases and should be helpful in designing more effective therapies for biliary disorders.
Core Tip: The biliary epithelia present important physiologic activities that are of interest with regard to chronic cholestatic liver diseases. Secondary bile acids (BAs) are derived by bacterial manipulation of the primary BAs produced by the liver. This review summarizes the most important recent findings with regard to secondary BAs interaction with biliary epithelia.
- Citation: Lenci I, Milana M, Signorello A, Grassi G, Baiocchi L. Secondary bile acids and the biliary epithelia: The good and the bad. World J Gastroenterol 2023; 29(2): 357-366
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.357
The biliary system is composed of a delicate structure of anastomosing ducts, leading the bile from the liver toward the intestine[1]. While for several years this anatomical apparatus was considered just as an inert route for bile transport, recently several studies have demonstrated that important qualitative/ quantitative bile changes occur within the biliary tract. The isolation and characterization of the biliary epithelia (composed of bile duct cells or cholangiocytes) has deepened our understanding of several important molecular process involving the biliary tree, also shedding some light on the mechanisms leading to chronic cholestatic liver diseases.
Bile acids (BAs) are the main organic molecules secreted in bile[2]. Their physiological importance, which in the beginning was identified only with regard to the physicochemical processes leading to micelle formation[3], nowadays has been expanded by the evidence that BAs are also essential signaling molecules[4]. In this minireview, the most important findings involving secondary BAs and biliary epithelia will be reported together with the possible implications of these mechanisms in human liver diseases.
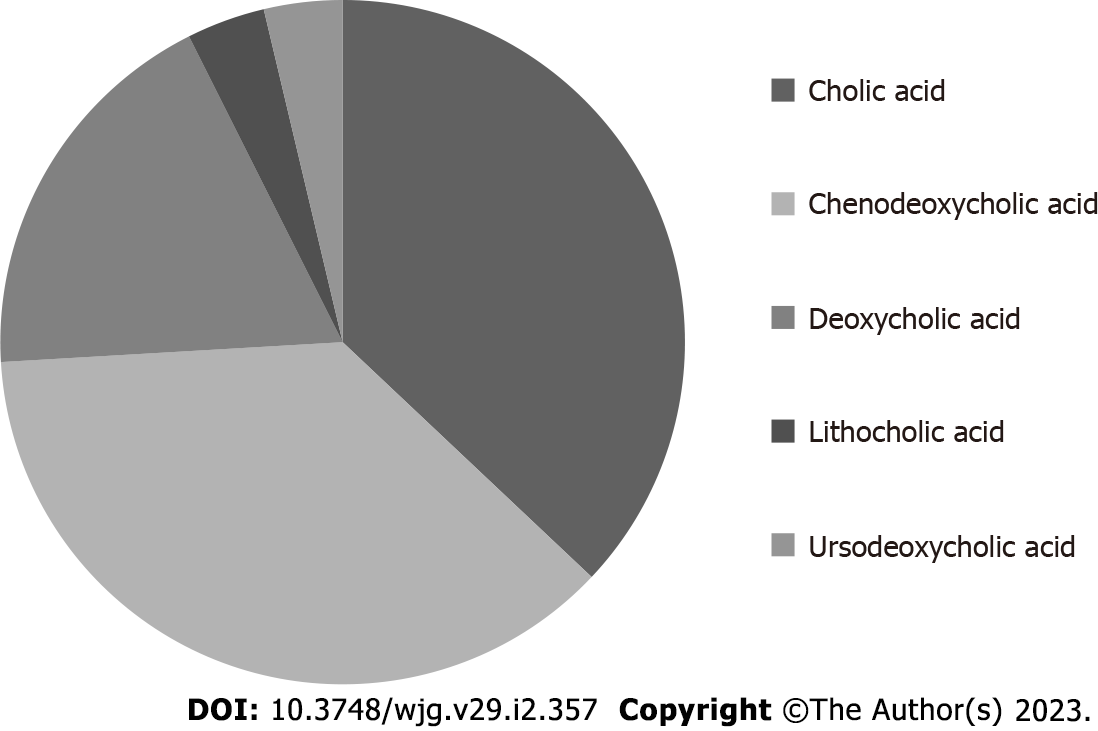
BAs are synthesized by the liver starting from cholesterol and are the most represented lipidic component in bile[5]. Taurine or glycine conjugation, occurring after synthesis, confers increased water solubility to these molecules in bile. BAs are traditionally classified as primary (produced by the liver) or secondary (derived by primary BAs after bacterial dehydroxylation in the intestine)[6]. In humans, the primary BAs are cholic (CA) and chenodeoxycholic (CDCA) acid, while the most represented secondary ones are deoxycholic (DCA) and lithocholic (LCA) acid. The removal of a hydroxyl group (C-7 position) in general determines reduced water solubility and increased detergency in comparison with primary precursors. The hydrophilic or hydrophobic character of a specific BA has been put in relation with its potential cytotoxicity and damaging effects[7]. In this perspective, secondary BAs are generally regarded as possibly damaging molecules when they reach adequate concentrations since their detergent/destabilizing effect on cell membranes. Being the bile a mixture of different (primary and secondary) BAs, the concept of hydrophilic/hydrophobic balance of the bile (and so the net concentration of secondary BAs) has been related with possible liver injury in some conditions[8]. The most hydrophobic human BA is the monohydrate LCA. Sulfation of this molecule by the liver greatly reduces its intestinal absorption (also enhancing its hydrophilicity and urine elimination) and the consequent damage induced by LCA enterohepatic recirculation[9]. It in fact represents less than 5% of total BAs in human bile[6]. The number of hydroxyl groups, however, is not the only determinant of the specific hydrophilic/hydrophobic character of a specific BA. In fact, another secondary BA, ursodeoxycholic acid (UDCA), despite having an equal number of OH groups (two) in comparison with CDCA and one less than CA, is more hydrophilic in comparison with the latter molecules. This physico-chemical characteristic is related to the fact that, differently from CDCA (3α, 7α), in UDCA, the two hydroxyl groups are not on the same plane (3α, 7β). See Table 1 for a quick reference on hydroxyl group number and position, together with some other features, of the principal BAs found in human bile. On Figure 1 the approximate amount of each individual BA in human bile is reported.
| Hydroxil groups number and position | Solubility in water (protonated form, µM)1 | Critical micellar concentration (sodium salt, mM)1 | Hydrophobicity index (taurine conjugated)2 | |
| Primary bile acids | ||||
| Cholic acid | 3 (3, 7, 12) | 273 | 13 | 0 |
| Chenodeoxycholic acid | 2 (3, 7) | 27 | 9 | 0.46 |
| Secondary bile acids | ||||
| Deoxycholic acid | 2 (3, 12) | 28 | 10 | 0.59 |
| Lithocholic acid | 1 (3) | 0.05 | 0.9 | 1 |
| Ursodeoxycolic acid | 2 (3, 7) | 0.9 | 19 | -0.47 |
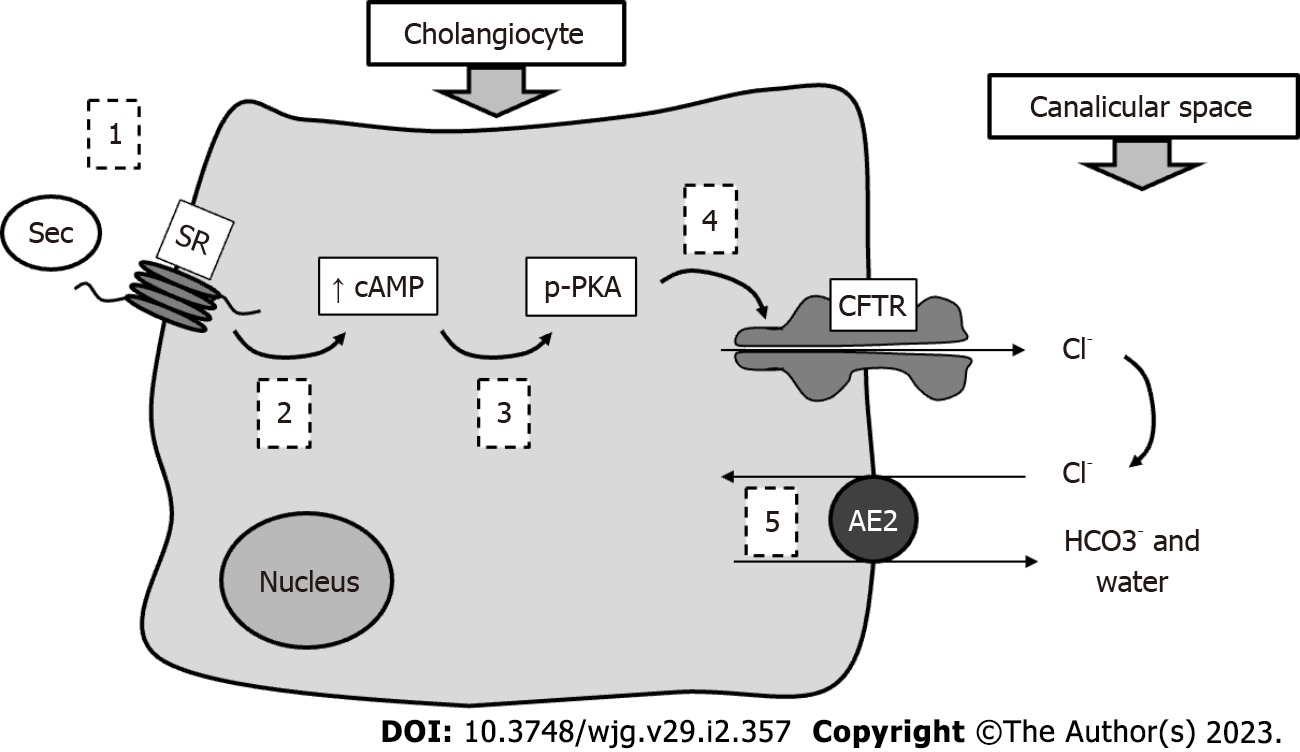
Together with hepatocytes, cholangiocytes constitute the liver epithelial compartment. These latter cells, lining the intrahepatic and extrahepatic biliary ducts, despite representing less than 10% of liver mass, are able to support nearly 50% of bile volume under stimulation[10]. They in fact contribute almost exclusively to the so-called BA-independent bile flow. Cholangiocytes are heterogeneous in size and function; the larger ones represent the physiologically functional compartment, while smaller cells (harboring small branches) may replace large ones when the latter are injured[11]. The most studied mechanism of bile duct secretion concerns the interaction between secretin (Sec) and a specific Sec receptor (SR) expressed by cholangiocytes only within the liver. The subsequent downstream molecular mechanisms are characterized by increased intracellular cAMP in bile duct cells, followed by PKC phosphorylation, extrusion of Cl- by the cystic fibrosis transmembrane regulator and finally its reabsorption and exchange with bicarbonate operated by the Cl-/HCO3- exchanger (AE2)[12]. With this process, a bicarbonate-enriched choleresis is obtained. See Figure 2 for a schematic representation of this mechanism. However, several hormones and neuropeptides (such as somatostatin, histamine, melatonin, gastrin and others) may regulate bile duct cell activity, as these cells have been demonstrated to express the corresponding receptors[13]. BA receptors and transporters are also present on cholangiocytes. They are responsible for important physiological mechanisms.
The biliary epithelium is constantly exposed to significant concentrations (mM) of BAs. This strict connection is at the basis of important processes under both normal and pathological conditions. As previously stated, BAs are mainly present in bile as glycine or taurine conjugates; however, more than 30 years ago, the possibility that unconjugated BAs may cross the biliary epithelium and recirculate in the liver (the so-called chole-hepatic shunt) was hypothesized, thereby inducing increased choleresis with multiple passages[14]. Later, uptake of BAs by the biliary epithelium was demonstrated by the identification of the apical sodium-dependent BA transporter (ASBT) on cholangiocytes[15]. ASBT in the same study was demonstrated to be expressed only by cholangiocytes within the liver and to prompt unidirectional BA transport from the apical to the basolateral cellular domain. ASBT is also expressed in the small intestine, actively reabsorbing BAs and having a major role in maintaining the appropriate entero-hepatic recirculation of these molecules. Gene disruption of this transporter, in fact, nearly completely abolished intestinal recovery of BAs[16], even if a reduced proportion of unconjugated protonated BAs is absorbed by passive uptake in the colon[17]. ASBT function in cholangiocytes, however, remains less clear. In one study, it was demonstrated that Sec stimulation of cholangiocytes was able to increase choleresis, also promoting the transfer of ASBT from the plasma membrane to the apical domain and supporting the original concept of the BA cholehepatic shunt[18]. In another study, a relationship between biliary BAs concentration and ASBT expression was found, suggesting a possible regulatory mechanism of this transporter in maintaining an appropriate biliary BAs concentration[19]. At present, ASBT inhibitors are under study to reduce the BA pool in diseases possibly related to its pathological increment, such as primary biliary cholangitis (PBC)[20].
Further information regarding BAs and cholangiocyte molecular interactions came after the identification of the TGR5, specific for BAs[20]. While in the liver the FXR is mainly expressed in the hepatocyte nucleus where it regulates the transcripts for the synthesis of these molecules[21], on the other hand, TGR5 is prevalently found on the cholangiocyte apical domain[22]. Studies on TGR5(-/-) mice showed an effect on body weight, the immune system and glucose homeostasis[23]. With regard to the biliary tree, TGR5 seems to be an important regulator of cell proliferation with the opposite effect when it is activated in ciliated vs non-ciliated cells[24]. In fact, activation of TGR5 on cholangiocyte cilia depresses cAMP formation and proliferation while the same signal in non-ciliated cholangiocytes enhances intracellular cAMP and cell growth. The important role of TGR5 as a possible regulator of biliary mass suggests that this receptor is a possible target in human diseases characterized by uncontrolled cholangiocyte growth, such as cholangiocarcinoma[25] or polycystic liver disease[26]. More recently, other BA receptors, such as the S1PR2, have been identified on cholangiocytes[27]. These signals enhance biliary growth upon stimulation with taurocholic acid (TCA), employing an ERK1/2 dependent mechanism. In conclusion, accumulating evidence demonstrates that the role of BAs in bile is not restricted to lipid dissolution. In fact, BAs are also important molecular signaling molecules.
As previously reported, secondary BAs originate from manipulation of the original molecules synthesized by the hepatocytes, by intestinal bacteria. However, within this family, molecules with opposite physicochemical and biological characteristics cohabit. The extremities of this class of organic compounds, in terms of heterogeneity, are represented by UDCA and LCA. At the same time, these two BAs seem particularly interesting and relevant with regard to human biliary diseases, as evidenced by several studies.
UDCA was first detected as primary BA in Chinese black bear bile, and later also identified in human bile as a secondary BA, in small amounts (≤ 3%)[28]. Interest in UDCA was first focused on its therapeutic potential for cholesterol gallstone dissolution[29,30]. However, its clinical efficacy for gallstone treatment is: (1) Limited to small (≤ 1 cm) non-calcified stones; and (2) affected by frequent recurrent disease when UDCA is withdrawn. On the other hand, early studies on gallstone dissolution, conducted in patients with concurrent chronic hepatitis, also demonstrated the capabilities of UDCA in improving liver function[31].
Some clinical studies specifically underscored the UDCA beneficial effects in diseases targeting biliary cells and causing an impaired biliary secretion (i.e. cholestasis), such as PBC[32]. UDCA (oral dose 13 to 15 mg/kg/day) is in fact, nowadays, a first line treatment for this disease[33,34]. Several mechanisms seem responsible for the improved clinical picture when UDCA is employed in biliary cholestasis[35]. First, due to its intrinsic hydrophilicity, UDCA seems able to reduce the cytotoxicity/hydrophobicity of the total BA pool against bile duct cells. Second, increased biliary secretion is observed if UDCA enrichment occurs in bile. Finally, immune-modulatory and antiapoptotic effects have been demonstrated[32]. Moreover, also regarding PBC, impairment of AE2 and consequent inadequate formation of a delicate bicarbonate film in the canalicular biliary space (the so-called bicarbonate umbrella) has been suggested to facilitate biliary damage by protonated BAs. In this setting, UDCA seems to be able to reconstitute adequate bicarbonate secretion, thus mitigating PBC injury[36] and also reducing the endoplasmic reticulum stress and autophagy acting as a chaperone[37]. With regard to the biliary epithelium, experimental studies have elucidated some important mechanisms.
In early research, conducted in the cholestatic model of the bile duct ligated (BDL) rat (a condition inducing a hyperplastic growth of the biliary tree), UDCA feeding was able to attenuate biliary mass proliferation[38]. A subsequent study using the same model (BDL) clarified that both pathologically enhanced proliferative and secretive processes of cholangiocytes were mitigated by UDCA, as demonstrated by reduced H3 histone, protein cellular nuclear antigen (PCNA) and SR gene expression, and decreased Sec-induced choleresis[39]. Decreased proliferation was not related to cholangiocyte apoptosis and was dependent (as was decreased secretion) on PKCα activation. Another molecular aspect characterizing the effects of UDCA was the decreased ASBT cholangiocyte expression leading to reduced intracellular BA influx. These findings were extended in a more complex model combining rat BDL and vagotomy. In fact, when vagotomy was performed in the BDL rat, the consequent lack of cholinergic stimuli impaired the hyperplastic cholangiocyte response to cholestasis and led to apoptosis in bile duct cells[40]. When UDCA was administered in this model, it was able to counterbalance bile duct cell loss and apoptosis by a PKCα/Ca2+ dependent mechanism[41].
Further information regarding UDCA and biliary epithelia came from the Mdr2(-/-) mice model. This mouse is not able to transport phospholipids in bile and develops a chronic cholestasis, resembling the human primary sclerosing cholangitis (PSC), with similar scars and strictures within the biliary tree[42]. In Mdr2(-/-) mice UDCA attenuated reactive cholangiocyte proliferation as well as inflammatory and fibrotic processes. These effects were in part related to the inhibition of mast cells, which are activated during experimental and human PSC[43].
LCA is a monohydrate secondary BA that is known for its particular hydrophobicity, remaining water insoluble in its free form while it presents a very low critical micellar concentration (concentration at which micelles are spontaneously formed) in saline[44]. According to its physico-chemical properties, LCA has longer been known as a cholestatic and injurious agent in animal experiments[45,46] and, in parallel with this, increased levels of this BA have been found in human with chronic liver disease[47].
Several mechanisms were identified at the basis of LCA-induced cholestasis such as: (1) Impairment of bile secretion (both BAs dependent and independent)[48]; (2) bile salt export pump translocation from apical membrane to cytosol with its consequent reduced activity[49]; (3) changes in apical membrane fluidity and tight junction permeability[50]; and (4) impairment of canalicular contraction[51].
With regard to biliary epithelia, a study on LCA feeding in Swiss albino mice evidenced interesting features[52]. After 4 d of a 1% LCA diet, destructive cholangitis characterized by stenosis of biliary ducts, solid crystal precipitation and bile infarcts was observed. Neutrophil infiltration surrounded the small biliary branches and periductal fibroblast activation with collagen deposition was reported. A subsequent study, conducted in the same experimental system, helped to clarify that LCA-related biliary damage was dependent on direct toxicity of this BA and not to the immune response since neutrophil inhibition did not significantly change the pathological picture[53]. With regard to secretive and proliferative cholangiocyte activities, in vitro experiments demonstrated that LCA and CA (taurine conjugated) had similar effects in promoting biliary growth and Sec-stimulated bile output[54]. These results were observed with the large cholangiocyte population, which is well-known as the main functional pool in the biliary tree. Similar results were later confirmed in in vivo experiments[55]. In fact, TCA or TLCA rat feeding (1% diet, 1-4 wk) both similarly increased biliary mass and enhanced cholangiocyte biliary secretion. Further experiments suggested that the TLCA stimulation of cholangiocytes function (similarly to TCA) was associated with increased ASBT activity and consequently enhanced intracellular (PKCα/Ca2+ dependent) BA trafficking[56]. This process, with regard to LCA, due to the changes in Ca2+ flux, was also related to impaired gap junction permeability and consequent cholestasis[57].
With regard to other secondary BAs that may play a role in human biliary physio-pathology, DCA is the only one possibly reaching significant concentrations (10%-35% of total BAs pool) in human bile[58]. DCA liver toxicity has been well-established since the early 1990s and, in a study on rat feeding, this was enhanced in comparison to LCA due to its increased intestinal reabsorption and bile enrichment[59]. Despite this and concerning the biliary epithelia, one study has raised interest by showing the suppression of gallbladder cancer growth by DCA, possibly due to interference with miR-92b-3p[60]. This miR in fact would be responsible of the activation PI3K/AKT pathway that is enhanced in several tumors and also represents a target for anticancer treatment[61]. Several other secondary BAs may be found in different species[62]. For instance in rodents the main represented primary BA is β-muricholic acid (β-MCA; 3α, 6β, 7β)[63]. Bacterial manipulation of β-MCA may give origin to different secondary BAs including HDCA (3α, 6α)[64]. HDCA is reported as the strongest regulator of BA-sensitive ion channel (BASIC) that is normally expressed in brain, intestine and cholangiocytes only, within the liver[65]. While the exact physiologic function of cholangiocyte BASIC has not been well established, evidences demonstrate enhanced activity of this channel, with increased trans-epithelial ion transport, after exposure to HDCA[66]. This suggests BASIC as a further possible regulator of biliary secretion. Table 2 summarizes the main findings regarding secondary human BAs and biliary epithelia.
| Model | Administration route | Main results | Main molecular, immunologic findings | Ref. |
| Ursodeoxycholic acid | ||||
| BDL rat | Feeding (both the unconjugated and taurine-conjugated form) | Decreased biliary proliferation and secretion. No apoptosis | Decreased H3-histone. PCNA, SR and ASBT expression. No apoptosis. Increased PKC α expression | [38,39] |
| BDL + vagotomy rat | Feeding (both unconjugated and taurine-conjugated form) | Reversal of duct loss and apoptosis induced by vagotomy | PKCα/Ca2+ dependent mechanism | [41] |
| Mdr2(-/-) mice | Feeding | Decreased proliferation, inflammation and fibrosis | Inhibition of mast cells activity | [43] |
| Lithocolic acid | ||||
| Mouse | Feeding | Destructive cholangitis, bile duct stenosis, bilary infarcts | Damage related to direct toxic effect and not to neutrophil infiltration | [52,53] |
| In vivo rat and isolated cholagiocytes | Feeding (cholic acid or Lithocholic acid both taurine conjugated) | Similar effect in increasing proliferation and secretion | Effect restricted to large cholangiocytes | [54,55] |
| In vivo rat and isolated cholagiocytes | Feeding (cholic acid or Lithocholic acid both taurine conjugated) | Cholangiocytes proliferation | Dependent by PKA-mediated ASBT expression | [56] |
| Deoxycholic acid | ||||
| Human gallbladder cancer (specimens and cell lines) | In vitro exposure | Increased concentration associated with inhibition of tumor growth | Reduced miR-92b-3p inhibits PI3K/AKT activity | [60] |
BAs are important organic molecules. For several decades, researchers have focused on their physico-chemical characteristics, due to their reported detergent properties. From this perspective, the hydrophilic or hydrophobic character of a BA has been considered in the past as the main determinants of physiologic effect. This preliminary view is clearly challenged nowadays, with many studies demonstrating the important molecular signaling systems activated by BAs, not only in the hepatocytes but also in the biliary epithelium. Artificial manipulation of native BA molecules, moreover, has led to the discovery of new agents, such as obeticholic acid, that may be helpful for human therapy[67]. Given all the above, it is clear that the original classification of BAs as primary and secondary compounds only expresses aspects of their synthesis and not necessarily beneficial or negative physiologic effects. Similarly, the division of secondary BAs as good or bad ones (as reported in this review) is questionable, since this does not adequately recapitulate the multitude of effects (probably discovered just in part at the present stage) these molecules may have. In fact, UDCA (generally supposed as beneficial) has been demonstrated to be detrimental in experimental obstructive cholestasis as it can lead to bile infarcts and should not be administered in this clinical condition[68]. On the other hand, LCA has shown interesting curative properties and anti-tumoral and anti-inflammatory effects on intestinal environment, in some studies[69]. In conclusion, UDCA and LCA clearly represent the extremities of a field in which research may growth and a revision in our present beliefs regarding these secondary BAs remains therefore possible in the near future. With regard to normal human physiology and in practice, however, LCA accumulation is prevented by a detoxification system while UDCA is formed only in trace amounts. However, bile enrichment is possible when BAs are exogenously administered to manipulate the BAs pool for therapeutic purposes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sanal MG, India; Wang X, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Healey JE Jr, Schroy PC. Anatomy of the biliary ducts within the human liver; analysis of the prevailing pattern of branchings and the major variations of the biliary ducts. AMA Arch Surg. 1953;66:599-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 432] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 446] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 3. | Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978;19:945-955. [PubMed] [Cited in This Article: ] |
| 4. | Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509-1520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 484] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 5. | Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina-Molina E, Bonfrate L, Wang DQ, Portincasa P. Bile Acid Physiology. Ann Hepatol. 2017;16:s4-s14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647-2658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 628] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 7. | Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 469] [Cited by in F6Publishing: 461] [Article Influence: 30.7] [Reference Citation Analysis (3)] |
| 8. | Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719-730. [PubMed] [Cited in This Article: ] |
| 9. | Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Tabibian JH, Masyuk AI, Masyuk TV, O'Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3:541-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Alpini G, Phinizy JL, Glaser S, Francis H, Benedetti A, Marucci L, LeSage G. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066-G1073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, Gaudio E. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013;1:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 23] [Reference Citation Analysis (0)] |
| 14. | Hofmann AF. Current concepts of biliary secretion. Dig Dis Sci. 1989;34:16S-20S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lazaridis KN, Pham L, Tietz P, Marinelli RA, deGroen PC, Levine S, Dawson PA, LaRusso NF. Rat cholangiocytes absorb bile acids at their apical domain via the ileal sodium-dependent bile acid transporter. J Clin Invest. 1997;100:2714-2721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920-33927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 244] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 18. | Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL, Mauldin J, Lesage G. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Duan S, Li X, Fan G, Liu R. Targeting bile acid signaling for the treatment of liver diseases: From bench to bed. Biomed Pharmacother. 2022;152:113154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 7] [Reference Citation Analysis (0)] |
| 21. | Jiang L, Zhang H, Xiao D, Wei H, Chen Y. Farnesoid X receptor (FXR): Structures and ligands. Comput Struct Biotechnol J. 2021;19:2148-2159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 22. | Keitel V, Häussinger D. TGR5 in the biliary tree. Dig Dis. 2011;29:45-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis. 2011;29:37-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, LaRusso NF. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1013-G1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Deutschmann K, Reich M, Klindt C, Dröge C, Spomer L, Häussinger D, Keitel V. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1319-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Masyuk AI, Masyuk TV, Trussoni CE, Pirius NE, LaRusso NF. Autophagy promotes hepatic cystogenesis in polycystic liver disease by depletion of cholangiocyte ciliogenic proteins. Hepatology. 2022;75:1110-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, Qiang X, Sun L, Gurley EC, Lai G, Zhang L, Liang G, Nagahashi M, Takabe K, Pandak WM, Hylemon PB, Zhou H. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 28. | Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 132] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. part I. Dig Dis Sci. 1982;27:737-761. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 201] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Bachrach WH, Hofmann AF. Ursodeoxycholic acid in the treatment of cholesterol cholelithiasis. Part II. Dig Dis Sci. 1982;27:833-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 120] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Leuschner U, Leuschner M, Sieratzki J, Kurtz W, Hübner K. Gallstone dissolution with ursodeoxycholic acid in patients with chronic active hepatitis and two years follow-up. A pilot study. Dig Dis Sci. 1985;30:642-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 133] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Trauner M, Graziadei IW. Review article: mechanisms of action and therapeutic applications of ursodeoxycholic acid in chronic liver diseases. Aliment Pharmacol Ther. 1999;13:979-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 34. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 727] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 35. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 449] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 36. | Úriz M, Sáez E, Prieto J, Medina JF, Banales JM. Ursodeoxycholic acid is conjugated with taurine to promote secretin-stimulated biliary hydrocholeresis in the normal rat. PLoS One. 2011;6:e28717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Sasaki M, Nakanuma Y. Bile Acids and Deregulated Cholangiocyte Autophagy in Primary Biliary Cholangitis. Dig Dis. 2017;35:210-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Poo JL, Feldmann G, Erlinger S, Braillon A, Gaudin C, Dumont M, Lebrec D. Ursodeoxycholic acid limits liver histologic alterations and portal hypertension induced by bile duct ligation in the rat. Gastroenterology. 1992;102:1752-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | LeSagE G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 140] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Marzioni M, Francis H, Benedetti A, Ueno Y, Fava G, Venter J, Reichenbach R, Mancino MG, Summers R, Alpini G, Glaser S. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 454] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 43. | Meng F, Kennedy L, Hargrove L, Demieville J, Jones H, Madeka T, Karstens A, Chappell K, Alpini G, Sybenga A, Invernizzi P, Bernuzzi F, DeMorrow S, Francis H. Ursodeoxycholate inhibits mast cell activation and reverses biliary injury and fibrosis in Mdr2(-/-) mice and human primary sclerosing cholangitis. Lab Invest. 2018;98:1465-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25:1477-1489. [PubMed] [Cited in This Article: ] |
| 45. | Javitt NB. Cholestasis in rats induced by taurolithocholate. Nature. 1966;210:1262-1263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 99] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Fisher MM, Magnusson R, Miyai K. Bile acid metabolism in mammals. I. Bile acid-induced intrahepatic cholestasis. Lab Invest. 1971;25:88-91. [PubMed] [Cited in This Article: ] |
| 47. | Fischer S, Beuers U, Spengler U, Zwiebel FM, Koebe HG. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | King JE, Schoenfield LJ. Cholestasis induced by sodium taurolithocholate in isolated hamster liver. J Clin Invest. 1971;50:2305-2312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Crocenzi FA, Mottino AD, Sánchez Pozzi EJ, Pellegrino JM, Rodríguez Garay EA, Milkiewicz P, Vore M, Coleman R, Roma MG. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52:1170-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Vu DD, Tuchweber B, Raymond P, Yousef IM. Tight junction permeability and liver plasma membrane fluidity in lithocholate-induced cholestasis. Exp Mol Pathol. 1992;57:47-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Watanabe N, Kagawa T, Kojima S, Takashimizu S, Nagata N, Nishizaki Y, Mine T. Taurolithocholate impairs bile canalicular motility and canalicular bile secretion in isolated rat hepatocyte couplets. World J Gastroenterol. 2006;12:5320-5325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, Trauner M. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168:410-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Woolbright BL, Li F, Xie Y, Farhood A, Fickert P, Trauner M, Jaeschke H. Lithocholic acid feeding results in direct hepato-toxicity independent of neutrophil function in mice. Toxicol Lett. 2014;228:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers RE, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol. 1997;273:G518-G529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Boucherie S, Koukoui O, Nicolas V, Combettes L. Cholestatic bile acids inhibit gap junction permeability in rat hepatocyte couplets and normal rat cholangiocytes. J Hepatol. 2005;42:244-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Gustafsson U, Sahlin S, Einarsson C. High level of deoxycholic acid in human bile does not promote cholesterol gallstone formation. World J Gastroenterol. 2003;9:1576-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol Lett. 1992;61:291-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 77] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Lin R, Zhan M, Yang L, Wang H, Shen H, Huang S, Huang X, Xu S, Zhang Z, Li W, Liu Q, Shi Y, Chen W, Yu J, Wang J. Deoxycholic acid modulates the progression of gallbladder cancer through N(6)-methyladenosine-dependent microRNA maturation. Oncogene. 2020;39:4983-5000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 61. | Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med. 2016;67:11-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 554] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 62. | Reiter S, Dunkel A, Dawid C, Hofmann T. Targeted LC-MS/MS Profiling of Bile Acids in Various Animal Tissues. J Agric Food Chem. 2021;69:10572-10580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in F6Publishing: 774] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 64. | Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, Che Q, Guo J, Su Z. Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. 2021;13:1949095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 65. | Wiemuth D, Sahin H, Lefèvre CM, Wasmuth HE, Gründer S. Strong activation of bile acid-sensitive ion channel (BASIC) by ursodeoxycholic acid. Channels (Austin). 2013;7:38-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Wiegreffe S, Löhrer D, Wirtz M, Wiemuth D. The bile acid-sensitive ion channel (BASIC) mediates bile acid-dependent currents in bile duct epithelial cells. Pflugers Arch. 2021;473:1841-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 67. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 678] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 68. | Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238-1251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 231] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 69. | Sheng W, Ji G, Zhang L. The Effect of Lithocholic Acid on the Gut-Liver Axis. Front Pharmacol. 2022;13:910493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |