Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.286
Peer-review started: September 18, 2022
First decision: October 19, 2022
Revised: November 6, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 14, 2023
Liver cancer is the sixth most commonly diagnosed cancer worldwide, with hepatocellular carcinoma (HCC) comprising most cases. Besides hepatitis B and C viral infections, heavy alcohol use, and nonalcoholic steatohepatitis (NASH)-associated advanced fibrosis/cirrhosis, several other risk factors for HCC have been identified (i.e. old age, obesity, insulin resistance, type 2 diabetes). These might in fact partially explain the occurrence of HCC in non-cirrhotic patients without viral infection. HCC surveillance through effective screening programs is still an unmet need for many nonalcoholic fatty liver disease (NAFLD) patients, and identification of pre-cirrhotic individuals who progress to HCC represents a substantial challenge in clinical practice at the moment. Patients with NASH-cirrhosis should undergo systematic HCC surveillance, while this might be considered in patients with advanced fibrosis based on individual risk assessment. In this context, interventions that potentially prevent NAFLD/ NASH-associated HCC are needed. This paper provided an overview of evidence related to lifestyle changes (i.e. weight loss, physical exercise, adherence to healthy dietary patterns, intake of certain dietary components, etc.) and pharmacological interventions that might play a protective role by targeting the underlying causative factors and pathogenetic mechanisms. However, well-designed prospective studies specifically dedicated to NAFLD/NASH patients are still needed to clarify the relationship with HCC risk.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is a public health problem, especially in developed countries. This condition, depending on certain associated risk factors, can ultimately lead to liver cirrhosis and hepatocellular carcinoma (HCC). Having the necessary tools and knowing the characteristics of patients in whom the disease progresses more quickly, effective monitoring programs can be developed. Primary prevention of NAFLD/nonalcoholic steatohepatitis (NASH)-associated HCC relies on controlling the main modifiable risk factors. Some pharmacological (e.g., metformin, statins, aspirin) and non-pharmacological interventions (weight loss, physical exercise, healthy diet, avoiding heavy drinking and smoking) might have protective effects. Herein, we emphasized the need for continued investigations to find the optimal methods for NAFLD/NASH-associated prevention.
- Citation: Cernea S, Onișor D. Screening and interventions to prevent nonalcoholic fatty liver disease/nonalcoholic steatohepatitis-associated hepatocellular carcinoma. World J Gastroenterol 2023; 29(2): 286-309
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.286
Primary liver cancer was estimated to be the fourth leading cause of cancer-related deaths and the sixth most commonly diagnosed cancer in 2018 worldwide, with hepatocellular carcinoma (HCC) comprising most cases (75%-85%)[1]. The main risk factors for HCC are chronic infection with hepatitis C virus (HCV) and hepatitis B virus (HBV), and non-viral factors, such as heavy alcohol drinking or nonal
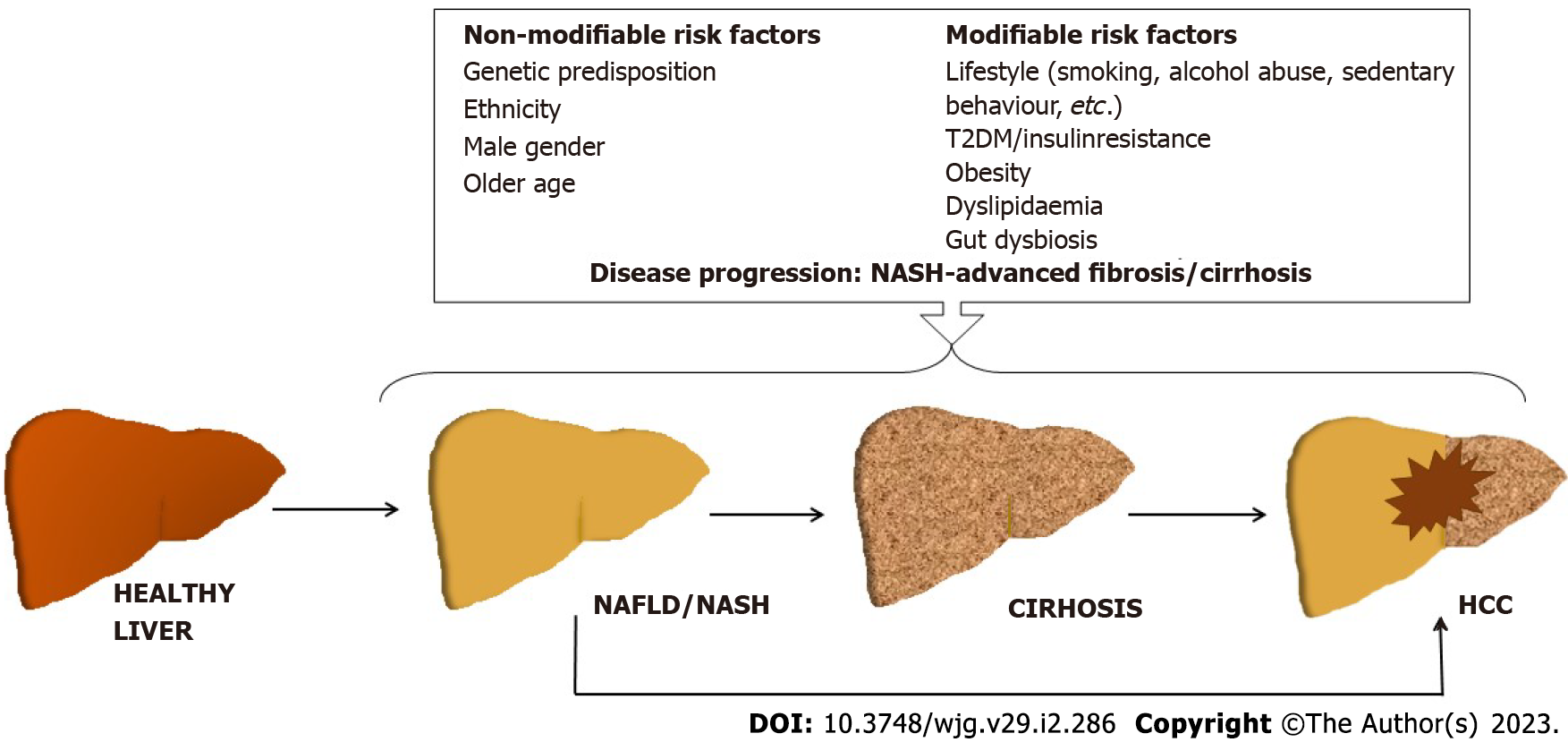
Besides NASH/advanced fibrosis and cirrhosis, several other risk factors for HCC have been identified (Figure 1). Among these, T2DM appears to be strongly and independently associated with both NAFLD/NASH and HCC[7]. In fact, the prevalence of NAFLD in patients with T2DM is twice as high, and the prevalence of NASH is about seven-ten times higher, while the risk of HCC is 2.0-2.5 fold higher than in the general population[4,8,9]. Some studies suggest that a longer duration of diabetes can increase the risk of HCC[10]. The underlying mechanisms that link T2DM to HCC are complex and not fully elucidated, but insulin resistance, chronic inflammation, lipotoxicity, and oxidative stress may play a substantial role by promoting DNA damage, angiogenesis, cellular growth and proliferation, and decreasing cellular apoptosis[11-13]. In fact, insulin resistance seems to play an important role in HCC development (through associated proinflammatory, vasoactive, and pro-oxidative environment), and it might explain in part the occurrence of HCC in non-cirrhotic NAFLD patients[6,13].
Although not unanimous, the overall evidence is suggestive of an increased risk of HCC in individuals with obesity [as evaluated by the body mass index (BMI)][7,14,15]. A case-control study performed in the United States has identified obesity in early adulthood (mid-20s to mid-40s) as a significant HCC risk factor [odds ratio (OR): 2.6, 95% confidence interval (CI): 1.4-4.4], as each unit of increased BMI was associated with a 3.89-mo decrease in age of HCC diagnosis (P < 0.001)[16]. Moreover, obesity in childhood (ages 7-13-years-old) was reported to be associated with higher HCC risk later in life in a retrospective cohort comprising 285884 Danish children[17]. Visceral obesity appears to be particularly significant as an HCC risk, regardless of BMI[18,19]. The gut-liver axis seems to play an important role in the obesity-associated HCC, as gut microbiota creates a tumor-promoting microenvironment by transferring its metabolites/components, which further trigger the release of proinflammatory cytokines (like tumor necrosis factor alpha, interleukin-1β, interleukin-6, etc.), suppress the anti-tumor immunity, and modify the bile acid metabolism[20-22].
Other risk factors for NAFLD-related HCC are male sex, older age, smoking, genetic predisposition (i.e. PNPLA3 polymorphism, etc.) (Figure 1)[6,23-27]. In addition, dyslipidemia, ethnicity, intestinal dysbiosis, and sedentary lifestyle may also contribute (Figure 1)[3,6,26,28-30]. Apparently, the presence of multiple risk factors increases the risk of liver cancer synergistically[6].
In fact, multiple hits drive the development of the NAFLD/NASH-associated HCC through activation of various metabolic, endocrine, and immunological pathways (i.e. increased free fatty acids levels/impaired lipid metabolism, hyperinsulinemia, oxidative stress, endoplasmic reticulum stress, and hyperleptinemia and increased production of proinflammatory cytokines, altered immune response, release of pro-fibrinogenic mediators, etc.) on a background of genetic/epigenetic alterations[31].
The goal of HCC surveillance in NAFLD/NASH patients is to reduce the HCC-related mortality by promoting early tumor detection[32]. Controversy still exists regarding which NAFLD patients would benefit most from the HCC surveillance[6]. In NAFLD patients, the risk of liver-related and all-cause mortality raises exponentially with higher fibrosis stage (from F1 to F4)[33]. The meta-analysis by Dulai et al[33] indicated that compared to F0, the rate ratio of the all-cause mortality was 1.58 (in stage 1), 2.52 (in stage 2), 3.48 (in stage 3), and 6.40 (in stage 4), and the same trend was seen for liver-related mortality. However, it should be noted that the evolution of fibrosis is not linear, as it progresses and regresses in about 20%-30% of patients over 5 years[34]. Among patients with NAFLD, those with cirrhosis are at greatest risk, with an annual HCC incidence rate of 10.6/1000 person-years (PY) compared to 0.08/1000 PY in patients without cirrhosis[5]. Furthermore, HCC incidence rates are higher in patients with decompensated cirrhosis than in those with compensated cirrhosis[28].
Nevertheless, NAFLD patients without cirrhosis are still at risk of developing HCC. The analysis of data obtained from a cohort of 1500 patients with HCC showed that about 13% of them did not have cirrhosis, and patients with NAFLD had a five-fold increased risk of developing HCC in the absence of cirrhosis compared with those with HCV-related HCC[35]. A lower proportion of patients with NAFLD-associated HCC presented cirrhosis than patients with HCV- or alcohol-related HCC (58% vs 85.6% and 72.4%, respectively)[35]. The same was basically shown by the meta-analysis by Tan et al[36] (61 studies; 94636 patients). They reported that NAFLD-related HCC patients were more likely to be non-cirrhotic (38.5% vs 14.6%, P < 0.0001) and had larger tumor diameters (P = 0.0087)[36]. Moreover, these patients had undergone surveillance in a lower proportion than patients with HCC secondary to other causes (32.8% vs 55.7%, P < 0.0001)[36].
Poor HCC surveillance is a significant problem for patients with NAFLD, and in fact, identification of pre-cirrhotic NAFLD individuals with high HCC risk remains a significant challenge at the moment. A prospective multicenter study performed in Italian secondary care centers that included 756 patients with NAFLD- or HCV-related HCC has shown that HCC was diagnosed though regular ultrasound/ specific surveillance in a lower proportion of NAFLD patients compared to HCV patients (47.7% vs 63.3%, P < 0.0001), resulting in a more advanced HCC burden at diagnosis in the former group[37]. Similarly, the analysis of data from the United States Veterans Administration HCC cohort study showed that more patients with NAFLD-HCC did not benefit from HCC surveillance 3 years before diagnosis (43.3%) compared to patients with alcohol abuse- or HCV-related HCC (40.2%, and 13.3%, respectively)[38].
Solid data and guidance regarding risk stratification in non-cirrhotic NAFLD patients who might benefit from HCC surveillance are limited, and specific recommendations in this area are urgently needed due to the growing epidemic of NAFLD[39].
Liver biopsy remains the “gold standard” for the diagnosis of NASH, but it cannot be routinely used in practice as a screening method to diagnose NAFLD, given its multiple limitations: It is expensive, the procedure is subject to interpretation errors, and it is potentially associated with adverse effects such as pain, bleeding, and infection[40,41]. The emergence of non-invasive methods for quantifying fibrosis and their validation has led to a decrease in the need for liver biopsies[42-44]. The Asia-Pacific and the American Gastroenterology Association guidelines agree that the combined use of serum tests and imaging tools may provide more reliable information than using either method alone[30,45]. The American Association for the Study of Liver Diseases guideline also consider the non-invasive methods as first-line tests for the investigation of fibrosis, but it does not recommend a specific diagnostic algorithm or follow-up strategies[43].
Identification of fibrosis and risk stratification is an essential step for HCC surveillance, as the guidelines clearly recommend screening in patients with cirrhosis, while patients with advanced fibrosis (F3) might also undergo surveillance based on individualized evaluation.
Currently, the primary imaging method for HCC detection is ultrasound (US)[40,46,47]. However, recent studies have highlighted the limitations of this examination[28]. For example, a study comprising 941 patients with cirrhosis who underwent ultrasonography reported that 20% of examinations had an inadequate quality to exclude images showing possible focal points[48]. Therefore, other methods (computer tomography, magnetic resonance imaging) might be used[45,47]. For these two investigations, the follow-up interval is not clearly established nor are the benefits of the association with measurement of alpha-fetoprotein (AFP) levels[49].
It has been questioned whether the addition of AFP quantification to routine biannual US examinations would increase the detection rate of HCC during screening. However, only about three-quarters of HCC patients are AFP positive[50]. In fact, the probability of having elevated AFP levels (i.e. > 10 ng/mL) in patients with early NAFLD-associated HCC without cirrhosis and normal transferase levels was only 17.5%-24.0% compared with 86.5%-90.5% in patients with viral-associated cirrhosis and advanced HCC with increased transaminases values, as showed in an Italian study that included 4123 HCC patients[51]. The use of AFP measurement as a screening tool for HCC might result in earlier diagnosis, but it does not seem to improve the mortality rate[52]. The European guidelines do not currently recommended AFP as a surveillance parameter for the detection of HCC in patients with NAFLD. The American guidelines recommend ultrasound screening with or without AFP, while Asia Pacific Society guidelines recommend screening with AFP[30,47,53]. Other biomarkers such as microRNAs (e.g., miR-34a, miR-221) are under investigation for early detection of HCC but need further validation[54].
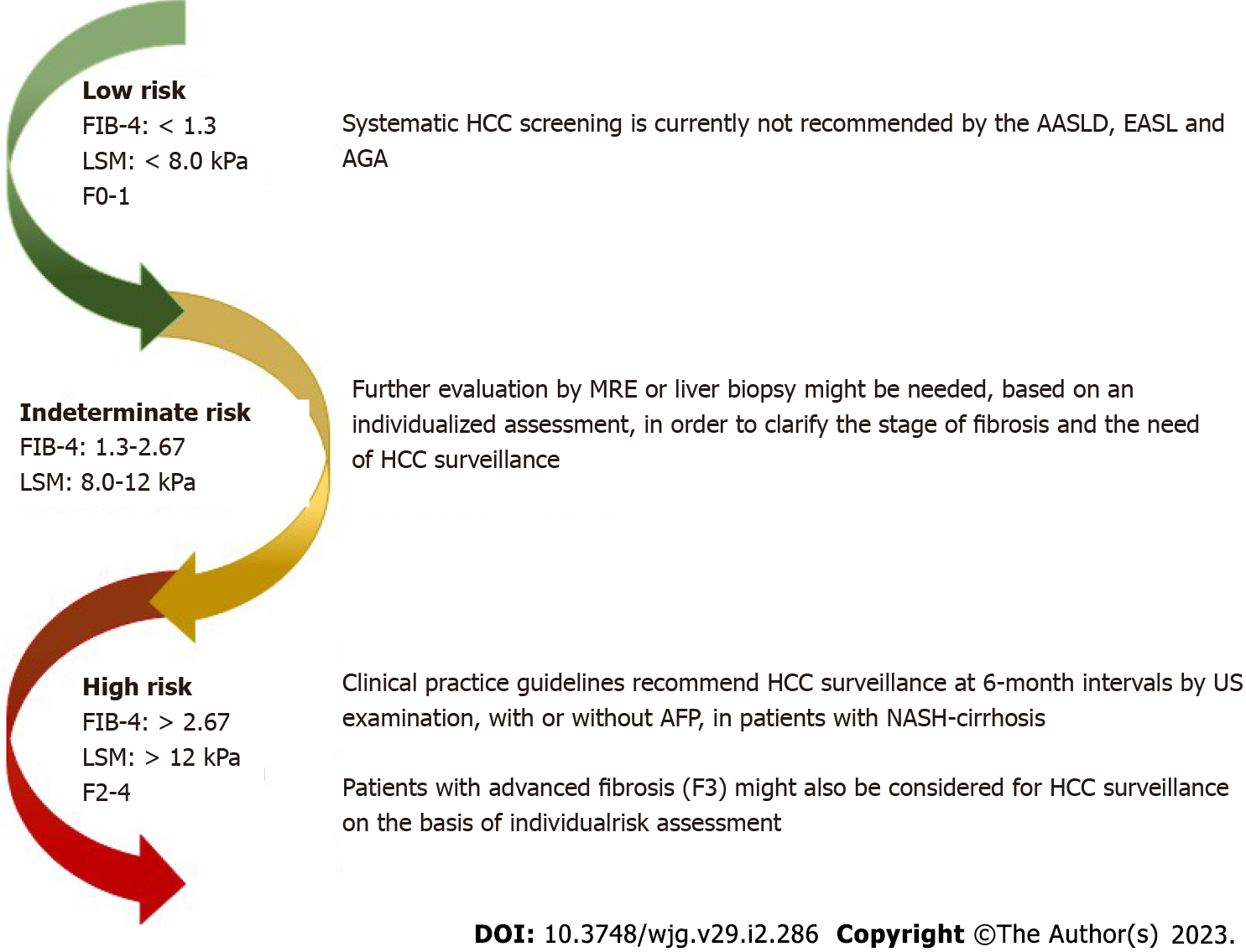
Most patients screened in a primary care setting have a low risk of clinically significant liver fibrosis, defined as having a Fibrosis-4 (FIB-4) score < 1.3, liver stiffness measurement (LSM) < 8.0 kPa on transient elastography, or a liver biopsy fibrosis stage of F0–F1[44,55-57]. Systematic HCC screening may not be prudent and is currently not recommended by the American Association for the Study of Liver Diseases, American Gastroenterology Association, and the European Association for the Study of the Liver in non-cirrhotic NAFLD patients (Figure 2)[42-44].
An estimated 30%–40% of screened patients have an indeterminate risk of clinically significant (advanced) liver fibrosis, defined as FIB-4 score of 1.3-2.67 and/or a LSM of 8.0-12.0 kPa on transient elastography[44,55-57]. The estimated incidence of HCC in non-cirrhotic NASH seems to be too low to justify systematic screening[58]. Apparently, non-cirrhotic NAFLD patients with multiple features of metabolic syndrome are at higher risk of HCC development and need special attention[5,35]. It is argued that additional triggers (such as active inflammation and fibrosis) are needed to promote carcinogenesis[6].
The current guidelines recommend the referral of these patients to a hepatologist for further evaluation by magnetic resonance elastography or liver biopsy[30,42,43]. The decision should be taken by mutual agreement and on the basis of an individual assessment (presence/absence of comorbidities, degree of fibrosis, etc.)[44]. The European Association for the Study of the Liver guidelines recommend that patients with liver disease and advanced fibrosis (F3) might be considered for HCC surveillance based on the individual risk, while the Asian Pacific and American Association for the Study of Liver Diseases clinical practice guidelines do not provide a specific recommendation for surveillance in patients with NAFLD without cirrhosis[30,43].
Nearly 10% of screened patients have a high risk of advanced liver fibrosis, defined as a FIB-4 score > 2.67, LSM > 12.0 kPa, or a liver biopsy showing clinically significant liver fibrosis (≥ F2)[44,55-57]. Patients with cirrhosis are at the highest risk for HCC. The meta-analysis and meta-regression by Orci et al[59] involving 470404 patients showed that the incidence rate of HCC was 0.03/100 PY in patients with NAFLD at a pre-cirrhotic stage and 3.78/100 PY in those with cirrhosis, while in patients with cirrhosis undergoing regular screening for HCC, it was 4.62/100 PY.
Some data suggested that HCC surveillance might not be associated with improved clinical outcomes. For example, a matched case-control study from the United States Veterans Affairs health system failed to find an association between screening (by US, AFP, either test, or both tests) and rate of HCC-related mortality[60]. However, the lack of benefit may have not been related to the failure of surveillance but rather to other causes, such as underuse of HCC treatment or applying surveillance in patients who were not candidates for HCC treatment. On the other hand, a meta-analysis of 59 cohort studies indicated that HCC surveillance was associated with improved early HCC detection, receiving curative therapy, and survival in patients with cirrhosis but with heterogeneity in pooled estimates[32]. Thus, available data is in favor of HCC surveillance in patients with cirrhosis, although it still needs further confirmation[32].
Surveillance programs by regular US (and AFP) in patients with compensated cirrhosis are cost effective[61]. In fact, cost-effectiveness analyses indicate that HCC screening should be considered for patients with Child-Pugh A and B (compensated) cirrhosis and decompensated liver cirrhosis patients waiting for liver transplantation[47].
In patients with NASH-cirrhosis, all three liver study societies recommend the use of an HCC surveillance program at 6-mo intervals, with US exams, with or without AFP[30,42,43]. The same was endorsed by the recommendations of American Gastroenterology Association Clinical Practice[40].
The NAFLD/NASH-associated HCC primary prevention relies on controlling the main modifiable risk factors, i.e. obesity, T2DM/insulin resistance, gut dysbiosis, disease activity/fibrosis (disease progression), that have been associated with activation of various oncogenic pathways finally leading to hepatocarcinogenesis[62]. There is no clearly effective intervention for HCC prevention available for NAFLD/NASH patients at the moment, although some pharmacological and non-pharmacological approaches might indeed be useful by addressing the predisposing factors/causes and underlying pathogenetic mechanisms (Table 1)[63].
| Interventions with potential protective effects | |
| Lifestyle interventions | Weight loss |
| Dietary changes | |
| Adherence to healthy eating patterns: Mediterranean diet, traditional Cantonese dietary pattern; Chinese Healthy Eating Index | |
| Reduced intake of: Saturated fats, sugar-sweetened beverages, alcohol | |
| Increased intake of: Vegetables, coffee; possibly fiber, white meat and fish, omega-3 polyunsaturated fatty acids, vitamin E | |
| Physical exercise | |
| Smoking cessation | |
| Pharmacological interventions | Metformin |
| Statins | |
| Aspirin | |
| Possibly also: Pioglitazone, GLP-1 RA, vitamin E (in nondiabetic individuals?), obeticholic acid | |
Weight loss through lifestyle intervention represents the cornerstone for NAFLD management, as it has been associated with the regression of steatosis, steatohepatitis, and even fibrosis (for > 10% weight loss)[64]. The analysis of two randomized controlled trials (RCTs) indicated that each lost kg was associated with a 7% increase in odds of obtaining NASH resolution without worsening of fibrosis, and a 5% increase in odds of obtaining fibrosis improvement without worsening of NASH[65]. The meta-analysis by Koutoukidis et al[66], which included 2588 NAFLD subjects who underwent weight loss inter
Preliminary results from a retrospective analysis of a database containing 72 million unique patients reported that weight loss medications reduced the risk of HCC in obese populations (OR: 0.07), with orlistat and liraglutide showing statistically significant decreases (OR: 0.13, and 0.35, respectively)[67]. In addition, the meta-analysis by Ramai et al[68], which included data from major databases, indicated that bariatric surgery was associated with a reduced risk of HCC (pooled unadjusted OR: 0.40), although with high heterogeneity (I2: 79%).
Although there is limited evidence regarding the impact of weight loss on the risk of developing HCC, it is intuitive and reasonable to encourage overweight/obese patients to decrease their weight. Weight loss is associated with the improvement of metabolic health (or indirect outcomes, such as the decrease of insulin resistance, inflammation, oxidative stress, etc.), which may translate into liver health benefits[69-72]. However, a recent RCT in patients with NAFLD demonstrated that a modest weight loss (-4 kg) through a calory-restricted diet was accompanied by reduction in transaminases, but the liver steatosis grade or the markers of oxidative stress were not significantly changed in comparison with the controls[73]. Thus, the liver benefits still have to be demonstrated by prospective data, which should clarify the direct effect of weight loss on long-term NAFLD/NASH progression and primary HCC prevention.
The effects of bariatric surgery on liver outcomes might be explained in part by weight loss, although other contributing mechanisms cannot be excluded [e.g., increase in glucagon-like peptide-1 (GLP-1) concentrations after intervention][74]. The meta-analysis by Lee et al[75], which analyzed the data of 32 cohort studies (n = 3093 liver biopsies from NAFLD patients with obesity that underwent bariatric surgery) showed that surgical intervention resulted in an absolute percentage BMI reduction of 24.98% (from 48.68 ± 2.92 to 34.2 ± 3.53 kg/m2). This was accompanied by steatosis resolution in 66% of patients as well as the resolution of inflammation (in 50% of patients), ballooning degeneration (in 76%), and fibrosis (in 40%)[75]. However, 12% of the subjects presented new or worsening fibrosis after the intervention[75].
The more recent meta-analysis by Ramai et al[68] included nine studies (19514750 patients) and reported that bariatric surgery was associated with a reduced risk of HCC (pooled unadjusted OR: 0.40, 95%CI: 0.28-0.57 and adjusted OR: 0.63, 95%CI: 0.53-0.75). So far there is no clear indication regarding the type of surgical intervention that would be most beneficial in terms of liver health.
A large prospective cohort study has demonstrated the association of unhealthy lifestyle (assessed by a composite score comprising BMI, alternative Mediterranean diet, alcohol intake, smoking, and sleep duration) with the risk of HCC: Higher composite scores (5, 6, 7, 8) representing healthier lifestyle were associated with a lower risk of HCC (0.67, 0.61, 0.49, and 0.13, respectively; Ptrend < 0.0001) compared with lower scores (0-4) over a mean follow-up of 17.7 years[76]. As unhealthy lifestyle is associated with a higher risk of NAFLD/NASH and HCC, it is reasonable to assume that correcting these behaviors will potentially protect against the development of HCC.
However, there is insufficient direct evidence to indicate that changes in lifestyle reduce the risk of HCC in NAFLD/NASH patients. A meta-analysis of 30 RCTs in NAFLD patients (n = 3280), which evaluated the effect of diet, exercise, or their combination on the liver and metabolic markers, reported that a combination of diet and exercise resulted in a greater decrease of ALT [mean difference (MD): -13.27], AST (MD: -7.02) and Homeostatic Model Assessment for insulin resistance (MD: -2.07) compared to either of them[77]. However, no histological or imaging data were available. Moreover, an umbrella review of evidence from observational studies and RCTs looking at the association between lifestyle and NAFLD with regards to risk and treatment (41 meta-analysis from observational studies and 81 meta-analysis from RCTs) suggested that some interventions [i.e. green tea, omega 3 polyunsaturated fatty acids (PUFA), and exercise] were associated with some improvement in metabolic and hepatic markers, but more robust RCTs are needed to investigate the effect of lifestyle changes on liver outcomes[78]. In addition, the network meta-analysis by Buzzetti et al[79] (59 RCTs, 3631 participants; 2-24 mo of follow-up) could not draw a definite conclusion regarding the effect of the lifestyle interventions on any of the clinical liver outcomes (including HCC), as the number of events was too low (probably due to short duration of follow-up).
Diet: There is limited information regarding the impact of diet/dietary components on liver histology in patients with NAFLD/NASH and on the risk of progression to HCC. Most studies report data related to liver biomarkers/fat content or risk of liver cancer in overall population/patients with chronic liver diseases, regardless of etiology. The relationship between HCC and several nutrients, foods, and dietary patterns has been evaluated mostly in observational studies, but few data exist exclusively in patients with NAFLD/NASH[80]. There is a lack of high-quality data from large RCTs in this population, but it should be noted that it is quite challenging to evaluate dietary determinants of the disease in the context of multiple confounding effects of other lifestyle factors[81].
Dietary patterns represent a complex combination of foods/nutrients and beverages that are habitually consumed, and their evaluation may capture in a more integrated way the effect of diet on health outcomes[82]. A recent systematic review of 30 observational studies (5222534 participants from Asia, America, and Europe) investigated the association between diet and risk of HCC and found differences with regards to geographical regions and dietary patterns[83]. Specifically, the Mediterranean diet appeared to be protective for the European and American populations, while the Chinese Healthy Eating Index and the Cantonese Dietary Pattern seemed to be associated with lower risk of HCC in Asian countries[83].
Analysis of combined data from two case-control studies demonstrated that better adherence to the Mediterranean diet was associated with a lower risk of HCC (ORs: 0.66 and 0.51, P < 0.001 for trend)[84]. In addition, the Alternate Mediterranean diet score (an adaptation of the original Mediterranean diet score) was associated with a decreased risk of HCC [hazard ratio (HR): 0.68; P = 0.02], as indicated by a multiethnic cohort prospective study[85]. Another study has shown a suggestive but nonsignificant association with lower risk (HR: 0.75; Ptrend = 0.18)[86]. The Singapore Chinese Health Study data also indicated that higher Alternate Mediterranean diet scores as well as higher scores of Alternative Healthy Eating Index-2010 (AHEI-2010) and Dietary Approaches to Stop Hypertension, representing a better dietary quality, were associated with a lower risk of HCC (Ptrend < 0.05)[76]. The results were in agreement with the report of the National Institutes of Health-AARP Diet and Health study, indicating that higher HEI-2010 and Alternate Mediterranean diet score were significantly associated with lower HCC risk (HR: 0.72; Ptrend = 0.03 and HR: 0.62; Ptrend = 0.0002, respectively)[87].
Similarly, better adherence to the Chinese Dietary Guidelines, as assessed by the Chinese Healthy Eating Index, was shown to be associated with a lower risk of HCC (OR: 0.43; P < 0.001), even in the stratified analysis by risk factors[88]. The study by Lan et al[89] that enrolled 782 patients with primary liver cancer and evaluated their habitual dietary intake found that an urban prudent dietary pattern (consisting of higher intake of eggs, mushrooms, dairy products, soy foods and nuts, and lower intake of refined grains) and a traditional Cantonese dietary pattern (characterized by a high intake of fruit and vegetables, Cantonese soup, fish, and Chinese herb tea) have been associated with a lower risk of primary liver cancer (OR: 0.25 and 0.61, respectively), while a diet rich in meat and preserved foods increased the risk (OR: 1.98). Moreover, a prospective study that enrolled 887 patients with newly diagnosed HCC suggested that a higher adherence to the 2016 Chinese Dietary Guidelines was associated with a lower risk of HCC-specific mortality (HR: 0.74) and all-cause mortality (HR: 0.75)[90].
Inflammation is a key pathogenetic mechanism that influences NASH progression and hepatocarcinogenesis. Two studies have evaluated the correlation between dietary inflammatory score/index (that reflect the overall inflammatory potential of a diet) and the risk of HCC/primary liver cancer mortality. The first one showed that higher dietary inflammatory index values (indicating a proinflammatory diet) were associated with an increased risk of liver cancer (HR: 2.05) and liver cancer-associated mortality (HR: 1.97)[91]. The second study reported that higher adherence to empirical dietary inflammatory pattern score (indicating a proinflammatory potential of the diet) was associated with an increased risk of HCC (HR: 2.03; Ptrend = 0.001)[92]. The same study reported that several other scores (empirical dietary index/lifestyle pattern score for hyperinsulinemia and insulin resistance) indicating a higher insulin resistance potential of a diet were also correlated with a higher risk of HCC[92].
Thus, a healthy dietary pattern (generally characterized by an increased intake of vegetables, fruits, nuts, and whole grains and a decreased intake of red and processed meats) appears to be protective against HCC and HCC-related mortality. However, more specific evaluations are needed to confirm the data and assess the strength of these associations in patients with NAFLD/NASH.
There is little evidence with respect to the impact of dietary macro- and micronutrients upon liver histology in patients with NAFLD/NASH (e.g., reversal of fibrosis) and the risk of progression to HCC.
Data coming from observational studies are heterogenous. Some studies showed that increased carbohydrate intake was associated with higher aminotransferases levels, liver fat, and NASH, while others indicated the opposite or were neutral[81,93-97]. A meta-analysis of ten RCTs concluded that low-carb diets significantly reduced the intrahepatic lipid content but did not change the serum liver enzyme concentrations in patients with NAFLD[98]. There is also sparse and inconsistent evidence regarding the association between food glycemic index and load with HCC[35]. Data from the European Prospective Investigation into Cancer and Nutrition cohort (EPIC) study (477206 subjects) did not find significant associations between dietary glycemic index or glycemic load or total carbohydrate intake with risk of liver cancer[99]. However, the risk of HCC in correlation with types of carbohydrates appeared to be divergent: 43% higher risk per 50 g total sugar intake/d and 30% lower per 50 g starch/d[99]. The analysis of the Shanghai Women’s Health Study and the Shanghai Men’s Health Study data also indicated no consistent association between dietary carbohydrates, glycemic index, and glycemic load and risk of liver cancer[100].
On the other hand, higher intake of dietary fructose/sugar-sweetened beverages (surrogate for free sugars) has been associated with NAFLD, independent of other risk factors in several studies, as well as with higher intrahepatic lipid content, mainly when consumed in the context of excessive caloric intake[81,101-105]. The meta-analysis by Li et al[106] (71 observational studies) reported that higher sugar-sweetened beverages intake was associated with higher overall cancer risk [relative risk (RR): 1.12; P = 0.000] and mortality risk (RR: 1.07; P = 0.029) as well as higher risk of HCC (RR: 2.00; P = 0.001) (but HCC results were based only on two studies). Interestingly, the EPIC study data suggested that consumption of > 6 servings of combined soft drinks per week was associated with higher HCC risk (HR: 1.83; Ptrend = 0.01) but artificially-sweetened rather than sugar-sweetened soft drinks intake appeared to be deleterious[107]. A significant positive association between carbonated beverages consumption and HCC risk was also seen in a case-control study of 582 cirrhotic patients (181 with HCC) (OR: 2.44; Ptrend = 0.021)[108].
The same study showed an inverse correlation of HCC risk with fiber intake (OR: 0.49; Ptrend = 0.012), which is in accordance with the EPIC study results indicating a 30% HCC risk reduction per 10 g/d of total dietary fiber intake, even in viral hepatitis-free individuals[99,108]. However, the analysis of data from two United States cohort studies (125455 participants) did not find a significant association of HCC risk with cereal, fruit, or vegetable fiber intake[109]. On the other hand, lower daily (mainly soluble) fiber intake was observed in patients with NAFLD/NASH in several observational studies[94,96,110].
There is inconsistent evidence with regards to the role of dietary proteins (types, amount) in the progression of NAFLD/NASH and occurrence of HCC. The Rotterdam Study which included 3882 individuals (1337 with NAFLD) showed that animal protein intake was significantly associated with overweight NAFLD after adjustment for metabolic covariates (OR: 1.36)[111]. Indirect evidence, in line with these results, was provided by another cross-sectional study that showed that total and animal protein consumption was positively associated with estimates of liver steatosis (OR: 1.25 and 1.27, respectively), while vegetable proteins had an inverse association with these (OR: 0.81)[112]. On the other hand, a recent RCT that evaluated the effect of a low-carbohydrate high-protein diet on liver fat in 72 T2DM patients demonstrated that it reduced the liver fat content to a slightly greater extent compared to a conventional diet (64% vs 51%, P = 0.051) beyond the effects of (similar) weight loss[73]. Similarly, a small prospective study in NAFLD patients with T2DM showed that high protein diets (30% of total caloric daily intake), either animal or plant-based, significantly reduced liver fat independent of change in body weight, and decreased the serum levels of fibroblast growth factor 21[113]. The animal protein diet determined a greater increase in postprandial levels of methionine and branched chain amino acids but this was not accompanied by beneficial or deleterious effects[113]. Some experimental data suggested potential benefits of branched chain amino acids in terms of hepatocarcinogenesis inhibition (changes in growth factors gene expression, inhibition of proliferation), increased apoptosis in liver cancer cell lines, and improvement of fibrosis[82,114,115]. In addition, few Japanese clinical studies suggested that branched chain amino acid supplementation in patients with HCC may reduce cancer recurrence after hepatic resection (mainly in patients with higher insulin resistance), but others showed no effect[116-119]. Certainly, additional good-quality evidence is needed regarding the role of dietary proteins/amino acids in the progression of NAFLD/NASH and associated HCC.
Most studies concerning dietary fats in NAFLD/NASH and HCC evaluated the role of type rather than amount of fats[120]. Observational studies seem to indicate that total dietary fat intake is not correlated with HCC risk[121-123]. Some but not all results suggested that vegetable fats might be associated with reduced HCC risk[121,123]. Higher consumption of saturated fatty acids however was shown to be associated with hepatic steatosis/NAFLD and NASH[81,95,124]. A large Chinese prospective cohort study also indicated that dietary saturated fats were associated with higher liver cancer risk [adjusted HR (aHR): 1.14; P = 0.005], but results from the EPIC study and a hospital-based case-control study from the United States did not support a direct association of saturated fats intake with HCC[125-127]. However, a meta-analysis of 14 studies by Zhao et al[128] demonstrated that higher dietary intake of saturated fats was associated with an increased risk of liver cancer (RR: 1.34 for highest vs lowest intake) in a dose-dependent manner.
On the other hand, the same United States case-control study showed that monounsaturated fatty acids (MUFA) but not total PUFA intake was inversely correlated with the risk of HCC (OR: 0.49 and 1.82)[122]. This was in contrast with the study by Yang et al[123] that showed that PUFA was inversely associated with HCC risk (aHR: 0.83; P = 0.03) (with MUFA being neutral). The study by Li et al[125] indicated that MUFA had a neutral effect (aHR: 1.26, 95%CI: 0.96-1.65; P = 0.034), while PUFA intake was associated with higher HCC risk (aHR: 1.41, 95%CI: 1.07-1.86 for highest vs lowest quartile; P = 0.005). Thus, data on MUFA and total PUFA seems quite heterogenous (maybe explained in part by dietary source, type of study, and study population).
In a NASH animal model, dietary intake of docosahexaenoic acid appeared to be superior to that of eicosapentaenoic acid in attenuating Western-diet induced liver injury, oxidative stress and fibrosis, and potentially in reducing the risk of HCC, thus suggesting differential effects of the two dietary omega-3 PUFAs[129]. Evidence is more convergent regarding the effect of omega-3 PUFA supplementation on liver fat content. Two meta-analyses actually showed that it decreased liver fat, and this was confirmed by a small histologic study in non-cirrhotic NASH patients that received 3000 mg/d omega-3 PUFA for 1 year[130-132]. However, the study did not reach the primary end-point (i.e. NAS reduction of ≥ 2 points without fibrosis progression)[132].
In addition, the evidence regarding the effect of omega-3 PUFA on HCC risk is not unequivocal. The case-control study by Moussa et al[127] demonstrated an inverse association between dietary omega-3 intake and HCC risk (OR: 0.50), but other epidemiological data seemed to indicate a neutral effect (HR: 0.63; Ptrend = 0.14 and aHR: 0.89, 95%CI: 0.75–1.04; P = 0.14, respectively)[122,123,125]. Some observational studies showed a positive relationship between dietary intake of omega-6 PUFA and HCC risk (adjusted OR: 2.29 for highest vs lowest tertile, and OR: 4.36), indicating a potentially negative effect, although other data showed an inverse association (HR: 0.54; Ptrend = 0.02)[123,127,133]. Linoleic acid intake might be inversely associated with HCC risk (OR: 0.35, P < 0.01)[122].
Even if the role of micronutrients in preventing the progression of NASH to HCC might be hypothetically explained from a pathogenetic perspective (i.e. antioxidant, anti-inflammatory, anti-fibrotic effects), and the experimental data is quite consistent. There is insufficient good quality clinical research regarding their dietary intake or supplementation effect in NAFLD patients[134].
Two United States prospective studies have shown an inverse relationship between (dietary and supplemental) magnesium intake and risk of liver cancer (HR: 0.65 and HR: 0.44; Ptrend = 0.0065, respectively)[135,136]. Results from two large cohort studies in China demonstrated an inverse association between dietary manganese intake and liver cancer risk long-term (HR: 0.51; Ptrend = 0.001), even after adjustment for HBV infection[137]. The case-control study by Rizk et al[108] also found that manganese intake was significantly lower in HCC patients vs controls (OR: 0.56; Ptrend = 0.038) as well as potassium intake (OR: 0.44; Ptrend = 0.004). On the other hand, the sodium intake was significantly higher[104]. Nevertheless, more studies are needed to clarify the role of minerals in NASH and HCC.
The same above-mentioned case-control study also indicated lower intake of vitamins E and B9 in HCC patients[108]. In the same sense, a report from two Chinese cohort studies showed that dietary vitamin E intake and supplement use were inversely associated with the risk of liver cancer (HR: 0.52)[138]. The effect of therapeutic intervention with vitamin E will be discussed below. An inverse association between HCC risk and (β) carotenes/vitamin A was noted in several studies (with OR: 0.48 for β carotene, 0.34 for vitamin A, and 0.35 for carotenes)[122,139].
Food items/groups have been evaluated with regards to their association with HCC risk. We will only briefly mention the main findings here. Several systematic reviews and meta-analyses have evaluated data regarding the association between meat consumption and risk of HCC. Even if not totally in agreement, they seem to indicate that read meat consumption is associated with increased HCC risk (RR: 1.22) or is neutral (RR: 1.04 and 1.10), and there is an increased HCC risk associated with processed meat consumption (RR: 1.20, and 1.01)[140-142]. Total meat intake had no significant effect[140-142]. On the contrary, white meat and fish intake were found to be inversely associated with the risk of HCC (RR: 0.69, 0.76 for white meat and RR: 0.78, 0.91 for fish)[141,142]. Some epidemiological data suggested that increased dairy product intake (mainly milk and high-fat diary) was associated with a higher risk of HCC, although not all studies agreed (yogurt seemed to be associated with a decreased risk or had a neutral effect)[121,143-145]. Two meta-analyses indicated that increased vegetable consumption was associated with a lower risk of HCC[146,147]. For other food items, there is insufficiently consistent evidence so far.
There have been suggestions for the use of many herbal and dietary natural compounds, such as prebiotics/polyphenols, resveratrol, and curcumin in NAFLD/NASH therapy, including for HCC prevention, with some small studies suggesting anti-inflammatory effects of prebiotics, but until now there is very limited data from clinical trials, and no clear conclusion can be drawn[134,148-150]. Preclinical data demonstrated the anti-inflammatory effects of the catechin-rich green tea extract that may attenuate NASH-associated liver injury through a decrease of hepatic nuclear factor kappa-B activation but also indirectly through prebiotic and antimicrobial effects on gut microbiota, resulting in decreased translocation of the gut-derived endotoxins[151]. Green tea contains several bioactive compounds that may exert anticarcinogenic properties (e.g., flavonoids, caffeine, polyphenols, etc.) through modulation of different signaling transduction and metabolic pathways (reduction of chronic inflammation, oxidative stress, insulin resistance, liver steatosis, etc.)[80,152]. The EPIC study data showed an inverse association between tea consumption and HCC risk (HR: 0.41, 95%CI: 0.22-0.78; Ptrend = 0.003), while a meta-analysis of 15 RCTs demonstrated that green tea reduced the liver enzymes values in NAFLD patients[153,154].
The use of probiotics in NAFLD/NASH, cirrhosis, and HCC has been reported in several studies and indicated that they can improve aminotransferases and insulin resistance and have anti-inflammatory effects, but there is no evidence so far with regards to HCC prevention[150,155]. A meta-analysis of 21 RCTs (1252 participants) suggested that the use of probiotics/synbiotics was associated with a decrease of inflammation markers, liver stiffness, and steatosis in subjects with NAFLD[156]. However, well-designed RCTs are further needed to fully understand their protective effect in patients with NASH.
Coffee: Eight meta-analyses of prospective cohort and case-control studies provided consistent evidence regarding an inverse relationship between coffee drinking and risk of HCC (RR ranging between 0.54 and 0.78), with only one meta-analysis indicating a neutral effect (RR: 0.93)[157-164]. Caffeinated rather than decaffeinated coffee seemed to have a more consistent effect on HCC[158,161]. Also, the association appeared to be related to the amount of daily coffee intake. The beneficial effects generally started at two cups/d. An extra cup of coffee/d reduced the cancer risk by about 15%-25% (RRs between 0.75 and 0.85), and two extra cups/d by about 14%-44% (RRs between 0.56 and 0.86)[157-164].
Alcohol: Alcohol is a major risk factor for HCC, and it has synergistic effects with the metabolic risk factors (T2DM, obesity) in inducing carcinogenic mechanisms[80,165]. Evidence suggests that a modest alcohol intake is protective against fatty liver, NASH, and fibrosis, but it was not firmly established if it is also protective against HCC[82,166]. A meta-analysis of 19 cohorts (4445 incident cases of liver cancer) showed neutral effects of moderate drinking (< 3 drinks/d) on liver cancer risk (RR: 0.91, 95%CI: 0.81-1.02) compared to no drinking, while heavy drinking (defined as ≥ 3 drinks/d) significantly increased the risk (RR: 1.16, 95%CI: 1.01-1.34)[167]. In line with these results, a prospective evaluation of 8345 subjects with hepatic steatosis (mean follow-up duration of 11.1 years) demonstrated a dose-response relationship for advanced liver outcomes/liver cancer that became significant at ≥ 10 g of alcohol intake/d (for liver outcomes) and ≥ 30 g/d (for liver cancer) after multivariate adjustments[168]. The decrease in risk of HCC after alcohol drinking cessation is uncertain, but a meta-analysis of four studies suggested a decline of HCC risk with 6%-7%/year, although caution was advised in data interpretation[169].
Physical activity: The benefits of physical activity in reducing insulin resistance and hepatic liver content in NAFLD patients are well known[170,171]. The meta-analysis by Golabi et al[170] (eight studies with 8 to 48 wk duration) reported that aerobic and resistance exercises determined a liver fat reduction of 30.2%.
Physical exercise intervenes at multiple levels in the pathogenic pathways of NAFLD by reducing the free fatty acid (FFA) flux to the liver, FFA hepatic synthesis, the mitochondrial and cellular damage, oxidative stress, damage-associated molecular patterns release, and hepatic stellate cell activation and by increasing the FFA oxidation and activating the AMP-activated protein kinase-regulated pathways, etc.[172]. Physical exercise also improved mitochondrial function (i.e. autophagy, biogenesis) and modulated the carcinogenic signaling pathways[80,173,174]. Taken together, these might explain the potential protective effects of exercise in NAFLD/NASH and HCC. Indeed, a meta-analysis of 14 prospective studies indicated that physical activity was inversely correlated with the risk of liver cancer (HR for high vs low physical activity: 0.75, 95%CI: 0.63-0.89)[175]. These results were in accordance with the EPIC study that reported an aHR of 0.55, 95%CI: 0.38–0.80 for HCC in active vs inactive individuals[176]. The associations seemed to be at least in part mediated by obesity[176]. However, no prospective study evaluated the effect of physical exercise on HCC risk, as this might be rather difficult to perform[172].
Smoking cessation: Data from the Liver Cancer Pooling Project, a consortium of 14 prospective cohort studies comprising 1518741 individuals indicated that cigarette smoking significantly increased the risk of HCC (HR: 1.86, 95%CI: 1.57–2.20)[177]. It also demonstrated that in individuals who quit smoking for > 30 years, the risk of HCC decreased to values almost similar to non-smokers (HR: 1.09, 95%CI: 0.74–1.61)[177]. In addition, quitters seemed to have a lower risk of HCC-related mortality (HR, 0.62, 95%CI: 0.39-0.97), but this was seen in subjects without diabetes[178].
Several drugs have been suggested to bring benefits for NAFLD/NASH and to potentially reduce the risk of associated HCC, although there is limited evidence for HCC chemoprevention. It is assumed however that by improving histological features associated with NASH and the primary drivers of fibrogenesis ultimately leading to cirrhosis (and HCC), the disease progression will be attenuated, and HCC occurrence eventually prevented[179].
Metformin: Metformin does not seem to improve NASH/fibrosis, but several meta-analyses suggested that it might reduce the risk of HCC in patients with T2DM[180-182]. A recent meta-analysis of 24 studies including 1.4 million individuals reported that metformin was associated with a 41% lower risk of HCC in DM patients treated with metformin (P < 0.001) and a significant reduction of all-cause mortality (HR: 0.74, 95%CI: 0.66-0.83; P = 0.037)[180]. Moreover, a network meta-analysis that compared several antidiabetic medications has shown that in comparison with sulphonylureas and insulin, metformin significantly decreased the risk of HCC (RR: 0.45, 95%CI: 0.27–0.74 and RR: 0.28, 95%CI: 0.17–0.47)[181]. Postulated mechanisms of chemoprotective effects of metformin are the activation of AMP-activated protein kinase and inhibition of the mammalian target of rapamycin pathway, the inhibition of angiogenesis and induction of apoptosis[183]. It was also suggested that metformin may inhibit the progression of high fat diet-induced HCC by modulating the innate immune-mediated inflammation and restoring tumor surveillance[184]. However, these data should be interpreted with caution, and there is still need for further substantiation.
Thiazolidinediones: The meta-analysis by Musso et al[185] has delineated the effects of the two thiazolidinediones (TZDs), indicating that only pioglitazone (30/45 mg/d, for 6 to 24 mo) was associated with improvement in fibrosis (even advanced fibrosis) and NASH resolution in patients with or without diabetes. In addition to increasing adiponectin levels and decreasing excessive lipolysis and FFA flux into the liver, pioglitazone attenuated oxidative stress and inflammation and, the activation and proliferation of hepatic stellate cells, extracellular matrix deposition, fibrosis, etc[12,186-188]. The possible role of the TZDs in hepatic chemoprevention is further suggested by in vitro data, showing that they may inhibit hepatocarcinogenesis by the regulation of nucleophosmin, a ubiquitously expressed cellular phosphoprotein involved in both proliferation and growth-suppression pathways[189,190]. Animal data also showed that pioglitazone reduced the HCC development, possibly through the upregulation of the AMP-activated protein kinase pathway and the reduction of the mitogen-activated protein kinase activation[191]. However, data in humans are scarce, and no definite conclusions can be drawn yet. The results of the network meta-analysis by Zhou et al[181] suggested possible beneficial effects of TZDs in reducing HCC incidence, but these were seen only in comparison with sulpho
GLP-1 receptor agonists: Two histological studies with GLP-1 receptor agonists (GLP-1 RAs) (liraglutide and semaglutide) in patients with or without T2DM have proven NASH resolution, but results regarding fibrosis were inconclusive[192,193]. Preclinical studies have suggested potential chemoprotective effects of the GLP-1 RAs through various mechanisms like enhancing natural killer cell-mediated cytotoxicity and, inducing autophagy and senescence of the HCC cells by the increase of transforming growth factor β1, promoting their apoptosis though activation of the JNK signaling pathway, etc[194-196]. There is very limited information regarding the long-term effect of GLP-1 RAs on HCC incidence in humans so far.
Statins: Two recent meta-analyses of observational and interventional studies have confirmed liver safety for statin use in patients with NAFLD and even a reduction of transaminases levels[197,198]. Moreover, the meta-analysis by Fatima et al[198], which also analyzed the liver histology outcomes, reported a significant reduction of steatosis grade, necro-inflammatory stage, and of significant fibrosis but not the fibrosis stage. Several meta-analyses (mostly of observational studies) have consistently reported reduced risk of HCC in statin users (RRs/ORs/HR between 0.52-0.75 for all statins), with some indication of differences between them[199-208]. In particular, it seemed that the lipophilic statins exert preventive effects (OR: 0.51, 95%CI: 0.46-0.57 and HR:0.49, 95%CI: 0.39–0.62) rather than the hydrophilic statins (OR: 0.77, 95%CI: 0.58-1.02 and HR: 0.73, 95%CI: 0.40–1.34)[205,208]. Higher doses appeared to be associated with better protective effects (HR: 0.38 vs 0.55)[195]. Moreover, a meta-analysis of nine retrospective cohort studies also reported a lower risk of HCC-related mortality (RR: 0.78, P = 0.001) and reduced HCC recurrence (RR: 0.55, P < 0.001) with statin use[208]. However, another meta-analysis indicated that statin usage decreased the risk of all-cause mortality (HR: 0.80, 95%CI: 0.68-0.94; P < 0.0001) but not of HCC-specific mortality (HR: 0.80, 95%CI: 0.62-1.03; P = 0.002)[200].
Apart from exerting lipid-lowering effects, statins have anti-inflammatory, antioxidant, anti-proliferative, and anti-angiogenic properties[12,209,210]. Their potential anti-tumor effects might be mediated through the downregulation of the RAF/mitogen-activated protein kinase 1/extracellular signal regulated kinase and nuclear factor kappa-B pathways, limitation of the cyclin-dependent kinase inhibitors (p21 and p27) degradation, prevention of the c-Myc activation, reduction of proinflammatory cytokines, etc., which determine apoptotic responses, tumor-suppressor effects, cell survival reduction, and cell growth inhibition[183,211]. Nevertheless, data from well-designed RCTs (that limit the effect of confounding factors) in support of the HCC chemopreventive effects of statins in NAFLD/NASH population is scant.
For the other anti-hyperglycemic and lipid-lowering drug classes there is no consistent evidence so far regarding potential HCC protective effects.
Resmetirom is a thyroid hormone receptor β-selective agonist, which was shown to significantly reduce the liver fat after 12 and 36 wk of treatment[212]. It is currently under evaluation for safety and efficacy in improving NASH and preventing progression to cirrhosis and/or advanced liver disease in a phase 3 RCT (MAESTRO-NASH; NCT03900429)[213].
Aspirin: Growing evidence coming from preclinical and clinical observational studies suggest that aspirin might play a role in HCC prevention[80,165]. The mechanisms are related to inhibition of selective cyclooxygenase-2 as well as of platelet-derived growth factor, P4HA2, nuclear factor kappa-B activation, and protein kinase 3 signaling, etc., which may prevent proliferation of liver cancer cells and angiogenesis and promote fibrosis resolution[80,165]. Several meta-analyses have explored the potential HCC protective effect of aspirin and have shown that it is associated with reduced incidence/risk of HCC (RRs/HRs/ORs between 0.74 and 0.51)[214-219]. The meta-analysis by Memel et al[214] implied stronger association after adjustment for metformin/statin use and accounting for cirrhosis. Also, an inverse relationship seems to exist between aspirin dose/duration of use and HCC risk, but this should be further confirmed[215,219]. There was no evidence of higher risk of bleeding with aspirin use, including in patients with HCC in most meta-analyses except one[216-220]. Moreover, the systematic review and meta-analysis by Tan et al[216] showed that aspirin use was associated with improved liver-related mortality (OR: 0.32, 95%CI: 0.15-0.70) and reduced risk of HCC recurrence (HR: 0.80, 95%CI: 0.75-0.86). The same was observed in the meta-analysis by Li et al[220], which demonstrated a reduced risk of HCC recurrence (RR: 0.74, 95%CI: 0.59-0.93; P = 0.01) and all-cause mortality (RR: 0.59, 95%CI: 0.47-0.73; P < 0.001). Although the evidence points toward a potential benefit of aspirin use in prevention of HCC, further prospective data is still necessary in the NAFLD/NASH population.
Vitamin E has anti-oxidative properties, and it might modulate fibrogenesis through inhibition of transforming growth factor β1[12,221]. A recent meta-analysis of eight RCTs reported that vitamin E supplementation (400-800 IU/d, between 8-96 wk) was associated with reduction of fibrosis score (MD vs placebo: -0.26, 95%CI: -0.47 to -0.04; P = 0.02) as well as a decrease in steatosis, lobular inflammation, and hepatocellular ballooning compared with placebo[222]. However, only one study included patients with T2DM. In fact, the study by Bril et al[223] that randomized 105 T2DM patients with biopsy-proven NASH to vitamin E 400 IU b.i.d., vitamin E 400 IU b.i.d. plus pioglitazone 45 mg/d, or placebo did not reach the primary outcome (two-point reduction of NAS from two different parameters, without worsening of fibrosis) in patients receiving vitamin E vs placebo but resulted in improvement of steatosis. Thus, it is not clear if the benefit of vitamin E supplementation is restricted to individuals without diabetes, and more data is needed in the T2DM population.
Experimental studies have suggested that antifibrotic therapies may serve as HCC preventive interventions by addressing the underlying cause of carcinogenesis onset[224,225]. Unfortunately, no anti-fibrotic drug has been approved so far by the European Medicines Agency or by the United States Food and Drug Administration for NASH treatment.
Obethicolic acid (OCA) is a farnesoid X receptor agonist shown to improve fibrosis without worsening NASH in an interim analysis of a phase 3 RCT (REGENERATE; NCT02548351)[226,227]. In this analysis, a significantly higher proportion of patients treated with OCA 25 mg daily presented improvement in fibrosis by ≥ 1 stage with no worsening of NASH (23% vs 12% in placebo group, P = 0.0002)[227]. In the analysis that included patients receiving at least 15 mo of therapy, three times more patients in the OCA 25 mg/d group obtained ≥ 1 stage improvement in fibrosis (38%) vs progression of fibrosis (13%) compared with the placebo group, in which similar proportions of patients presented improvement (23%) vs worsening (21%) of fibrosis[227]. The FLINT (Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment) trial had previously shown that treatment with OCA 25 mg/d for 72 wk improved liver histology (RR: 1.9, 95%CI: 1.3-2.8; P = 0.0002) in patients with biopsy-proven NASH[228]. However, in both trials, a higher proportion of patients treated with OCA presented pruritus (23% in FLINT study and 51% in REGENERATE study) in a dose-dependent manner[227,228]. In addition, OCA therapy was associated with increased low-density lipoprotein cholesterol levels, but this could be attenuated by concomitant statin therapy[80,229]. HCC animal model data suggested that the OCA might attenuate the development and progression of NASH-associated HCC by upregulating sirtuin-1 and modulating the SOCS3/Jak2/STAT3 pathway[230].
Pentoxifylline: Although few small histological studies have suggested liver-related benefits with pentoxifylline 400 mg t.i.d. treatment [i.e. improvement of steatosis, lobular inflammation, and liver fibrosis score (mean change: -0.2 vs +0.4 on placebo, P = 0.038)] and regression of fibrosis when combined with vitamin E (P = 0.003), data is still scarce and not consistent[231,232]. Four meta-analyses have reported conflicting results with regards to the effect of pentoxifylline on liver fibrosis, with only two of them suggesting benefits[233-236]. Therefore, additional data is required.
Two other initially promising anti-fibrotic medications have failed to prove a significant impact on hepatic fibrosis in phase 3 clinical trials. Elafibranor, an agonist of PPAR-α/δ, reduced steatohepatitis without worsening fibrosis in a phase 2 trial in NASH patients, but the primary end-point was not met[237]. The phase 3 RCT (RESOLVE-IT-NCT02704403) was prematurely discontinued due to limited efficacy at the time of the interim analysis[238]. Cenicriviroc, a dual inhibitor of C–C motif chemokine receptor 2/5, reduced liver fibrosis in NASH patients but did not reach the primary outcome in a phase 2b study[239]. This was followed by a phase 3 trial (AURORA-NCT03028740), which was also terminated early due to lack of efficacy resulting from the planned interim analysis[240]. Several other drugs with potential anti-fibrotic effects are currently in development/evaluation, and the results are expected with interest[241].
Regardless of the risk status, all NAFLD/NASH patients should consider adopting lifestyle changes (healthy diet and physical exercise) and controlling their body weight, as these are the cornerstone interventions for NAFLD/NASH management and possibly through altering the natural course of the disease for HCC prevention.
Data suggest a possible role of comprehensive lifestyle changes in reducing the risk of HCC, but specific evidence in NAFLD/NASH patients is rather limited at this point and not sufficient to clearly indicate preventive effects on NAFLD/NASH-associated HCC. Moreover, there is no consensus regarding the composition of a protective diet but decreasing the intake of deleterious nutrients/foods and beverages (i.e. saturated fats, sugar-sweetened beverages, alcohol), increasing the beneficial nutrients/foods/beverages (vegetables, coffee, dietary fiber, omega-3 PUFA), and adherence to a healthy dietary pattern (such as the Mediterranean diet or traditional Cantonese dietary pattern) are reasonable and safe approaches. However, their role in HCC prevention still needs to be confirmed by further well-designed prospective studies and experimental research.
Several drug classes (metformin, statins, and aspirin and possibly TZDs, GLP-1 RA, vitamin E, and obeticholic acid) might exert chemopreventive effects by addressing the underlying mechanisms of the disease, but direct evidence regarding their role in NAFLD/NASH-associated HCC prevention is insufficient at the moment.
A timing combination of therapies/non-pharmacological interventions and perhaps adapting them to the stage of disease and/or patient particularities will be necessary to obtain disease resolution and prevention of cirrhosis/NASH-associated HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Limaiem F, Tunisia; Wei W, China S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51049] [Article Influence: 8508.2] [Reference Citation Analysis (122)] |
| 2. | Moriguchi M, Seko Y, Takahashi A, Itoh Y. Epidemiology of hepatocellular carcinoma in nonalcoholic fatty liver disease. Hepatoma Res. 2019;5:43. [DOI] [Cited in This Article: ] |
| 3. | Younossi ZM, Henry L. Epidemiology of non-alcoholic fatty liver disease and hepatocellular carcinoma. JHEP Rep. 2021;3:100305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6295] [Article Influence: 786.9] [Reference Citation Analysis (0)] |
| 5. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 444] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 6. | Plaz Torres MC, Bodini G, Furnari M, Marabotto E, Zentilin P, Strazzabosco M, Giannini EG. Surveillance for Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: Universal or Selective? Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477-491.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 1000] [Article Influence: 200.0] [Reference Citation Analysis (1)] |
| 8. | Cernea S, Raz I. NAFLD in type 2 diabetes mellitus: Still many challenging questions. Diabetes Metab Res Rev. 2021;37:e3386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 553] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 10. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, Vauthey JN. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Li X, Wang X, Gao P. Diabetes Mellitus and Risk of Hepatocellular Carcinoma. Biomed Res Int. 2017;2017:5202684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Cernea S, Cahn A, Raz I. Pharmacological management of nonalcoholic fatty liver disease in type 2 diabetes. Expert Rev Clin Pharmacol. 2017;10:535-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5:270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Kanwal F, Shubrook JH, Younossi Z, Natarajan Y, Bugianesi E, Rinella ME, Harrison SA, Mantzoros C, Pfotenhauer K, Klein S, Eckel RH, Kruger D, El-Serag H, Cusi K. Preparing for the NASH epidemic: A call to action. Metabolism. 2021;122:154822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther. 2010;31:1051-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Hassan MM, Abdel-Wahab R, Kaseb A, Shalaby A, Phan AT, El-Serag HB, Hawk E, Morris J, Singh Raghav KP, Lee JS, Vauthey JN, Bortus G, Torres HA, Amos CI, Wolff RA, Li D. Obesity Early in Adulthood Increases Risk but Does Not Affect Outcomes of Hepatocellular Carcinoma. Gastroenterology. 2015;149:119-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Berentzen TL, Gamborg M, Holst C, Sørensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60:325-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Hwang S, Park YM, Han KD, Yun JS, Ko SH, Ahn YB, Han JH. Associations of general obesity and central obesity with the risk of hepatocellular carcinoma in a Korean population: A national population-based cohort study. Int J Cancer. 2021;148:1144-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, Boffetta P, Dahm CC, Overvad K, Tjønneland A, Halkjær J, Fagherazzi G, Boutron-Ruault MC, Carbonnel F, Kaaks R, Lukanova A, Boeing H, Trichopoulou A, Bamia C, Lagiou P, Palli D, Grioni S, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, van den Berg S, Peeters PH, Braaten T, Weiderpass E, Quirós JR, Travier N, Sánchez MJ, Navarro C, Barricarte A, Dorronsoro M, Lindkvist B, Regner S, Werner M, Sund M, Khaw KT, Wareham N, Travis RC, Norat T, Wark PA, Riboli E, Nöthlings U. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer. 2013;132:645-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Loo TM, Kamachi F, Watanabe Y, Yoshimoto S, Kanda H, Arai Y, Nakajima-Takagi Y, Iwama A, Koga T, Sugimoto Y, Ozawa T, Nakamura M, Kumagai M, Watashi K, Taketo MM, Aoki T, Narumiya S, Oshima M, Arita M, Hara E, Ohtani N. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 21. | Yamagishi R, Kamachi F, Nakamura M, Yamazaki S, Kamiya T, Takasugi M, Cheng Y, Nonaka Y, Yukawa-Muto Y, Thuy LTT, Harada Y, Arai T, Loo TM, Yoshimoto S, Ando T, Nakajima M, Taguchi H, Ishikawa T, Akiba H, Miyake S, Kubo M, Iwakura Y, Fukuda S, Chen WY, Kawada N, Rudensky A, Nakae S, Hara E, Ohtani N. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci Immunol. 2022;7:eabl7209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 22. | Takahashi S, Tanaka N, Fukami T, Xie C, Yagai T, Kim D, Velenosi TJ, Yan T, Krausz KW, Levi M, Gonzalez FJ. Role of Farnesoid X Receptor and Bile Acids in Hepatic Tumor Development. Hepatol Commun. 2018;2:1567-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 986] [Cited by in F6Publishing: 1350] [Article Influence: 192.9] [Reference Citation Analysis (0)] |
| 24. | Marengo A, Jouness RI, Bugianesi E. Progression and Natural History of Nonalcoholic Fatty Liver Disease in Adults. Clin Liver Dis. 2016;20:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Degasperi E, Colombo M. Distinctive features of hepatocellular carcinoma in non-alcoholic fatty liver disease. Lancet Gastroenterol Hepatol. 2016;1:156-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 799] [Article Influence: 266.3] [Reference Citation Analysis (0)] |
| 27. | Krawczyk M, Stokes CS, Romeo S, Lammert F. HCC and liver disease risks in homozygous PNPLA3 p.I148M carriers approach monogenic inheritance. J Hepatol. 2015;62:980-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol. 2022;76:195-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Sharpton SR, Ajmera V, Loomba R. Emerging Role of the Gut Microbiome in Nonalcoholic Fatty Liver Disease: From Composition to Function. Clin Gastroenterol Hepatol. 2019;17:296-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 30. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 375] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 31. | Raza S, Rajak S, Anjum B, Sinha RA. Molecular links between non-alcoholic fatty liver disease and hepatocellular carcinoma. Hepatoma Res. 2019;5:42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, Yang JD, Reig M, Cabibbo G, Nahon P, Parikh ND, Marrero JA. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 33. | Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557-1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 984] [Cited by in F6Publishing: 1166] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 34. | Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, Cummings O, Yeh M, Gill R, Chalasani N, Neuschwander-Tetri BA, Diehl AM, Dasarathy S, Terrault N, Kowdley K, Loomba R, Belt P, Tonascia J, Lavine JE, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open. 2019;2:e1912565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 35. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:124-31.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 415] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 36. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 106] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 37. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 407] [Cited by in F6Publishing: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 38. | Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594-601.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 39. | Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68:326-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 40. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 275] [Article Influence: 137.5] [Reference Citation Analysis (1)] |
| 41. | Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 213] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 42. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016;9:65-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 43. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3544] [Cited by in F6Publishing: 4089] [Article Influence: 681.5] [Reference Citation Analysis (7)] |
| 44. | Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, Abdelmalek MF, Harrison SA, Loomba R, Mantzoros CS, Bugianesi E, Eckel RH, Kaplan LM, El-Serag HB, Cusi K. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161:1657-1669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 214] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 45. | Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 186] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 46. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 594] [Article Influence: 198.0] [Reference Citation Analysis (0)] |
| 47. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3934] [Cited by in F6Publishing: 4938] [Article Influence: 823.0] [Reference Citation Analysis (0)] |
| 48. | Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 49. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 638] [Cited by in F6Publishing: 617] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 50. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 294] [Cited by in F6Publishing: 324] [Article Influence: 36.0] [Reference Citation Analysis (5)] |
| 51. | Giannini EG, Sammito G, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Gasbarrini A, Sacco R, Foschi FG, Missale G, Morisco F, Svegliati Baroni G, Virdone R, Trevisani F; Italian Liver Cancer (ITA. LI.CA) Group. Determinants of alpha-fetoprotein levels in patients with hepatocellular carcinoma: implications for its clinical use. Cancer. 2014;120:2150-2157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 53. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2121] [Cited by in F6Publishing: 2685] [Article Influence: 447.5] [Reference Citation Analysis (1)] |
| 54. | Elemeery MN, Mohamed MA, Madkour MA, Shamseya MM, Issa NM, Badr AN, Ghareeb DA, Pan CH. MicroRNA signature in patients with hepatocellular carcinoma associated with type 2 diabetes. World J Gastroenterol. 2019;25:6322-6341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Lomonaco R, Godinez Leiva E, Bril F, Shrestha S, Mansour L, Budd J, Portillo Romero J, Schmidt S, Chang KL, Samraj G, Malaty J, Huber K, Bedossa P, Kalavalapalli S, Marte J, Barb D, Poulton D, Fanous N, Cusi K. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diabetes Care. 2021;44:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 161] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 56. | Ciardullo S, Monti T, Perseghin G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults With Type 2 Diabetes. Diabetes Care. 2021;44:519-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 57. | Barb D, Repetto EM, Stokes ME, Shankar SS, Cusi K. Type 2 diabetes mellitus increases the risk of hepatic fibrosis in individuals with obesity and nonalcoholic fatty liver disease. Obesity (Silver Spring). 2021;29:1950-1960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 58. | Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 59. | Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, Bruix J, Reig M, Toso C. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol. 2022;20:283-292.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 60. | Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, Lowy E, Berry K, Ioannou GN. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155:1128-1139.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 61. | Parikh ND, Singal AG, Hutton DW, Tapper EB. Cost-Effectiveness of Hepatocellular Carcinoma Surveillance: An Assessment of Benefits and Harms. Am J Gastroenterol. 2020;115:1642-1649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 62. | Baffy G. Hepatocellular Carcinoma in Non-alcoholic Fatty Liver Disease: Epidemiology, Pathogenesis, and Prevention. J Clin Transl Hepatol. 2013;1:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1343] [Cited by in F6Publishing: 2454] [Article Influence: 818.0] [Reference Citation Analysis (1)] |
| 64. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1181] [Cited by in F6Publishing: 1302] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 65. | Koutoukidis DA, Jebb SA, Tomlinson JW, Cobbold JF, Aveyard P. Association of Weight Changes With Changes in Histological Features and Blood Markers in Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol. 2022;20:e538-e547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Koutoukidis DA, Astbury NM, Tudor KE, Morris E, Henry JA, Noreik M, Jebb SA, Aveyard P. Association of Weight Loss Interventions With Changes in Biomarkers of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. JAMA Intern Med. 2019;179:1262-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 67. | Trujillo S, Kamionkowski S, Walsh E, Davila J, Asaad I. S1175 Do Weight Loss Medications Reduce the Risk of Cirrhosis and HCC?. Am J Gastroenterol 2021; 116: S547-S548. [DOI] [Cited in This Article: ] |
| 68. | Ramai D, Singh J, Lester J, Khan SR, Chandan S, Tartaglia N, Ambrosi A, Serviddio G, Facciorusso A. Systematic review with meta-analysis: bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;53:977-984. [PubMed] [Cited in This Article: ] |
| 69. | He S, Wang J, Zhang J, Xu J. Intermittent Versus Continuous Energy Restriction for Weight Loss and Metabolic Improvement: A Meta-Analysis and Systematic Review. Obesity (Silver Spring). 2021;29:108-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Forsythe LK, Wallace JM, Livingstone MB. Obesity and inflammation: the effects of weight loss. Nutr Res Rev. 2008;21:117-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 71. | Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016;23:591-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 506] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 72. | Tumova E, Sun W, Jones PH, Vrablik M, Ballantyne CM, Hoogeveen RC. The impact of rapid weight loss on oxidative stress markers and the expression of the metabolic syndrome in obese individuals. J Obes. 2013;2013:729515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Thomsen MN, Skytte MJ, Samkani A, Carl MH, Weber P, Astrup A, Chabanova E, Fenger M, Frystyk J, Hartmann B, Holst JJ, Larsen TM, Madsbad S, Magkos F, Thomsen HS, Haugaard SB, Krarup T. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia. 2022;65:506-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 74. | Larraufie P, Roberts GP, McGavigan AK, Kay RG, Li J, Leiter A, Melvin A, Biggs EK, Ravn P, Davy K, Hornigold DC, Yeo GSH, Hardwick RH, Reimann F, Gribble FM. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell Rep. 2019;26:1399-1408.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 75. | Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1040-1060.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 76. | Luu HN, Behari J, Goh GB, Wang R, Jin A, Thomas CE, Clemente JC, Odegaard AO, Koh WP, Yuan JM. Composite Score of Healthy Lifestyle Factors and Risk of Hepatocellular Carcinoma: Findings from a Prospective Cohort Study. Cancer Epidemiol Biomarkers Prev. 2021;30:380-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Fernández T, Viñuela M, Vidal C, Barrera F. Lifestyle changes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2022;17:e0263931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 78. | Peng X, Li J, Zhao H, Lai J, Lin J, Tang S. Lifestyle as well as metabolic syndrome and non-alcoholic fatty liver disease: an umbrella review of evidence from observational studies and randomized controlled trials. BMC Endocr Disord. 2022;22:95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Buzzetti E, Linden A, Best LM, Madden AM, Roberts D, Chase TJG, Freeman SC, Cooper NJ, Sutton AJ, Fritche D, Milne EJ, Wright K, Pavlov CS, Davidson BR, Tsochatzis E, Gurusamy KS. Lifestyle modifications for nonalcohol-related fatty liver disease: a network meta-analysis. Cochrane Database Syst Rev. 2021;6:CD013156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Lange NF, Radu P, Dufour JF. Prevention of NAFLD-associated HCC: Role of lifestyle and chemoprevention. J Hepatol. 2021;75:1217-1227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Hydes TJ, Ravi S, Loomba R, E Gray M. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin Mol Hepatol. 2020;26:383-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 82. | Yang WS, Zeng XF, Liu ZN, Zhao QH, Tan YT, Gao J, Li HL, Xiang YB. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124:330-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 83. | George ES, Sood S, Broughton A, Cogan G, Hickey M, Chan WS, Sudan S, Nicoll AJ. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 84. | Turati F, Trichopoulos D, Polesel J, Bravi F, Rossi M, Talamini R, Franceschi S, Montella M, Trichopoulou A, La Vecchia C, Lagiou P. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 85. | Bogumil D, Park SY, Le Marchand L, Haiman CA, Wilkens LR, Boushey CJ, Setiawan VW. High-Quality Diets Are Associated With Reduced Risk of Hepatocellular Carcinoma and Chronic Liver Disease: The Multiethnic Cohort. Hepatol Commun. 2019;3:437-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Ma Y, Yang W, Simon TG, Smith-Warner SA, Fung TT, Sui J, Chong D, VoPham T, Meyerhardt JA, Wen D, Giovannucci EL, Chan AT, Zhang X. Dietary Patterns and Risk of Hepatocellular Carcinoma Among U.S. Men and Women. Hepatology. 2019;70:577-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 87. | Li WQ, Park Y, McGlynn KA, Hollenbeck AR, Taylor PR, Goldstein AM, Freedman ND. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. 2014;60:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 88. | Chen PY, Fang AP, Wang XY, Lan QY, Liao GC, Liu ZY, Zhang DM, Zhang YY, Chen YM, Zhu HL. Adherence to the Chinese or American Dietary Guidelines is Associated with a Lower Risk of Primary Liver Cancer in China: A Case-Control Study. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Lan QY, Liao GC, Zhou RF, Chen PY, Wang XY, Chen MS, Chen YM, Zhu HL. Dietary patterns and primary liver cancer in Chinese adults: a case-control study. Oncotarget. 2018;9:27872-27881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Luo Y, Zhang YJ, Zhang DM, Yishake D, Liu ZY, Chen MS, Wang F, Zhou ZG, Long JA, Zhong RH, Chen S, Lu XT, Li SY, He TT, Luo Y, Fang AP, Zhu HL. Association between dietary patterns and prognosis of hepatocellular carcinoma in the Guangdong liver cancer cohort study. Hepatol Res. 2020;50:1164-1175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Zhong GC, Wang K, Peng Y, Shivappa N, Hébert JR, Wu YQ, Gong JP. Dietary inflammatory index and incidence of and death from primary liver cancer: A prospective study of 103,902 American adults. Int J Cancer. 2020;147:1050-1058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 92. | Yang W, Sui J, Zhao L, Ma Y, Tabung FK, Simon TG, Lee DH, Zeng X, Nguyen LH, Meyerhardt JA, Chan AT, Giovannucci EL, Zhang X. Association of Inflammatory and Insulinemic Potential of Diet and Lifestyle with Risk of Hepatocellular Carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30:789-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Kwon OW, Jun DW, Lee SM, Lee KN, Lee HL, Lee OY, Yoon BC, Choi HS. Carbohydrate but not fat is associated with elevated aminotransferases. Aliment Pharmacol Ther. 2012;35:1064-1072. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Cortez-Pinto H, Jesus L, Barros H, Lopes C, Moura MC, Camilo ME. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 95. | Papandreou D, Karabouta Z, Pantoleon A, Rousso I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite. 2012;59:939-944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Wehmeyer MH, Zyriax BC, Jagemann B, Roth E, Windler E, Schulze Zur Wiesch J, Lohse AW, Kluwe J. Nonalcoholic fatty liver disease is associated with excessive calorie intake rather than a distinctive dietary pattern. Medicine (Baltimore). 2016;95:e3887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 97. | Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 98. | Haghighatdoost F, Salehi-Abargouei A, Surkan PJ, Azadbakht L. The effects of low carbohydrate diets on liver function tests in nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. J Res Med Sci. 2016;21:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Fedirko V, Lukanova A, Bamia C, Trichopolou A, Trepo E, Nöthlings U, Schlesinger S, Aleksandrova K, Boffetta P, Tjønneland A, Johnsen NF, Overvad K, Fagherazzi G, Racine A, Boutron-Ruault MC, Grote V, Kaaks R, Boeing H, Naska A, Adarakis G, Valanou E, Palli D, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HBA, Siersema PD, Peeters PH, Weiderpass E, Skeie G, Engeset D, Quirós JR, Zamora-Ros R, Sánchez MJ, Amiano P, Huerta JM, Barricarte A, Johansen D, Lindkvist B, Sund M, Werner M, Crowe F, Khaw KT, Ferrari P, Romieu I, Chuang SC, Riboli E, Jenab M. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol. 2013;24:543-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 100. | Vogtmann E, Li HL, Shu XO, Chow WH, Ji BT, Cai H, Gao J, Zhang W, Gao YT, Zheng W, Xiang YB. Dietary glycemic load, glycemic index, and carbohydrates on the risk of primary liver cancer among Chinese women and men. Ann Oncol. 2013;24:238-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452-1455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 102. | Ma J, Fox CS, Jacques PF, Speliotes EK, Hoffmann U, Smith CE, Saltzman E, McKeown NM. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J Hepatol. 2015;63:462-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 103. | Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol. 2008;22:811-816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 104. | Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95:283-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 409] [Cited by in F6Publishing: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 105. | Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 627] [Cited by in F6Publishing: 569] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 106. | Li Y, Guo L, He K, Huang C, Tang S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: a systematic review and dose-response meta-analysis of observational studies. J Cancer. 2021;12:3077-3088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 107. | Stepien M, Duarte-Salles T, Fedirko V, Trichopoulou A, Lagiou P, Bamia C, Overvad K, Tjønneland A, Hansen L, Boutron-Ruault MC, Fagherazzi G, Severi G, Kühn T, Kaaks R, Aleksandrova K, Boeing H, Klinaki E, Palli D, Grioni S, Panico S, Tumino R, Naccarati A, Bueno-de-Mesquita HB, Peeters PH, Skeie G, Weiderpass E, Parr CL, Quirós JR, Buckland G, Molina-Montes E, Amiano P, Chirlaque MD, Ardanaz E, Sonestedt E, Ericson U, Wennberg M, Nilsson LM, Khaw KT, Wareham N, Bradbury KE, Ward HA, Romieu I, Jenab M. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur J Nutr. 2016;55:7-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Rizk M, Guilloteau A, Mouillot T, Thiefin G, Bronowicki JP, Richou C, Doffoel M, Diab Assaf M, Hillon P, Cottet V. Dietary components modulate the risk of hepatocellular carcinoma in cirrhotic patients. Nutr Res. 2019;61:82-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Yang W, Ma Y, Liu Y, Smith-Warner SA, Simon TG, Chong DQ, Qi Q, Meyerhardt JA, Giovannucci EL, Chan AT, Zhang X. Association of Intake of Whole Grains and Dietary Fiber With Risk of Hepatocellular Carcinoma in US Adults. JAMA Oncol. 2019;5:879-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 110. | Zolfaghari H, Askari G, Siassi F, Feizi A, Sotoudeh G. Intake of Nutrients, Fiber, and Sugar in Patients with Nonalcoholic Fatty Liver Disease in Comparison to Healthy Individuals. Int J Prev Med. 2016;7:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Alferink LJ, Kiefte-de Jong JC, Erler NS, Veldt BJ, Schoufour JD, de Knegt RJ, Ikram MA, Metselaar HJ, Janssen H, Franco OH, Darwish Murad S. Association of dietary macronutrient composition and non-alcoholic fatty liver disease in an ageing population: the Rotterdam Study. Gut. 2019;68:1088-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 112. | Rietman A, Sluik D, Feskens EJM, Kok FJ, Mensink M. Associations between dietary factors and markers of NAFLD in a general Dutch adult population. Eur J Clin Nutr. 2018;72:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Markova M, Pivovarova O, Hornemann S, Sucher S, Frahnow T, Wegner K, Machann J, Petzke KJ, Hierholzer J, Lichtinghagen R, Herder C, Carstensen-Kirberg M, Roden M, Rudovich N, Klaus S, Thomann R, Schneeweiss R, Rohn S, Pfeiffer AF. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology. 2017;152:571-585.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 114. | Cha JH, Bae SH, Kim HL, Park NR, Choi ES, Jung ES, Choi JY, Yoon SK. Branched-chain amino acids ameliorate fibrosis and suppress tumor growth in a rat model of hepatocellular carcinoma with liver cirrhosis. PLoS One. 2013;8:e77899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Takegoshi K, Honda M, Okada H, Takabatake R, Matsuzawa-Nagata N, Campbell JS, Nishikawa M, Shimakami T, Shirasaki T, Sakai Y, Yamashita T, Takamura T, Tanaka T, Kaneko S. Branched-chain amino acids prevent hepatic fibrosis and development of hepatocellular carcinoma in a non-alcoholic steatohepatitis mouse model. Oncotarget. 2017;8:18191-18205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Ichikawa K, Okabayashi T, Maeda H, Namikawa T, Iiyama T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K. Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today. 2013;43:720-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Yoshiji H, Noguchi R, Namisaki T, Moriya K, Kitade M, Aihara Y, Douhara A, Yamao J, Fujimoto M, Toyohara M, Mitoro A, Sawai M, Yoshida M, Morioka C, Uejima M, Uemura M, Fukui H. Branched-chain amino acids suppress the cumulative recurrence of hepatocellular carcinoma under conditions of insulin-resistance. Oncol Rep. 2013;30:545-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 118. | Tada T, Kumada T, Toyoda H, Yasuda S, Koyabu T, Nakashima M. Impact of Branched-Chain Amino Acid Granule Therapy in Patients with Hepatocellular Carcinoma Who Have Normal Albumin Levels and Low Branched-Chain Amino Acid to Tyrosine Ratios. Nutr Cancer. 2019;71:1132-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Hachiya H, Aoki T, Iso Y, Shimizu T, Tago K, Park KH, Sakuraoka Y, Shiraki T, Mori S, Kubota K. Effects of branched-chain amino acids on postoperative tumor recurrence in patients undergoing curative resection for hepatocellular carcinoma: A randomized clinical trial. J Hepatobiliary Pancreat Sci. 2020;27:819-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Zelber-Sagi S, Noureddin M, Shibolet O. Lifestyle and Hepatocellular Carcinoma What Is the Evidence and Prevention Recommendations. Cancers (Basel). 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Kuper H, Tzonou A, Lagiou P, Mucci LA, Trichopoulos D, Stuver SO, Trichopoulou A. Diet and hepatocellular carcinoma: a case-control study in Greece. Nutr Cancer. 2000;38:6-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Polesel J, Talamini R, Montella M, Maso LD, Crovatto M, Parpinel M, Izzo F, Tommasi LG, Serraino D, La Vecchia C, Franceschi S. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur J Cancer. 2007;43:2381-2387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 123. | Yang W, Sui J, Ma Y, Simon TG, Petrick JL, Lai M, McGlynn KA, Campbell PT, Giovannucci EL, Chan AT, Zhang X. High Dietary Intake of Vegetable or Polyunsaturated Fats Is Associated With Reduced Risk of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2020;18:2775-2783.e11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 124. | Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909-916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 508] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 125. | Ji XW, Wang J, Shen QM, Li ZY, Jiang YF, Liu DK, Tan YT, Li HL, Xiang YB. Dietary fat intake and liver cancer incidence: A population-based cohort study in Chinese men. Int J Cancer. 2021;148:2982-2996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Duarte-Salles T, Fedirko V, Stepien M, Aleksandrova K, Bamia C, Lagiou P, Laursen AS, Hansen L, Overvad K, Tjønneland A, Boutron-Ruault MC, Fagherazzi G, His M, Boeing H, Katzke V, Kühn T, Trichopoulou A, Valanou E, Kritikou M, Masala G, Panico S, Sieri S, Ricceri F, Tumino R, Bueno-de-Mesquita HB, Peeters PH, Hjartåker A, Skeie G, Weiderpass E, Ardanaz E, Bonet C, Chirlaque MD, Dorronsoro M, Quirós JR, Johansson I, Ohlsson B, Sjöberg K, Wennberg M, Khaw KT, Travis RC, Wareham N, Ferrari P, Freisling H, Romieu I, Cross AJ, Gunter M, Lu Y, Jenab M. Dietary fat, fat subtypes and hepatocellular carcinoma in a large European cohort. Int J Cancer. 2015;137:2715-2728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Moussa I, Day RS, Li R, Kaseb A, Jalal PK, Daniel-MacDougall C, Hatia RI, Abdelhakeem A, Rashid A, Chun YS, Li D, Hassan MM. Association of dietary fat intake and hepatocellular carcinoma among US adults. Cancer Med. 2021;10:7308-7319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Zhao L, Deng C, Lin Z, Giovannucci E, Zhang X. Dietary Fats, Serum Cholesterol and Liver Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 129. | Jump DB, Depner CM, Tripathy S, Lytle KA. Potential for dietary ω-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer. Adv Nutr. 2015;6:694-702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 130. | Parker HM, Johnson NA, Burdon CA, Cohn JS, O'Connor HT, George J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 131. | Lu W, Li S, Li J, Wang J, Zhang R, Zhou Y, Yin Q, Zheng Y, Wang F, Xia Y, Chen K, Liu T, Lu J, Guo C. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol Res Pract. 2016;2016:1459790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 132. | Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, Shah NL, Al-Osaimi AM, Pramoonjago P, Jayakumar S, Binder LP, Simmons-Egolf WD, Burks SG, Bao Y, Taylor AG, Rodriguez J, Caldwell SH. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol. 2015;62:190-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 133. | Koh WP, Dan YY, Goh GB, Jin A, Wang R, Yuan JM. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36:893-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 134. | Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 135. | Shah SC, Zhu X, Dai Q, Peek RM, Shrubsole MJ. Magnesium intake is associated with a reduced risk of incident liver cancer, based on an analysis of the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study prospective cohort. Am J Clin Nutr. 2021;113:630-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Zhong GC, Peng Y, Wang K, Wan L, Wu YQ, Hao FB, Hu JJ, Gu HT. Magnesium intake and primary liver cancer incidence and mortality in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Int J Cancer. 2020;147:1577-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 137. | Ma X, Yang Y, Li HL, Zheng W, Gao J, Zhang W, Yang G, Shu XO, Xiang YB. Dietary trace element intake and liver cancer risk: Results from two population-based cohorts in China. Int J Cancer. 2017;140:1050-1059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Zhang W, Shu XO, Li H, Yang G, Cai H, Ji BT, Gao J, Gao YT, Zheng W, Xiang YB. Vitamin intake and liver cancer risk: a report from two cohort studies in China. J Natl Cancer Inst. 2012;104:1173-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 139. | Lan QY, Zhang YJ, Liao GC, Zhou RF, Zhou ZG, Chen YM, Zhu HL. The Association between Dietary Vitamin A and Carotenes and the Risk of Primary Liver Cancer: A Case-Control Study. Nutrients. 2016;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 140. | Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36:937-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 141. | Yu J, Liu Z, Liang D, Li J, Ma S, Wang G, Chen W. Meat Intake and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Nutr Cancer. 2022;74:3340-3350. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 142. | Luo J, Yang Y, Liu J, Lu K, Tang Z, Liu P, Liu L, Zhu Y. Systematic review with meta-analysis: meat consumption and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2014;39:913-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 143. | Duarte-Salles T, Fedirko V, Stepien M, Trichopoulou A, Bamia C, Lagiou P, Lukanova A, Trepo E, Overvad K, Tjønneland A, Halkjaer J, Boutron-Ruault MC, Racine A, Cadeau C, Kühn T, Aleksandrova K, Trichopoulos D, Tsiotas K, Boffetta P, Palli D, Pala V, Tumino R, Sacerdote C, Panico S, Bueno-de-Mesquita HB, Dik VK, Peeters PH, Weiderpass E, Torhild Gram I, Hjartåker A, Ramón Quirós J, Fonseca-Nunes A, Molina-Montes E, Dorronsoro M, Navarro Sanchez C, Barricarte A, Lindkvist B, Sonestedt E, Johansson I, Wennberg M, Khaw KT, Wareham N, Travis RC, Romieu I, Riboli E, Jenab M. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2014;135:1662-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 144. | Yang W, Sui J, Ma Y, Simon TG, Chong D, Meyerhardt JA, Willett WC, Giovannucci EL, Chan AT, Zhang X. A prospective study of dairy product intake and the risk of hepatocellular carcinoma in U.S. men and women. Int J Cancer. 2020;146:1241-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 145. | Talamini R, Polesel J, Montella M, Dal Maso L, Crispo A, Tommasi LG, Izzo F, Crovatto M, La Vecchia C, Franceschi S. Food groups and risk of hepatocellular carcinoma: A multicenter case-control study in Italy. Int J Cancer. 2006;119:2916-2921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 146. | Yang Y, Zhang D, Feng N, Chen G, Liu J, Zhu Y. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 147. | Guo XF, Shao XF, Li JM, Li S, Li KL, Li D. Fruit and vegetable intake and liver cancer risk: a meta-analysis of prospective cohort studies. Food Funct. 2019;10:4478-4485. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 148. | Mandlik DS, Mandlik SK. Herbal and Natural Dietary Products: Upcoming Therapeutic Approach for Prevention and Treatment of Hepatocellular Carcinoma. Nutr Cancer. 2021;73:2130-2154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 149. | Nagashimada M, Honda M. Effect of Microbiome on Non-Alcoholic Fatty Liver Disease and the Role of Probiotics, Prebiotics, and Biogenics. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 150. | Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 232] [Cited by in F6Publishing: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 151. | Hodges JK, Sasaki GY, Bruno RS. Anti-inflammatory activities of green tea catechins along the gut-liver axis in nonalcoholic fatty liver disease: lessons learned from preclinical and human studies. J Nutr Biochem. 2020;85:108478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Shimizu M, Shirakami Y, Sakai H, Kubota M, Kochi T, Ideta T, Miyazaki T, Moriwaki H. Chemopreventive potential of green tea catechins in hepatocellular carcinoma. Int J Mol Sci. 2015;16:6124-6139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 153. | Bamia C, Lagiou P, Jenab M, Trichopoulou A, Fedirko V, Aleksandrova K, Pischon T, Overvad K, Olsen A, Tjønneland A, Boutron-Ruault MC, Fagherazzi G, Racine A, Kuhn T, Boeing H, Floegel A, Benetou V, Palli D, Grioni S, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, Dik VK, Bhoo-Pathy N, Uiterwaal CS, Weiderpass E, Lund E, Quirós JR, Zamora-Ros R, Molina-Montes E, Chirlaque MD, Ardanaz E, Dorronsoro M, Lindkvist B, Wallström P, Nilsson LM, Sund M, Khaw KT, Wareham N, Bradbury KE, Travis RC, Ferrari P, Duarte-Salles T, Stepien M, Gunter M, Murphy N, Riboli E, Trichopoulos D. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int J Cancer. 2015;136:1899-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 154. | Mahmoodi M, Hosseini R, Kazemi A, Ofori-Asenso R, Mazidi M, Mazloomi SM. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Phytother Res. 2020;34:1587-1598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 155. | Hrncir T, Hrncirova L, Kverka M, Hromadka R, Machova V, Trckova E, Kostovcikova K, Kralickova P, Krejsek J, Tlaskalova-Hogenova H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms. 2021;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 156. | Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Am J Clin Nutr. 2019;110:139-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 157. | Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017;26:368-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 80] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 158. | Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7:e013739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 159. | Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 160. | Di Maso M, Boffetta P, Negri E, La Vecchia C, Bravi F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: A Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv Nutr. 2021;12:1160-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 161. | Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1413-1421.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 162. | Bhurwal A, Rattan P, Yoshitake S, Pioppo L, Reja D, Dellatore P, Rustgi V. Inverse Association of Coffee with Liver Cancer Development: An Updated Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2020;29:421-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 163. | Larsson SC, Wolk A. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 164. | Yu C, Cao Q, Chen P, Yang S, Deng M, Wang Y, Li L. An updated dose-response meta-analysis of coffee consumption and liver cancer risk. Sci Rep. 2016;6:37488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 165. | Simon TG, Chan AT. Lifestyle and Environmental Approaches for the Primary Prevention of Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:549-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 166. | Petroni ML, Brodosi L, Marchignoli F, Musio A, Marchesini G. Moderate Alcohol Intake in Non-Alcoholic Fatty Liver Disease: To Drink or Not to Drink? Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 167. | Turati F, Galeone C, Rota M, Pelucchi C, Negri E, Bagnardi V, Corrao G, Boffetta P, La Vecchia C. Alcohol and liver cancer: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2014;25:1526-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 168. | Åberg F, Puukka P, Salomaa V, Männistö S, Lundqvist A, Valsta L, Perola M, Färkkilä M, Jula A. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology. 2020;71:835-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 169. | Heckley GA, Jarl J, Asamoah BO, G-Gerdtham U. How the risk of liver cancer changes after alcohol cessation: a review and meta-analysis of the current literature. BMC Cancer. 2011;11:446. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 170. | Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J Gastroenterol. 2016;22:6318-6327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 111] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 171. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, Chan FK, Sung JJ, Woo J, Chan HL. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 172. | van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018;18:89-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 173. | Guarino M, Kumar P, Felser A, Terracciano LM, Guixé-Muntet S, Humar B, Foti M, Nuoffer JM, St-Pierre MV, Dufour JF. Exercise Attenuates the Transition from Fatty Liver to Steatohepatitis and Reduces Tumor Formation in Mice. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 174. | Saran U, Guarino M, Rodríguez S, Simillion C, Montani M, Foti M, Humar B, St-Pierre MV, Dufour JF. Anti-tumoral effects of exercise on hepatocellular carcinoma growth. Hepatol Commun. 2018;2:607-620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 175. | Baumeister SE, Leitzmann MF, Linseisen J, Schlesinger S. Physical Activity and the Risk of Liver Cancer: A Systematic Review and Meta-Analysis of Prospective Studies and a Bias Analysis. J Natl Cancer Inst. 2019;111:1142-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 176. | Baumeister SE, Schlesinger S, Aleksandrova K, Jochem C, Jenab M, Gunter MJ, Overvad K, Tjønneland A, Boutron-Ruault MC, Carbonnel F, Fournier A, Kühn T, Kaaks R, Pischon T, Boeing H, Trichopoulou A, Bamia C, La Vecchia C, Masala G, Panico S, Fasanelli F, Tumino R, Grioni S, Bueno de Mesquita B, Vermeulen R, May AM, Borch KB, Oyeyemi SO, Ardanaz E, Rodríguez-Barranco M, Dolores Chirlaque López M, Felez-Nobrega M, Sonestedt E, Ohlsson B, Hemmingsson O, Werner M, Perez-Cornago A, Ferrari P, Stepien M, Freisling H, Tsilidis KK, Ward H, Riboli E, Weiderpass E, Leitzmann MF. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J Hepatol. 2019;70:885-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 177. | Petrick JL, Campbell PT, Koshiol J, Thistle JE, Andreotti G, Beane-Freeman LE, Buring JE, Chan AT, Chong DQ, Doody MM, Gapstur SM, Gaziano JM, Giovannucci E, Graubard BI, Lee IM, Liao LM, Linet MS, Palmer JR, Poynter JN, Purdue MP, Robien K, Rosenberg L, Schairer C, Sesso HD, Sinha R, Stampfer MJ, Stefanick M, Wactawski-Wende J, Zhang X, Zeleniuch-Jacquotte A, Freedman ND, McGlynn KA. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer. 2018;118:1005-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 178. | Chiang CH, Lu CW, Han HC, Hung SH, Lee YH, Yang KC, Huang KC. The Relationship of Diabetes and Smoking Status to Hepatocellular Carcinoma Mortality. Medicine (Baltimore). 2016;95:e2699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 179. | Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA; Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in Histologic Features and Diagnosis Associated With Improvement in Fibrosis in Nonalcoholic Steatohepatitis: Results From the Nonalcoholic Steatohepatitis Clinical Research Network Treatment Trials. Hepatology. 2019;70:522-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 180. | Li Q, Xu H, Sui C, Zhang H. Impact of metformin use on risk and mortality of hepatocellular carcinoma in diabetes mellitus. Clin Res Hepatol Gastroenterol. 2022;46:101781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 181. | Zhou YY, Zhu GQ, Liu T, Zheng JN, Cheng Z, Zou TT, Braddock M, Fu SW, Zheng MH. Systematic Review with Network Meta-Analysis: Antidiabetic Medication and Risk of Hepatocellular Carcinoma. Sci Rep. 2016;6:33743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 182. | Zhang H, Gao C, Fang L, Zhao HC, Yao SK. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol. 2013;48:78-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 183. | Choi J, Roberts LR. Statins and metformin for chemoprevention of hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2016;8:48-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 184. | de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 185. | Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern Med. 2017;177:633-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 186. | Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315:187-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 187. | van der Veen JN, Lingrell S, Gao X, Quiroga AD, Takawale A, Armstrong EA, Yager JY, Kassiri Z, Lehner R, Vance DE, Jacobs RL. Pioglitazone attenuates hepatic inflammation and fibrosis in phosphatidylethanolamine N-methyltransferase-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2016;310:G526-G538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 188. | Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924-1940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 329] [Cited by in F6Publishing: 334] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 189. | Galli A, Ceni E, Mello T, Polvani S, Tarocchi M, Buccoliero F, Lisi F, Cioni L, Ottanelli B, Foresta V, Mastrobuoni G, Moneti G, Pieraccini G, Surrenti C, Milani S. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor gamma-independent regulation of nucleophosmin. Hepatology. 2010;52:493-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 190. | Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 636] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 191. | Li S, Ghoshal S, Sojoodi M, Arora G, Masia R, Erstad DJ, Lanuti M, Hoshida Y, Baumert TF, Tanabe KK, Fuchs BC. Pioglitazone Reduces Hepatocellular Carcinoma Development in Two Rodent Models of Cirrhosis. J Gastrointest Surg. 2019;23:101-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 192. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1100] [Cited by in F6Publishing: 1185] [Article Influence: 148.1] [Reference Citation Analysis (1)] |
| 193. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 745] [Article Influence: 248.3] [Reference Citation Analysis (0)] |
| 194. | Lu X, Xu C, Dong J, Zuo S, Zhang H, Jiang C, Wu J, Wei J. Liraglutide activates nature killer cell-mediated antitumor responses by inhibiting IL-6/STAT3 signaling in hepatocellular carcinoma. Transl Oncol. 2021;14:100872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 195. | Krause GC, Lima KG, Dias HB, da Silva EFG, Haute GV, Basso BS, Gassen RB, Marczak ES, Nunes RSB, de Oliveira JR. Liraglutide, a glucagon-like peptide-1 analog, induce autophagy and senescence in HepG2 cells. Eur J Pharmacol. 2017;809:32-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 196. | Li Q, Xue AY, Li ZL, Yin Z. Liraglutide promotes apoptosis of HepG2 cells by activating JNK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:3520-3526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 197. | Pastori D, Pani A, Di Rocco A, Menichelli D, Gazzaniga G, Farcomeni A, D'Erasmo L, Angelico F, Del Ben M, Baratta F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br J Clin Pharmacol. 2022;88:441-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 198. | Fatima K, Moeed A, Waqar E, Atif AR, Kamran A, Rizvi H, Suri NF, Haider H, Shuja SH, Khalid M, Minhas AMK. Efficacy of statins in treatment and development of non-alcoholic fatty liver disease and steatohepatitis: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2022;46:101816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Zhou YY, Zhu GQ, Wang Y, Zheng JN, Ruan LY, Cheng Z, Hu B, Fu SW, Zheng MH. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016;7:21753-21762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 200. | Wang J, Li X. Impact of statin use on the risk and prognosis of hepatocellular carcinoma: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1603-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 201. | Wong YJ, Qiu TY, Ng GK, Zheng Q, Teo EK. Efficacy and Safety of Statin for Hepatocellular Carcinoma Prevention Among Chronic Liver Disease Patients: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2021;55:615-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 202. | Chang Y, Liu Q, Zhou Z, Ding Y, Yang M, Xu W, Chen K, Zhang Q, Wang Z, Li H. Can Statin Treatment Reduce the Risk of Hepatocellular Carcinoma? Technol Cancer Res Treat. 2020;19:1533033820934881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 203. | Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144:323-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 204. | Wang Y, Wang W, Wang M, Shi J, Jia X, Dang S. A Meta-Analysis of Statin Use and Risk of Hepatocellular Carcinoma. Can J Gastroenterol Hepatol. 2022;2022:5389044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 205. | Pradelli D, Soranna D, Scotti L, Zambon A, Catapano A, Mancia G, La Vecchia C, Corrao G. Statins and primary liver cancer: a meta-analysis of observational studies. Eur J Cancer Prev. 2013;22:229-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 206. | Gu Y, Yang X, Liang H, Li D. Comprehensive evaluation of effects and safety of statin on the progression of liver cirrhosis: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 207. | Facciorusso A, Abd El Aziz MA, Singh S, Pusceddu S, Milione M, Giacomelli L, Sacco R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 208. | Li X, Liu L, Hu Y. Statin use and the prognosis of patients with hepatocellular carcinoma: a meta-analysis. Biosci Rep. 2020;40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 209. | Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65:1183-1195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 210. | Moctezuma-Velázquez C, Abraldes JG, Montano-Loza AJ. The Use of Statins in Patients With Chronic Liver Disease and Cirrhosis. Curr Treat Options Gastroenterol. 2018;16:226-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 211. | Li S, Saviano A, Erstad DJ, Hoshida Y, Fuchs BC, Baumert T, Tanabe KK. Risk Factors, Pathogenesis, and Strategies for Hepatocellular Carcinoma Prevention: Emphasis on Secondary Prevention and Its Translational Challenges. J Clin Med. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 212. | Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012-2024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 350] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 213. | Taub R. A Phase 3 Study to Evaluate the Efficacy and Safety of MGL-3196 (Resmetirom) in Patients With NASH and Fibrosis (MAESTRO-NASH). [accessed 2022 Sep 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03900429 ClinicalTrials.gov Identifier: NCT03900429. [Cited in This Article: ] |
| 214. | Memel ZN, Arvind A, Moninuola O, Philpotts L, Chung RT, Corey KE, Simon TG. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Hepatol Commun. 2021;5:133-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 215. | Wang S, Yu Y, Ryan PM, Dang M, Clark C, Kontogiannis V, Rahmani J, Varkaneh HK, Salehisahlabadi A, Day AS, Zhang Y. Association of aspirin therapy with risk of hepatocellular carcinoma: A systematic review and dose-response analysis of cohort studies with 2.5 million participants. Pharmacol Res. 2020;151:104585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 216. | Tan RZH, Lockart I, Abdel Shaheed C, Danta M. Systematic review with meta-analysis: The effects of non-steroidal anti-inflammatory drugs and anti-platelet therapy on the incidence and recurrence of hepatocellular carcinoma. Aliment Pharmacol Ther. 2021;54:356-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 217. | Wang Y, Wang M, Liu C, Wang W, Shi J, Dang S. Aspirin Use and the Risk of Hepatocellular Carcinoma: A Meta-analysis. J Clin Gastroenterol. 2022;56:e293-e302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 218. | Liu Y, Ren T, Xu X, Jin J. Association of aspirin and nonaspirin NSAIDs therapy with the incidence risk of hepatocellular carcinoma: a systematic review and meta-analysis on cohort studies. Eur J Cancer Prev. 2022;31:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 219. | Yi M, Feng X, Peng W, Teng F, Tang Y, Chen Z. Aspirin for the prevention of hepatocellular carcinoma: an updated meta-analysis with particular focus on patients with chronic liver disease. Eur J Clin Pharmacol. 2022;78:647-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 220. | Li X, Yu Y, Liu L. Influence of aspirin use on clinical outcomes of patients with hepatocellular carcinoma: a meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45:101545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 221. | Mehta SR. Advances in the treatment of nonalcoholic fatty liver disease. Ther Adv Endocrinol Metab. 2010;1:101-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 222. | Vadarlis A, Antza C, Bakaloudi DR, Doundoulakis I, Kalopitas G, Samara M, Dardavessis T, Maris T, Chourdakis M. Systematic review with meta-analysis: The effect of vitamin E supplementation in adult patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2021;36:311-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 223. | Bril F, Biernacki DM, Kalavalapalli S, Lomonaco R, Subbarayan SK, Lai J, Tio F, Suman A, Orsak BK, Hecht J, Cusi K. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care. 2019;42:1481-1488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 224. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 530] [Cited by in F6Publishing: 813] [Article Influence: 116.1] [Reference Citation Analysis (0)] |
| 225. | Athuluri-Divakar SK, Hoshida Y. Generic chemoprevention of hepatocellular carcinoma. Ann N Y Acad Sci. 2019;1440:23-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 226. | Shiff S. Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment (REGENERATE). [accessed 2022 Sep 17]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02548351 Accessed on 17.09.2022. ClinicalTrials.gov Identifier: NCT02548351. [Cited in This Article: ] |
| 227. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 724] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 228. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1569] [Cited by in F6Publishing: 1613] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 229. | Pockros PJ, Fuchs M, Freilich B, Schiff E, Kohli A, Lawitz EJ, Hellstern PA, Owens-Grillo J, Van Biene C, Shringarpure R, MacConell L, Shapiro D, Cohen DE. CONTROL: A randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082-2093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 230. | Attia YM, Tawfiq RA, Gibriel AA, Ali AA, Kassem DH, Hammam OA, Elmazar MM. Activation of FXR modulates SOCS3/Jak2/STAT3 signaling axis in a NASH-dependent hepatocellular carcinoma animal model. Biochem Pharmacol. 2021;186:114497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 231. | Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 232. | Kedarisetty CK, Bhardwaj A, Kumar G, Rastogi A, Bihari C, Kumar M, Sarin SK. Efficacy of combining pentoxiphylline and vitamin E versus vitamin E alone in non-alcoholic steatohepatitis- A randomized pilot study. Indian J Gastroenterol. 2021;40:41-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 233. | Du J, Ma YY, Yu CH, Li YM. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 234. | Singh S, Khera R, Allen AM, Murad MH, Loomba R. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology. 2015;62:1417-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 235. | Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, Chaiyakunapruk N. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore). 2016;95:e4529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 236. | Zeng T, Zhang CL, Zhao XL, Xie KQ. Pentoxifylline for the treatment of nonalcoholic fatty liver disease: a meta-analysis of randomized double-blind, placebo-controlled studies. Eur J Gastroenterol Hepatol. 2014;26:646-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 237. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 719] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 238. | Addy C. Phase 3 Study to Evaluate the Efficacy and Safety of Elafibranor Versus Placebo in Patients With Nonalcoholic Steatohepatitis (NASH) (RESOLVE-IT). [accessed 2022 Sep 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02704403 ClinicalTrials.gov Identifier: NCT02704403. [Cited in This Article: ] |
| 239. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 468] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 240. | Rodriguez G. AURORA: A Study for the Efficacy and Safety of Cenicriviroc (CVC) for the Treatment of Liver Fibrosis in Adults With Nonalcoholic Steatohepatitis (NASH) (AURORA). [accessed 2022 Sep 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03028740 ClinicalTrials.gov Identifier: NCT03028740. [Cited in This Article: ] |
| 241. | Shan L, Wang F, Zhai D, Meng X, Liu J, Lv X. New Drugs for Hepatic Fibrosis. Front Pharmacol. 2022;13:874408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |