Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2798
Peer-review started: December 13, 2022
First decision: February 23, 2023
Revised: March 8, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: May 14, 2023
Hepatic fibrosis is a serious condition, and the development of hepatic fibrosis can lead to a series of complications. However, the pathogenesis of hepatic fibrosis remains unclear, and effective therapy options are still lacking. Our group identified hepatitis C virus nonstructural protein 3-transactivated protein 1 (NS3TP1) by suppressive subtractive hybridization and bioinformatics analysis, but its role in diseases including hepatic fibrosis remains undefined. Therefore, additional studies on the function of NS3TP1 in hepatic fibrosis are urgently needed to provide new targets for treatment.
To elucidate the mechanism of NS3TP1 in hepatic fibrosis and the regulatory effects of calcitriol on NS3TP1.
Twenty-four male C57BL/6 mice were randomized and separated into three groups, comprising the normal, fibrosis, and calcitriol treatment groups, and liver fibrosis was modeled by carbon tetrachloride (CCl4). To evaluate the level of hepatic fibrosis in every group, serological and pathological examinations of the liver were conducted. TGF-β1 was administered to boost the in vitro cultivation of LX-2 cells. NS3TP1, α-smooth muscle actin (α-SMA), collagen I, and collagen III in every group were examined using a Western blot and real-time quantitative polymerase chain reaction. The activity of the transforming growth factor beta 1 (TGFβ1)/Smad3 and NF-κB signaling pathways in each group of cells transfected with pcDNA-NS3TP1 or siRNA-NS3TP1 was detected. The statistical analysis of the data was performed using the Student’s t test.
NS3TP1 promoted the activation, proliferation, and differentiation of hepatic stellate cells (HSCs) and enhanced hepatic fibrosis via the TGFβ1/Smad3 and NF-κB signaling pathways, as evidenced by the presence of α-SMA, collagen I, collagen III, p-smad3, and p-p65 in LX-2 cells, which were upregulated after NS3TP1 overexpression and downregulated after NS3TP1 interference. The proliferation of HSCs was lowered after NS3TP1 interference and elevated after NS3TP1 overexpression, as shown by the luciferase assay. NS3TP1 inhibited the apoptosis of HSCs. Moreover, both Smad3 and p65 could bind to NS3TP1, and p65 increased the promoter activity of NS3TP1, while NS3TP1 increased the promoter activity of TGFβ1 receptor I, as indicated by coimmunoprecipitation and luciferase assay results. Both in vivo and in vitro, treatment with calcitriol dramatically reduced the expression of NS3TP1. Calcitriol therapy-controlled HSCs activation, proliferation, and differentiation and substantially suppressed CCl4-induced hepatic fibrosis in mice. Furthermore, calcitriol modulated the activities of the above signaling pathways via downregulation of NS3TP1.
Our results suggest that calcitriol may be employed as an adjuvant therapy for hepatic fibrosis and that NS3TP1 is a unique, prospective therapeutic target in hepatic fibrosis.
Core Tip: We proved that hepatitis C virus nonstructural protein 3-transactivated protein 1 (NS3TP1) promoted hepatic fibrosis via the transforming growth factor beta 1/Smad3 and NF-κB signaling pathways. Calcitriol attenuates liver fibrosis through NS3TP1-mediated above both signaling pathways. These novel findings profoundly expand our knowledge about the mechanisms underlying the role and function of NS3TP1 in hepatic fibrosis. The relationship between NS3TP1 and liver fibrosis was discussed for the first time and provided a foundation for research related to liver fibrosis by targeting NS3TP1. We first showed that calcitriol alleviated hepatic fibrosis through the above signaling pathways via NS3TP1.
- Citation: Shi L, Zhou L, Han M, Zhang Y, Zhang Y, Yuan XX, Lu HP, Wang Y, Yang XL, Liu C, Wang J, Liang P, Liu SA, Liu XJ, Cheng J, Lin SM. Calcitriol attenuates liver fibrosis through hepatitis C virus nonstructural protein 3-transactivated protein 1-mediated TGF β1/Smad3 and NF-κB signaling pathways. World J Gastroenterol 2023; 29(18): 2798-2817
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2798.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2798
Hepatic fibrosis is a dynamic process that occurs in any type of chronic liver damage that causes a net increase in the extracellular matrix. Further development of liver fibrosis can lead to liver cirrhosis and a series of complications[1,2]. Early intervention for liver fibrosis is crucial, but currently, there is no effective treatment. Therefore, additional studies on the mechanism of liver fibrosis are urgently needed to provide new targets for treatment.
The open reading framework region of the hepatitis C virus (HCV) genome consists of a core protein region, envelope protein region and a nonstructural protein region. The NS3 gene is located in the nonstructural protein region of the HCV genome and it has serine protease activity and RNA helicase activity. HCV NS3 is crucial for the maturation and replication of the HCV RNA protein[3]. Moreover, this gene contributes to the occurrence of hepatic fibrosis through interaction with host cell components[4]. In this study, we screened and cloned HCV nonstructural protein 3-transactivated protein 1 (NS3TP1) by suppressive subtractive hybridization and bioinformatics analysis. This protein is also referred to asparagine synthetase domain containing 1 (ASNSD1) and registered in GenBank as NBLA00058. The registration number is AY11696. This gene is located on human chromosome 2q32.2. The total length of the gene coding sequence was 1932 nucleotides, and the coding product was 643 amino acid residues. NS3TP1 is widely distributed in the human body[5-8]. NS3 regulates the occurrence of liver fibrosis by interacting with host cells. Whether NS3TP1, as a trans-activator of NS3, can also affect liver fibrosis should be further investigated. Meienberg et al[9] demonstrated that NS3TP1 may interact with type 3 collagen alpha 1 chain (COL3A1). In our studies, we found that the NS3TP1 protein increased the expression levels of transforming growth factor beta receptor I (TGFβRI) by gene chip technology. Moreover, TGFβRI is one of the key molecules involved in the activation of the classic transforming growth factor beta 1 (TGFβ1)/Smad3 pathway in liver fibrosis[10]. Therefore, we speculate that NS3TP1 is related to the occurrence of liver fibrosis.
Furthermore, calcitriol was shown to downregulate the expression of NS3TP1 at the mRNA level in the Comparative Toxicogenomics Database (CTD)[11]. Thus, a correlation was established between calcitriol, NS3TP1, and liver fibrosis. As a conventional drug for treating vitamin D deficiency-related rickets, calcitriol regulates biological effects by binding to the vitamin D receptor (VDR). Pop et al[12] discovered that VDR was substantially generated in HSCs and Kupffer cells but weakly expressed in hepatic cells. Moreover, VDR is fully functional in HSCs and Kupffer cells. The role of vitamin D in the emergence of chronic hepatic fibrosis was highlighted by the identification of VDR as a crucial endocrine checkpoint for the fibrogenic activity of HSCs[13,14]. This finding indicated that the active component of vitamin D is a vital regulator of hepatic fibrosis. The activating of VDR in HSCs inhibits liver inflammation and fibrosis through the TGFβ1/Smad3 signaling pathway[15]. Moreover, activation of VDR reduces hepatic fibrosis by alleviating inflammation[16,17]. The activation of hepatic stellate cells (HSCs) has been proven to be a main driving factor of liver fibrosis[18]. The TGFβ1/Smad3 signaling pathway is the classical pathway of liver fibrosis activation[19,20]. In addition, NF-κB regulates cell death, inflammation, and wound healing and is therefore a crucial regulator of the progression of hepatic fibrosis[21,22].
The mechanism and mutual regulation between NS3TP1 and calcitriol in liver fibrosis have not been elucidated. However, based on previous studies, it was speculated that NS3TP1 increases hepatic fibrosis by triggering the TGFβ1/Smad3 and NF-κB signaling pathways. Moreover, calcitriol can regulate NS3TP1, but further studies are necessary for verification.
Calcitriol was purchased from MedChemExpress (United States).
Liver fibrosis was induced by carbon tetrachloride (CCl4) solubilized in corn oil (Sigma-Aldrich, Germany). Male C57BL/6 mice aged 8 wk were bought from Beijing Weitonglihua Laboratory Corporation (China). They experienced a week in a clean animal room at 24 °C with unrestricted access to food and water prior to the trials. The average weight of mice before modeling was 23-25 g, and mice were given an intraperitoneal (i.p.) injection with CCl4 three times/wk (0.5 μL/g) for 4 wk to induce fibrosis[21,23,24]. Mice with liver fibrosis were successfully established and randomly separated into calcitriol and negative control (NC) groups. Mice in the control group were administered normal saline (10 μL/g/d, 5 times/wk) intragastrically for 4 wk and injected (i.p.) with corn oil or CCl4, while mice in the calcitriol group were intragastrically administered calcitriol (1 μg/kg/d, 5 times/wk) and intraperitoneally injected with CCl4 for 4 wk. Finally, 48 h after being injected with CCl4, the mice were sacrificed. Normal saline was used to dilute 100% avertin to a 2.5% solution, and the anesthesia dose for mice was an i.p. injection of 100-200 µL/10 g. The mice were euthanized by deep anesthesia, followed by cervical dislocation. The Xi’an Jiaotong University Medical Science Center’s specific pathogen free Animal Laboratory Center served as the site for all investigations. The Research Ethics Council of the Xi’an Jiaotong University Medical Science Center (Xi’an, China) gave its approval to all animal trials. All animals were treated humanely, and the experimental protocols were carried out in line with the regulations of the institution.
LX-2 cells are hepatogenic mesenchymal human cells and were obtained from Xiang Ya Central Laboratory (Xiangya Medical College, China). The cells were transfected using jetPRIME reagent (PolyPlus Transfection SA, NY, United States) and treated with calcitriol. Recombinant human TGFβ1 (BioLegend, CA, United States) was administered to the cell cultivation medium at 2.5 ng/mL or 5.0 ng/mL for 24 h[18,25]. All procedures were executed in accordance with the manufacturer’s guidelines.
PcDNA3.1/mycHis(-)NS3TP1, pGL4.10-NS3TP1 promoter, pGL4.10-p65 promoter, and pGL4.10-TGFβ1R promoter were constructed by Beijing Genomics Institute (BGI, China). NS3TP1-siRNA was purchased from Gene Pharma (Hong Xun, Suzhou, Jiangsu Province, China).
Total RNA (Total RNA Kit, Omega, United States) was extracted from LX-2 cells and reverse-transcribed into single-stranded cDNA (Prime Script RT Reagent Kit, TaKaRa, China). Real-time quantitative polymerase chain reaction (RT-qPCR) was used to amplify the genes using specific primers (Hong Xun, Suzhou, Jiangsu Province, China), and β-actin was used as the internal control gene. The level of the target gene was estimated using the ΔΔCT method and normalized to that of the control. Supplementary Table 1 includes a list of the primer sequences.
Proteins were extracted from mouse livers or LX-2 cells and isolated using 10% BIS-Tris gel/MOPS (Invitrogen, NY, United States) in MOPS SDS-PAGE (Thermo Fisher, United States). Transferring the separated proteins to a membrane made of polyvinylidene fluoride (PVDF) (Millipore, United States), which was incubated with secondary antibodies for 1.5 h after being exposed to primary antibodies (Supplementary Table 2) for 12 h at 4 °C. The immunoreactive bands were created using intensified chemiluminescence (Thermo Fisher Scientific) and visualized using the ChemiDoc™ touch imaging system (Bio-Rad, Hercules, CA, United States). ImageJ was used to assess the pictures.
Ice-cold M-PERTM mammalian protein extraction reagent was used to lyse LX-2 cells, and it also contained a mixture of protease and phosphatase inhibitors. Whole cell lysates (500 μL) were incubated with 50 μL protein magnetic beads for 2 h at room temperature and centrifuged, and the supernatant was transferred to a new 1.5 mL centrifuge tube. After that, the beads were mixed for 2 h at room temperature with whole-cell lysates containing 2 μL of anti-HIS antibody and 3 μL of regular mouse IgG antibody. The beads were eluted in DTT-free Laemmli buffer and then washed three times with PBST before being subjected to western blotting analysis.
On a 96-well plate, LX-2 cells were exposed to various calcitriol concentrations for 24 h. PBS was used to wash the cells twice after the initial medium was removed. Each well received a 1:100 addition of CCK-8 (Dojindo, Kumamoto, Japan) reagent before being cultured for 1 h at 37 °C. At 450 nm, the optical density was calculated.
LX-2 cells were treated with various concentrations of calcitriol for 48 h. The original medium was washed twice with PBS. The cells were collected by flow cytometry, treated with the reagents from an Annexin V-FITC/7-AAD apoptosis detection kit (BioLegend, CA, United States), and measured by a FACSCalibur flow cytometer (Beckman Coulter, CA, United States).
Hematoxylin was obtained from Yili Reagent Company (Beijing, China). Eosin was obtained from Zhongshan Jinqiao Biotechnology Company (Beijing, China). A Masson Tricolor staining kit was bought from Bogoo Corporation (Shanghai, China). A Pico Sirius Red Staining Kit was bought from Ruisai Biologicals (Shanghai, China). Oil red dye was obtained from Sigma-Aldrich (MO, United States). The manufacturer’s instructions were strictly adhered to the conduct of every experiment.
Liver slices underwent an overnight incubation at 4 °C with a primary antibody and a 40-minute incubation at room temperature with an enzyme-labeled anti-rabbit antibody (PV-6001, ZSGB-BIO, Beijing, China). The slices were then developed using DAB substrate (Gene Science and Technology Company, China) after 30 min at room temperature incubation with avidin-biotin complex (PK-6100, Vectastain Elite ABC Standard kit, Vector Laboratories, Burlingame, CA, United States).
HepG2 cells were transiently transfected with different promoter plasmids. A renal cell luciferase vector plasmid was used as a control. Dual-luciferase reporter gene activity was evaluated using a dual-luciferase reporter gene detection kit (Promega, United States).
Statistical analyses were performed using SPSS 24.0. The Student’s t test was used to statistically examine all data. A P value < 0.05 was regarded as statistically significant. The data is shown as the mean ± SE.
Herein, we confirmed the in vivo and in vitro roles of NS3TP1 in hepatic fibrosis. The level of NS3TP1 was measured in mice with CCl4-induced hepatic fibrosis and TGF β1-activated LX-2 cells. Hema
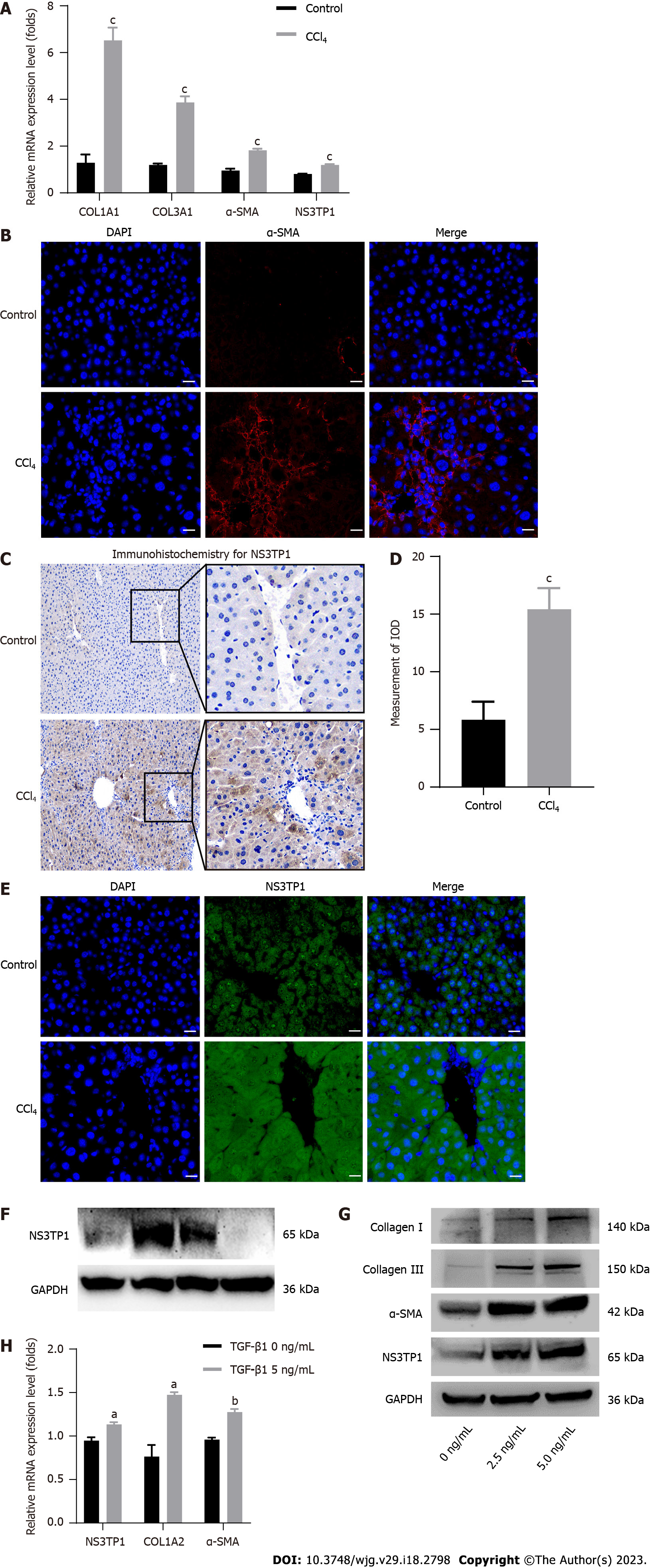
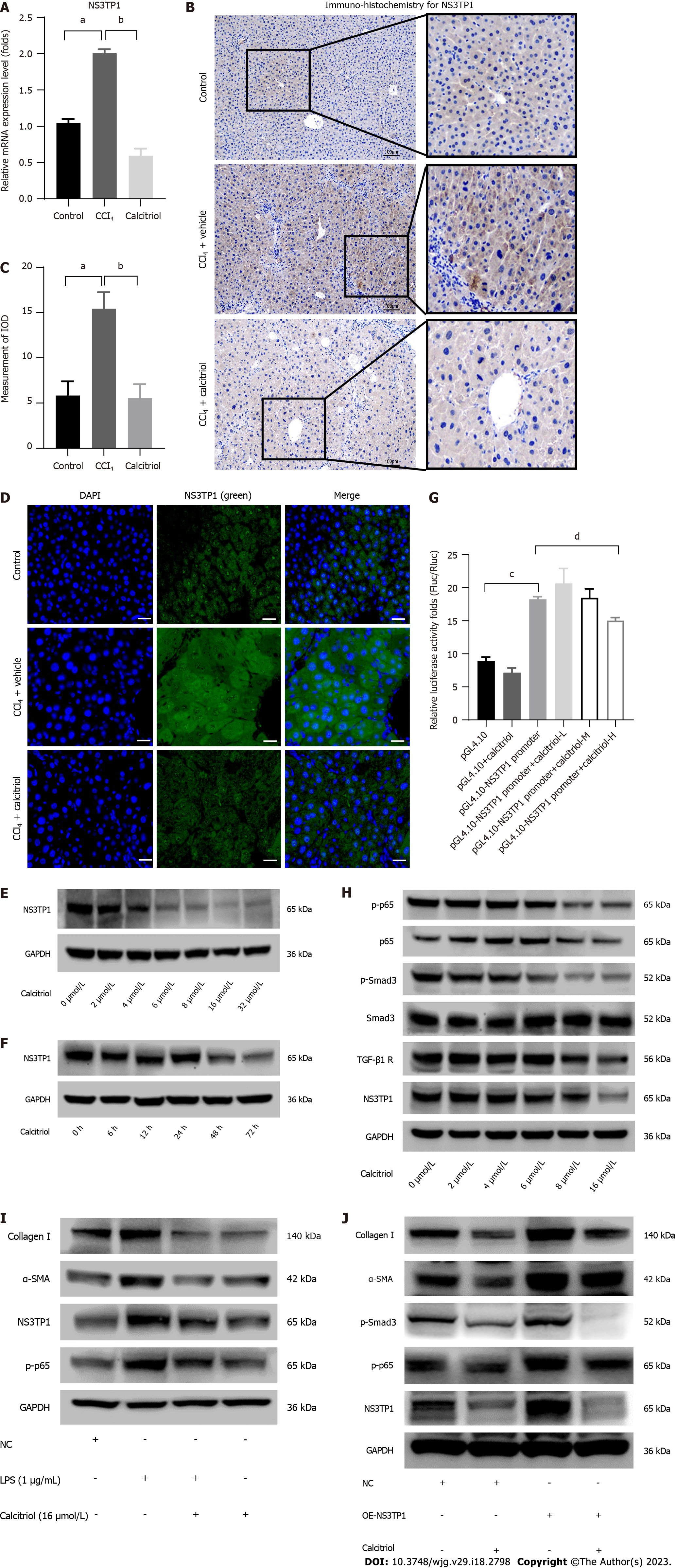
In vivo, COL1A1, COL3A1, α-SMA, and NS3TP1 were considerably elevated at the mRNA level in CCl4-treated mouse livers (Figure 1A). The level of α-SMA in the CCl4-treated mouse livers was upregulated, as indicated by immunofluorescence (Figure 1B). NS3TP1 in CCl4-handled mouse livers was upregulated, as indicated by immunohistochemistry (Figure 1C and D) and immunofluorescence (Figure 1E).
In vitro, Western blotting was performed to monitor the level of NS3TP1 in various cell lines, such as L02, LX-2, Huh7, and HepG2, and NS3TP1 was significantly raised in LX-2 cells (Figure 1F). In addition, LX-2 cells were administered with recombinant human TGFβ1 protein for 24 h. Then, α-SMA, collagen I, collagen III, and NS3TP1 were found to be significantly upregulated at the protein and mRNA levels (Figure 1G and H).
Therefore, in vivo and in vitro investigations validated the elevation of NS3TP1 in hepatic fibrosis.
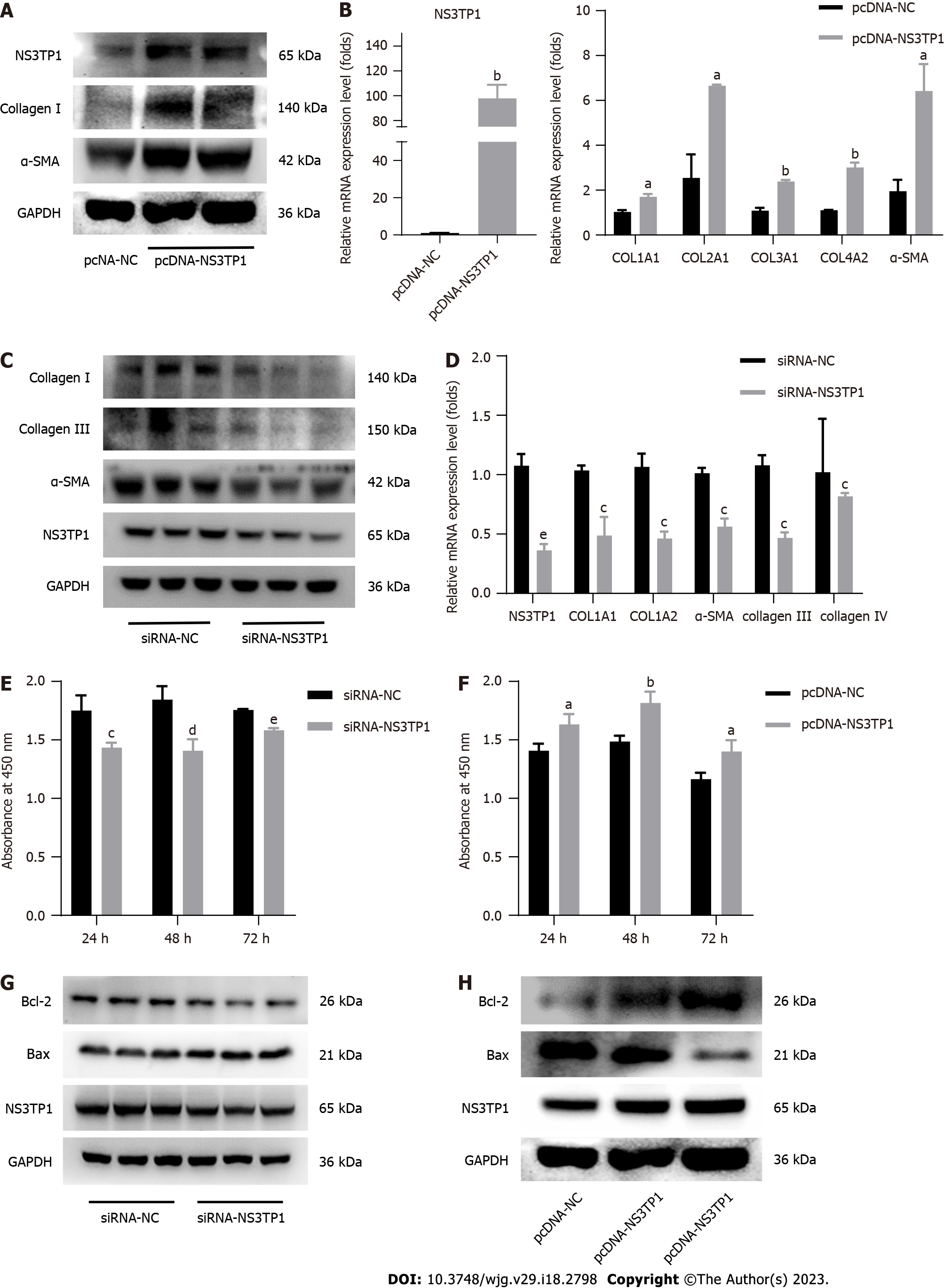
The pcDNA-NS3TP1 plasmid was constructed and transiently transfected into LX-2 cells. The expression levels of fibrosis-related proteins were detected by Western blotting after 48 h. Compared to those in the negative control group, the levels of collagen I and α-SMA in the NS3TP1 overexpression group were increased (Figure 2A). The mRNA levels of COL1A1, COL2A1, COL3A1, COL4A2, and -SMA were monitored using RT-qPCR, and the results matched those of the Western blotting (Figure 2B). The levels of collagen I, collagen III, collagen IV, and α-SMA in NS3TP1 knockdown LX-2 cells were measured by Western blotting and RT-qPCR after 48 h. The results showed that in the NS3TP1 gene interference group, the expression of collagen I, collagen III, collagen IV, and α-SMA at the protein and mRNA levels was significantly downregulated (Figure 2C and D). This finding was the opposite of that of the NS3TP1 overexpression group.
NS3TP1 was interfered or overexpressed in LX-2 cells, the impact of NS3TP1 on HSC growth was then examined after 24 h, 48 h, and 72 h using a Cell Counting Kit-8 (CCK-8) cell proliferation and activity detection kit. Compared to the control group’s results, the proliferation of HSCs in the NS3TP1 interference group decreased, while it increased after NS3TP1 overexpression, indicating that NS3TP1 increased the proliferation of HSCs (Figure 2E and F).
In LX-2 cells, after NS3TP1 was knocked down, the level of Bcl-2 was downregulated, the level of Bax was upregulated, apoptosis was enhanced, and the effects induced by overexpression were reversed. These results indicated that NS3TP1 inhibited the apoptosis of HSCs (Figure 2G and H).
Consequently, in vitro research supported NS3TP1’s significance in fostering hepatic fibrosis.
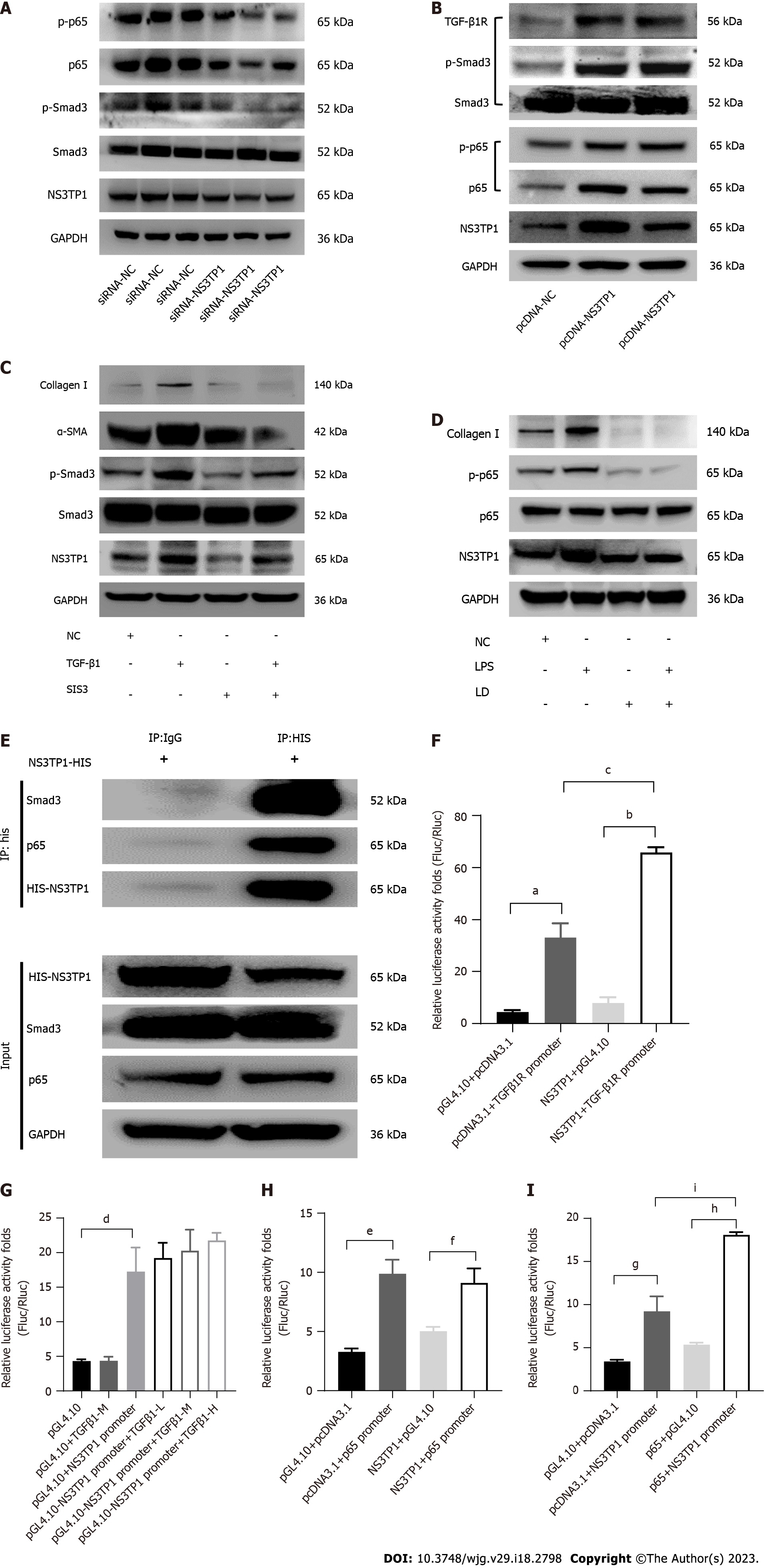
After transient transfection of LX-2 cells with the NS3TP1-overexpressing plasmid or siRNA-NS3TP1 for 48 h, the activity of the TGFβ1/Smad3 and NF-κB signaling pathways was evaluated. In comparison to that of the control group, the activity of both signaling pathways was elevated in the NS3TP1 overexpression group and decreased in the gene interference group. These results suggested that NS3TP1 promoted hepatic fibrosis via both signaling pathways (Figure 3A and B).
We applied 3 μmol/L Smad3-specific inhibitor (SIS3) and 2 μmol/L licochalcone D (LD, inhibition of the phosphorylation of NF-κB at serine 276) to inhibit the above pathways and then added TGFβ1 (5 ng/mL) or LPS (1 μg/mL). NS3TP1 and downstream collagen I, α-SMA, p-smad3, and p-p65 Levels were decreased, indicating that NS3TP1 was localized downstream of Smad3 and p65 (Figure 3C and D).
Using coimmunoprecipitation (Co-IP), we found that NS3TP1 could bind to Smad3 and p65 (Figure 3E). The dual luciferase assay revealed that NS3TP1 enhanced TGFβRI promoter activity (Figure 3F), while TGFβ1 had no effect on NS3TP1 promoter activity (Figure 3G). Moreover, NS3TP1 had no effect on p65 promoter activity (Figure 3H), while p65 increased NS3TP1 promoter activity (Figure 3I). Therefore, NS3TP1 may inhibit the activity of both signaling pathways by suppressing the phosphorylation of Smad3 or p65 at the protein level. In addition, NS3TP1 may regulate the TGFβ1/Smad3 signaling pathway at the mRNA level by upregulating the TGFβRI promoter, and the regulation of the NS3TP1 and NF-κB signaling pathways at the mRNA level may be realized through the upregulation of the NS3TP1 promoter by p65.
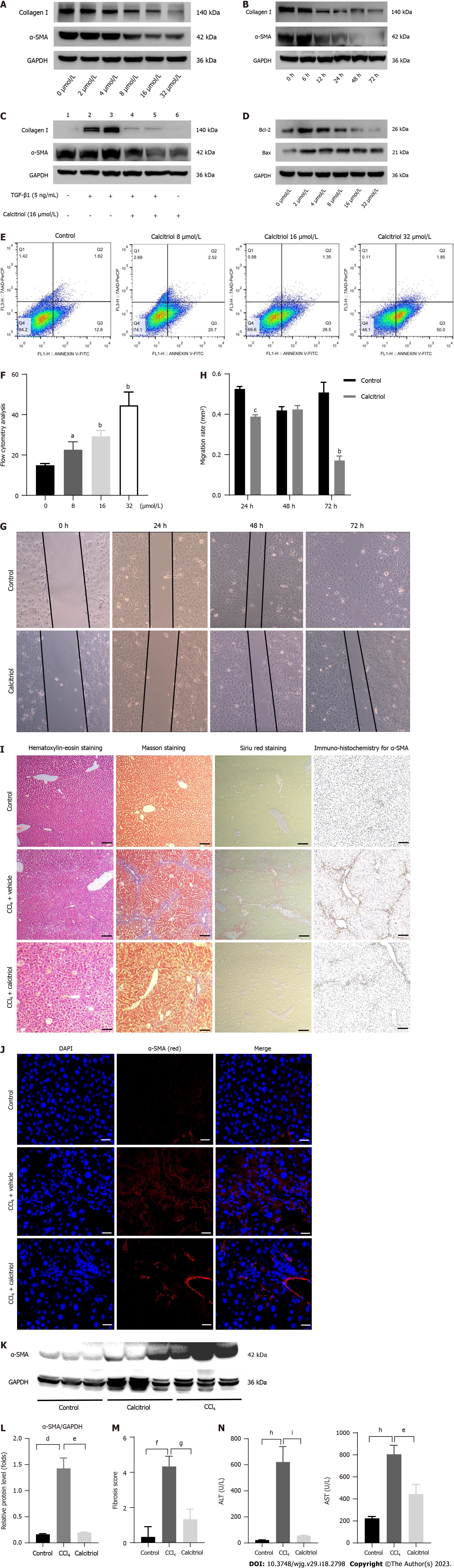
The CCK-8 kit was used to detect LX-2 cells stimulated with various concentrations of calcitriol. After incubation for 48 h with the CCK-8 reagent, the absorbance value decreased with increasing calcitriol concentration, which could be attributed to calcitriol-inhibited cell proliferation (SupplementaryFigure 4). Calcitriol reduced the activation of HSCs in a concentration- and time-dependent manner. The optimum concentration of calcitriol was 16 μmol/L (Figure 4A). The optimum time for calcitriol treatment was 48 h (Figure 4B). Calcitriol promoted the return of TGFβ1-activated HSCs to quiescent HSCs (qHSCs) (Figure 4C). Following calcitriol administration, a Western blot analysis revealed concentration-dependently elevated Bax and diminished Bcl-2 Levels (Figure 4D). Flow cytometry showed that LX-2 cell apoptosis increased with increasing calcitriol concentration (Figure 4E and F). Oil red O staining showed an increased number of fat droplets in LX-2 cells (Supplementary Figure 5). Calcitriol reduced the migration of activated HSCs (Figure 4G and H).
In vivo, the dose and the protocol for this study were chosen based on the results of previous studies[26-28]. The optimal dosage of calcitriol was identified as 1 μg/kg/d, 5 times/wk according to the preliminary experimental results, and subsequent experiments were carried out with this dosage.
Calcitriol treatment reduced CCl4-induced collagen accumulation in the mouse liver, as shown by hematoxylin-eosin staining, Masson staining, Sirius red staining, immunohistochemistry for α-SMA (Figure 4I), and immunofluorescence for α-SMA (Figure 4J). Western blot results further support these conclusions (Figure 4K and L). Liver fibrosis was improved by calcitriol treatment, according to the Ishak scoring system (Figure 4M). Plasma ALT and AST levels also demonstrated that calcitriol decreased CCl4-induced inflammation in the mouse liver (Figure 4N). These findings showed that calcitriol prevented hepatic fibrosis both in vivo and in a lab setting.
Calcitriol prevented the expression of NS3TP1 at the mRNA level in the CCl4-treated mouse liver, as shown by RT-qPCR (Figure 5A) and by immunohistochemistry (Figure 5B and C). Moreover, calcitriol inhibited the expression of NS3TP1 at the protein level, as shown by immunofluorescence staining for NS3TP1 (Figure 5D). In LX-2 cells, the inhibition of NS3TP1 by calcitriol at the protein level was time- and concentration dependent, with an optimal concentration of 16 μmol/L and an optimal treatment time of 48 h (Figure 5E and F). In addition, the activity of the NS3TP1 promoter was inhibited by 32 μmol/L calcitriol in HepG2 cells, as validated by dual-luciferase reporter gene assay (Figure 5G).
Both signaling pathways were inhibited in a dose- and time-dependent manner after 12 h of TGFβ1 treatment and 48 h of calcitriol treatment in LX-2 cells (Figure 5H). Calcitriol significantly inhibited the LPS-activated NF-κB signaling pathway in HSCs (Figure 5I). PcDNA 3.1/myC-His(-)-NS3TP1 was transfected into LX-2 cells for 24 h, and then the cells were stimulated with calcitriol for 48 h. Calcitriol inhibited LX-2 cell activation following NS3TP1 overexpression (Figure 5J).
In conclusion, these results confirm that calcitriol prevented hepatic fibrosis through the above signaling pathways via NS3TP1.
The present study first showed that NS3TP1 promoted hepatic fibrosis by enhancing the TGFβ1/Smad3 and NF-κB signaling pathways. NS3TP1 controlled HSC activation, proliferation, and differentiation. These results provide solid proof for the role of NS3TP1 contributing to hepatic fibrosis.
Both in vivo and in vitro experiments confirmed that NS3TP1 was elevated in liver fibrosis (Figure 1), which was consistent with our group’s previous results. TGF-β1 activates hepatic stellate cells effectively[29], and the CCl4-induced liver fibrosis model is one of the classical models of liver fibrosis[30]. There was an increase in NS3TP1 expression levels in the treated cells and tissues, indicating that NS3TP1 plays a role in liver fibrosis development. Moreover, NS3TP1 promoted the activation and proliferation but inhibited the apoptosis of LX-2 cells (Figure 2), Meienberg et al[9] demonstrated that NS3TP1 may interact with COL3A1, which further supports that NS3TP1 regulates the occurrence of hepatic fibrosis. The TGFβ1/Smad3 and NF-κB signaling pathways have been well established as two classical pathways for hepatic stellate cell activation[20,22,31]. To confirm the correlation between NS3TP1 and the activity of both signaling pathways, we overexpressed or interfered with NS3TP1 in LX-2 cells. We found that the activities of both signaling pathways were enhanced in the NS3TP1 overexpression group. However, the activities of both signaling pathways were decreased in the gene interference group, suggesting that NS3TP1 regulated hepatic fibrosis through both the above signaling pathways. To further elucidate the specific mechanisms between NS3TP1 and both signaling pathways, we used Co-IP and dual-luciferase assays. Co-IP analysis demonstrated that Smad3 and p65 could bind to NS3TP1. Therefore, NS3TP1 may decrease the activity of both signaling pathways by inhibiting the phosphorylation of Smad3 or p65. It is necessary to examine the possible colocalization of NS3TP1 and Smad3 or p65 in LX-2 cells by confocal microscopy. The dual-luciferase assay revealed that NS3TP1 increased the activity of the TGFβRI promoter and that p65 increased the activity of the NS3TP1 promoter. Altogether, NS3TP1 regulated both signaling pathways at protein and mRNA levels (Figure 3). The key targets of both signaling pathways are p-smad3 and p65 respectively, and p65 is the classic regulatory factor of the inflammatory pathway[32,33], which further supports our research.
We confirmed that calcitriol inhibited hepatic fibrosis in vitro and in vivo (Figure 4). This finding is consistent with previous research[26,34,35]. In vivo experiments were conducted using CCl4-induced model mice, wherein calcitriol was found to attenuate liver fibrosis. The optimal dosage of calcitriol to treat liver fibrosis was 1 μg/kg/d, administered five times per week, which also reduced CCl4-induced inflammation in the mouse liver. Calcitriol decreased the deposition of extracellular matrix following HSC activation, prevented the proliferation, activation, and migration of HSCs, and promoted cell apoptosis, and these in vitro experiments were primarily conducted using LX-2 cells. In addition, HSCs were also able to accumulate lipid droplets when exposed to calcitriol, which may be related to the dedifferentiation of activated HSCs into qHSCs, suggesting that calcitriol reversed hepatic fibrosis as described previously[25,36]. In conclusion, calcitriol inhibited liver fibrosis, however, the precise mechanism was uncertain. Therefore, we investigated the mechanisms by which calcitriol prevented hepatic fibrosis.
We demonstrated that calcitriol downregulated NS3TP1 at the mRNA level in CTD[11]. Moreover, we first proved that calcitriol inhibited NS3TP1 in vivo and in vitro (Figure 5) in a dose-dependent manner, and the increased NS3TP1 expression levels were found to be suppressed by calcitriol in mouse liver fibrotic tissues. The dual-luciferase assay showed that calcitriol blocked the promoter activity of NS3TP1, which conforms with the research of Wang et al[11]. Finally, we first demonstrated that calcitriol prevented hepatic fibrosis through the above signaling pathways via NS3TP1. Calcitriol inhibited liver fibrosis by binding to VDR, and NS3TP1 may be one of the targets. Whether other molecules that can bind to VDR can also reduce the expression of NS3TP1 will be the focus of further research. The results by Hah et al[15] supported this phenomenon. It was found that activating VDR in HSCs prevented liver inflammation and fibrosis through the TGFβ1/Smad3 signaling pathway. Activation of the TGFβ1 signaling pathway leads to genome-wide reallocation of VDR binding through TGFβ1-dependent chromatin remodeling in the presence of VDR ligands. By attaching to Smad3, VDR decreases Smad3 occupancy at these locations and inhibits fibrosis[15]. We proved that calcitriol decreased liver fibrosis through the NF-κB signaling pathway via NS3TP1. This finding is consistent with previous studies. Calcitriol suppresses the NF-κB signaling pathway, which confirms its anti-inflammatory effect, and activated HSCs are involved in inflammation[37].
Regarding the limitations of this study, animal experiments were not completed on NS3TP1-KO mice. In our research, we discovered that NS3TP1-KO mice could not give birth normally. Combined with the results of GeneCards database retrieval, we found that the NS3TP1 content in the reproductive system is quite rich. We therefore speculated that knocking down NS3TP1 would also have an impact on reproductive functions. To learn more about how the protein contributes to liver fibrosis, we will concentrate on NS3TP1 knockouts that target the liver.
In conclusion, NS3TP1 regulates TGFβ1/Smad3 and NF-κB signaling pathways to induce liver fibrosis. These results contribute to NS3TP1 as a novel, prospective therapeutic target for the treatment of hepatic fibrosis, and calcitriol may be employed as an adjuvant therapy.
Despite the lack of a specific therapeutic medicine, liver fibrosis still constitutes a serious hazard to human health, hence it is critical to identify novel targets for its therapy. After screening using suppressive subtractive hybridization and bioinformatics analysis, our research team discovered that hepatitis C virus nonstructural protein 3-transactivated protein 1 (NS3TP1) may be involved in the occurrence of liver fibrosis. As a result, the role of NS3TP1 in liver fibrosis was investigated to provide a new target for the treatment of liver fibrosis.
A potential new target for the treatment of hepatic fibrosis is provided by this work.
To determine whether NS3TP1 can promote liver fibrosis and whether calcitriol can inhibit the occurrence of liver fibrosis through NS3TP1.
In vitro experiments were performed on carbon tetrachloride mouse liver, and NS3TP1 and fibrosis-related indexes were studied through serological and pathological tests. In vivo experiments were performed on LX-2 cells, and siRNA-NS3TP1 and pcDNA-NS3TP1 were constructed and transfected into LX-2 cells, respectively. Collagen I, collagen III, α-smooth muscle actin (α-SMA), TGFβ1/Smad3 and NF-κB signaling pathways were detected by western blot, RT-PCR, Co-imunoprecipitation and luciferase assays.
Collagen I, collagen III, α-SMA, transforming growth factor beta (TGFβ1)/Smad3, and NF-κB signaling pathways were found to be up-regulated following overexpression of NS3TP1, whereas the aforementioned indices were shown to be down-regulated after NS3TP1 interference in vitro. Results from Co-IP and Luciferase assays confirmed that Smad3 and p65 could both bind to NS3TP1, and that p65 boosted NS3TP1’s promoter activity while NS3TP1 increased the promoter activity of TGFβ receptor I (TGFβ-RI). NS3TP1 and fibrosis-related indicators decreased after calcitriol therapy both in vitro and in vivo, and calcitriol restrained the expression of TGFβ1/Smad3 and NF-κB signaling pathways via NS3TP1.
NS3TP1 promotes hepatic fibrosis through TGFβ1/Smad3 and NF-κB signaling pathways. Calcitriol further inhibits TGFβ1/Smad3 and NF-κB signaling pathways to reduce liver fibrosis by down-regulating NS3TP1.
NS3TP1 provides a novel target for the treatment of liver fibrosis and a direction for the research of potential drug targets. Calcitriol is endowed with new functions as an adjunct therapeutic drug for liver fibrosis.
We would like to thank all the coauthors who participated in this study: Feng Ye, Lei Shi, Yun-Ru Chen, Xi Zhang, Jian-Zhou Li, and Yi-Fan Xu. Thank you to all the teachers at the Institute of Infectious Diseases at Beijing Ditan Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andersen JB, Denmark; Heij LR, Germany; Mohamed GA, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 573] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 2. | Du QH, Zhang CJ, Li WH, Mu Y, Xu Y, Lowe S, Han L, Yu X, Wang SY, Li Y, Li J. Gan Shen Fu Fang ameliorates liver fibrosis in vitro and in vivo by inhibiting the inflammatory response and extracellular signal-regulated kinase phosphorylation. World J Gastroenterol. 2020;26:2810-2820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ul Haq A, Sheikh A, Naeem S, Abidi SH. Molecular docking analysis of fluoroquinolones and other natural and synthetic compounds with the HCV NS3 helicase. Bioinformation. 2022;18:147-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Li HC, Yang CH, Lo SY. Hepatitis C Viral Replication Complex. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Powell DR, Revelli JP, Doree DD, DaCosta CM, Desai U, Shadoan MK, Rodriguez L, Mullens M, Yang QM, Ding ZM, Kirkpatrick LL, Vogel P, Zambrowicz B, Sands AT, Platt KA, Hansen GM, Brommage R. High-Throughput Screening of Mouse Gene Knockouts Identifies Established and Novel High Body Fat Phenotypes. Diabetes Metab Syndr Obes. 2021;14:3753-3785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Vogel P, Ding ZM, Read R, DaCosta CM, Hansard M, Small DL, Ye GL, Hansen G, Brommage R, Powell DR. Progressive Degenerative Myopathy and Myosteatosis in ASNSD1-Deficient Mice. Vet Pathol. 2020;57:723-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Vogel P, Read RW, Hansen GM, Powell DR. Histopathology is required to identify and characterize myopathies in high-throughput phenotype screening of genetically engineered mice. Vet Pathol. 2021;58:1158-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Theusch E, Chen YI, Rotter JI, Krauss RM, Medina MW. Genetic variants modulate gene expression statin response in human lymphoblastoid cell lines. BMC Genomics. 2020;21:555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Meienberg J, Rohrbach M, Neuenschwander S, Spanaus K, Giunta C, Alonso S, Arnold E, Henggeler C, Regenass S, Patrignani A, Azzarello-Burri S, Steiner B, Nygren AO, Carrel T, Steinmann B, Mátyás G. Hemizygous deletion of COL3A1, COL5A2, and MSTN causes a complex phenotype with aortic dissection: a lesson for and from true haploinsufficiency. Eur J Hum Genet. 2010;18:1315-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Wang T, Zhang C, Meng X, Zhu B, Wang S, Yuan W, Zhang S, Xu J. Long Noncoding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 in Extracellular Vesicles Promotes Hepatic Stellate Cell Activation, Liver Fibrosis and β-Catenin Signaling Pathway. Front Physiol. 2022;13:792182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Wang WL, Chatterjee N, Chittur SV, Welsh J, Tenniswood MP. Effects of 1α,25 dihydroxyvitamin D3 and testosterone on miRNA and mRNA expression in LNCaP cells. Mol Cancer. 2011;10:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int J Mol Sci. 2022;23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 19] [Reference Citation Analysis (0)] |
| 13. | Lu W, Li X, Liu N, Zhang Y, Li Y, Pan Y, Yang J, Liu Z, Kong J. Vitamin D alleviates liver fibrosis by inhibiting histidine-rich calcium binding protein (HRC). Chem Biol Interact. 2021;334:109355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Okubo T, Atsukawa M, Tsubota A, Yoshida Y, Arai T, Iwashita AN, Itokawa N, Kondo C, Iwakiri K. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol Res. 2020;50:588-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hah N, Sherman MH, Yu RT, Downes M, Evans RM. Targeting Transcriptional and Epigenetic Reprogramming in Stromal Cells in Fibrosis and Cancer. Cold Spring Harb Symp Quant Biol. 2015;80:249-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | He W, Ni W, Zhao L, Wang X, Liu L, Fan Z. MicroRNA-125a/VDR axis impaired autophagic flux and contributed to fibrosis in a CCL4-induced mouse model and patients with liver cirrhosis. Life Sci. 2021;264:118666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Gu L, Xu Q, Cao H. 1,25(OH)2D3 Protects Liver Fibrosis Through Decreasing the Generation of TH17 Cells. Med Sci Monit. 2017;23:2049-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Chen C, Li X, Wang L. Thymosinβ4 alleviates cholestatic liver fibrosis in mice through downregulating PDGF/PDGFR and TGFβ/Smad pathways. Dig Liver Dis. 2020;52:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zhou MY, Cheng ML, Huang T, Hu RH, Zou GL, Li H, Zhang BF, Zhu JJ, Liu YM, Liu Y, Zhao XK. Transforming growth factor beta-1 upregulates glucose transporter 1 and glycolysis through canonical and noncanonical pathways in hepatic stellate cells. World J Gastroenterol. 2021;27:6908-6926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Tang M, Guo C, Sun M, Zhou H, Peng X, Dai J, Ding Q, Wang Y, Yang C. Effective delivery of osteopontin small interference RNA using exosomes suppresses liver fibrosis via TGF-β1 signaling. Front Pharmacol. 2022;13:882243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 21. | Luo Q, Ling Y, Li Y, Qu X, Shi Q, Zheng S, Huang Y, Zhou X. Phosphatidylethanolamine-binding protein 4 deficiency exacerbates carbon tetrachloride-induced liver fibrosis by regulating the NF-κB signaling pathway. Front Pharmacol. 2022;13:964829. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 22. | Masamune A, Hamada S. Editorial: Mechanisms of Inflammation and Fibrosis Interplays in the Digestive Diseases. Front Physiol. 2022;13:906742. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 23. | Xie S, Qiu C, Sun Y, Yu Y, Hu Z, Zhang K, Chen L, Cheng Y, Bao M, Zhang Q, Zhu J, Grimm R, Shen W. Assessment of Fibrotic Liver Regeneration After Partial Hepatectomy With Intravoxel Incoherent Motion Diffusion-Weighted Imaging: An Experimental Study in a Rat Model With Carbon Tetrachloride Induced Liver Injury. Front Physiol. 2022;13:822763. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 24. | Higashiyama M, Tomita K, Sugihara N, Nakashima H, Furuhashi H, Nishikawa M, Inaba K, Wada A, Horiuchi K, Hanawa Y, Shibuya N, Kurihara C, Okada Y, Nishii S, Mizoguchi A, Hozumi H, Watanabe C, Komoto S, Yamamoto J, Seki S, Miura S, Hokari R. Chitinase 3-like 1 deficiency ameliorates liver fibrosis by promoting hepatic macrophage apoptosis. Hepatol Res. 2019;49:1316-1328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Udomsinprasert W, Jittikoon J. Vitamin D and liver fibrosis: Molecular mechanisms and clinical studies. Biomed Pharmacother. 2019;109:1351-1360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Reiter FP, Ye L, Bösch F, Wimmer R, Artmann R, Ziesch A, Kanitz V, Mayr D, Steib CJ, Trauner M, Regel I, Gerbes AL, Mayerle J, Hohenester S, de Toni EN, Denk G. Antifibrotic effects of hypocalcemic vitamin D analogs in murine and human hepatic stellate cells and in the CCl(4) mouse model. Lab Invest. 2019;99:1906-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Tsai TH, Lin CJ, Hang CL, Chen WY. Calcitriol Attenuates Doxorubicin-Induced Cardiac Dysfunction and Inhibits Endothelial-to-Mesenchymal Transition in Mice. Cells. 2019;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Gong J, Gong H, Liu Y, Tao X, Zhang H. Calcipotriol attenuates liver fibrosis through the inhibition of vitamin D receptor-mediated NF-κB signaling pathway. Bioengineered. 2022;13:2658-2672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 260] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 30. | Delire B, Stärkel P, Leclercq I. Animal Models for Fibrotic Liver Diseases: What We Have, What We Need, and What Is under Development. J Clin Transl Hepatol. 2015;3:53-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 31. | Zhang YZ, Yao JN, Zhang LF, Wang CF, Zhang XX, Gao B. Effect of NLRC5 on activation and reversion of hepatic stellate cells by regulating the nuclear factor-κB signaling pathway. World J Gastroenterol. 2019;25:3044-3055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Rodríguez MJ, Sabaj M, Tolosa G, Herrera Vielma F, Zúñiga MJ, González DR, Zúñiga-Hernández J. Maresin-1 Prevents Liver Fibrosis by Targeting Nrf2 and NF-κB, Reducing Oxidative Stress and Inflammation. Cells. 2021;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | ShamsEldeen AM, Al-Ani B, Ebrahim HA, Rashed L, Badr AM, Attia A, Farag AM, Kamar SS, Haidara MA, Al Humayed S, Ali Eshra M. Resveratrol suppresses cholestasis-induced liver injury and fibrosis in rats associated with the inhibition of TGFβ1-Smad3-miR21 axis and profibrogenic and hepatic injury biomarkers. Clin Exp Pharmacol Physiol. 2021;48:1402-1411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Ma L, Ishigami M, Honda T, Yokoyama S, Yamamoto K, Ishizu Y, Kuzuya T, Hayashi K, Hirooka Y, Goto H. Antifibrotic Effects of 1,25(OH)(2)D(3) on Nonalcoholic Steatohepatitis in Female Mice. Dig Dis Sci. 2019;64:2581-2590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Arai T, Atsukawa M, Tsubota A, Koeda M, Yoshida Y, Okubo T, Nakagawa A, Itokawa N, Kondo C, Nakatsuka K, Masu T, Kato K, Shimada N, Hatori T, Emoto N, Kage M, Iwakiri K. Association of vitamin D levels and vitamin D-related gene polymorphisms with liver fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease. Dig Liver Dis. 2019;51:1036-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Megahed A, Gadalla H, Abdelhamid FM, Almehmadi SJ, Khan AA, Albukhari TA, Risha EF. Vitamin D Ameliorates the Hepatic Oxidative Damage and Fibrotic Effect Caused by Thioacetamide in Rats. Biomedicines. 2023;11. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 37. | Gupta G, Khadem F, Uzonna JE. Role of hepatic stellate cell (HSC)-derived cytokines in hepatic inflammation and immunity. Cytokine. 2019;124:154542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |