Published online Apr 21, 2023. doi: 10.3748/wjg.v29.i15.2222
Peer-review started: September 20, 2022
First decision: October 20, 2022
Revised: November 7, 2022
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: April 21, 2023
Worldwide, gastric cancer (GC) is the fifth most commonly diagnosed mali
Core Tip: Premalignant cascade of gastric cancer starts with a chronic gastritis, and evolves to atrophic gastritis, intestinal metaplasia, dysplasia and finally the carcinoma. During the process, different immune responses contribute to inflammation of the gastric epithelium. Our work compiles studies related to the innate immune response with a focus on molecular and cellular features such as, Toll-like receptors, neutrophils, cytokines and socioeconomic factors, as crucial players during the precancerous cascade and the cancer onset.
- Citation: Villarroel-Espindola F, Ejsmentewicz T, Gonzalez-Stegmaier R, Jorquera RA, Salinas E. Intersections between innate immune response and gastric cancer development. World J Gastroenterol 2023; 29(15): 2222-2240
- URL: https://www.wjgnet.com/1007-9327/full/v29/i15/2222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i15.2222
Globally, gastric cancer (GC) is the fifth most commonly diagnosed malignancy. Although there has been a reduction in its incidence, its poor prognosis makes it the fourth leading cause of cancer related deaths per year[1], and around 86% of all GC cases in 2018 occurred in countries with a high or very high Human Development Index, where 60% of the total cases occurred in Eastern and South-Eastern Asia[2]. GC development is a multistep process initiated by the transition of normal mucosa to non-atrophic gastritis. This superficial gastritis may progress to atrophic gastritis, then intestinal metaplasia and finally to dysplasia and adenocarcinoma[3]. Overall, GC is viewed as the consequence of a multifactorial process involving environmental factors (socioeconomic status, smoking and alcohol consumption), dietary habits (diets rich in salt and poor in antioxidants) and intrinsic factors (ethnicity, genetic background, age and sex)[4,5]. Recently, a meta-analysis and prospective cohort study demonstrated in the Chinese population, that healthy lifestyle factors such as abstention from smoking, non-consumption of alcohol, low consumption of preserved foods, and frequent intake of fresh fruits and vegetables and all of these factors in combination can significantly reduce the relative and absolute risk of incidence of GC. Although, the individual carries a high polygenic risk of GC based on the presence of 112 single-nucleotide polymorphisms[6]. This observation suggests that some intrinsic host factors, like the genetic background, may be secondary to external or environmental aspects during GC onset.
Most of the malignant gastric tumors correspond to the histological type of adenocarcinoma (approximately 90%), with a lower percentage of lymphomas of the mucosa-associated lymphoid tissue, leiomyosarcomas and other rarer tumors[7,8]. The adenocarcinomas have been divided classically into two histological subtypes: Diffuse and intestinal, each of which have differences in their presentations depending on the anatomic subsite, age when diagnosed, sex, race, demographical distribution and socio-economic situation[7-9]. More recent molecular and genomic classifications have defined four major genomic subtypes of GC: The Epstein-Barr virus (EBV) infected tumors; genomically stable tumors; chromosomally unstable tumors; and tumors with microsatellite instability (MSI)[5], all of which offer a poor prognosis and different molecular profiles.
It is very well documented that GC is strongly associated with infectious agents such as the bacterium Helicobacter pylori (H. pylori) and, recently, the EBV[5]. Approximately 15%–20% of human cancers are provoked by cancer-causing viruses[10]; however, the specific role of EBV in GC development is not clear as of yet. Although the World Health Organization has categorized H. pylori as a group 1 carcinogen[11], the role of other bacteria in causing cancer is controversial; studies have shown that some bacteria, such as Fusobacterium nucleatum[12], and Porphyromonas spp.[13,14] play a role in the development of colon, oral and other digestive cancers. Nevertheless, all those microorganisms can promote a local inflammatory status and a parallel activation of protumoral pathways.
Innate immunity represents the first barrier against pathogens, and epithelial cells of the gastric mucosa are the first line of immunity against, for example, an H. pylori infection. In response to an infection, many physiological adaptations are observed, such as an increase in vascular diameter and permeability along with an overexpression of cell-adhesion molecules on endothelial cells which promotes the extravasation of myeloid cells into the inflamed site of infection. The characteristics of an inflamed microenvironment are low levels of glucose and a scarcity of oxygen due to an altered metabolism, increased oxygen consumption by neutrophils, and a reduced oxygen supply due to disrupted perfusion[15]. It is within such a hypoxic microenvironment that immune cells kill and prevent the spread of invading microorganisms. Accumulating evidence suggests that chronic inflammation, either non-infectious such as in autoimmune disorders or, as a result of a pathogen infection, is connected to cancer development. At the same time, the crosstalk between innate and adaptive immunity is critical for the successful eradication of different pathogens and tumor cells.
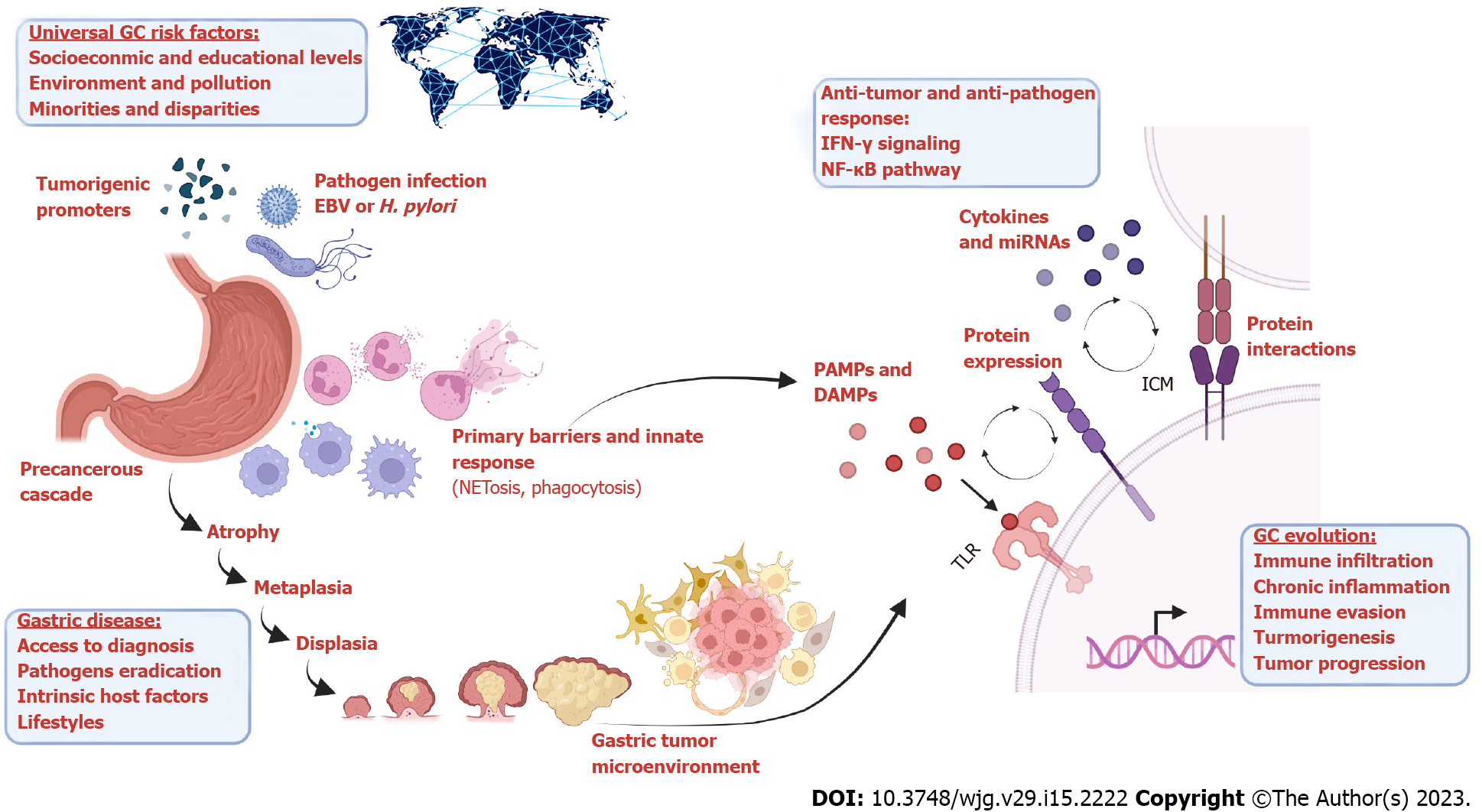
The aim of this review is to provide an overview of innate immune activation in the context of gastrointestinal malignancies, focusing on the premalignant lesions of the epithelium and the gastric tumor microenvironment. During the transition from atrophic gastritis to the final carcinoma, some microorganisms will play a determinant role promoting the neoplastic transformation or contributing with a particular tumor phenotype. This work compiles studies related to Toll-like receptors (TLRs), neutrophils, cytokines and pathogens, as crucial players during the precancerous cascade and the cancer onset (Figure 1), allowing the correlation of those aspects with clinical and socioeconomic variables.
Gastro-intestinal epithelial cells express several TLRs that can respond to exogenous infectious ligands or pathogen-associated molecular patterns (PAMPs). TLRs are the most important class of pattern recognition receptors (PRRs). These transmembrane proteins present a distinctive Leucine-Rich Repeat extracellular domain that confers specificity to their ligands[16], and a cytoplasmic signaling domain homologous to that of the interleukin 1 receptor (IL-1R), termed the toll/IL-1R homology domain[17]. Up to now, the TLR family consists of ten (TLR1-TLR10) and twelve (TLR1-TLR9 and TLR11-TLR13) members identified in humans and mice respectively[18]. These receptors are expressed in various immune cells, including macrophages, Dendritic cells (DCs), B cells, specific types of T cells, and even in non-immune cells such as fibroblasts and epithelial cells; their activation leads to the induction of inflammatory cytokines, chemokines, antigen-presenting molecules, and costimulatory molecules[15,17,19].
Epithelial cells from the gastric mucosa are considered as the first line of innate immunity against gastrointestinal pathogens, including H. pylori infection, and the PRRs have shown a wide range of expression in normal and pathological tissue (Table 1).
| PRR | Organ | Model | Method | Ligand | Observation | Reference |
| TLR1/2 | Esophagus | Esophageal carcinoma and premalignant lesions | IHQ | Triacyl lipopeptide | Receptor upregulation in tumor and dysplasia | [22,171] |
| TLR2 | Stomach; esophagus | Human ADC and premalignant lesions; H. pylori infection mice model andin vitro culture | IHQ; RT-qPCR | Microbial lipopeptide | Receptor upregulation in tumor cores. Increased tumorigenesis; constitutive expression in TE-1 cell line | [21,22,27,32,172-174] |
| TLR3 | Stomach; esophagus | Human gastric and esophageal carcinoma | IHQ; RT-qPCR | dsRNA | Increased receptor levels correlate with poor prognosis; increased expression on EAC-derived cell lines | [25,174-176] |
| TLR4 | Stomach; esophagus | Human ADC and premalignant lesions; H. pylori infection; esophageal carcinoma | IHQ; RT-qPCR | LPS | Upregulation in tumor cores; weak association with clinicopathologic variables; high expression correlates with poor prognosis; upregulation of IL-8 and COX-2 in BE | [21,24,25,27,32,171,173-179,182] |
| TLR5 | Stomach; esophagus | Human ADC and premalignant lesions; H pylori infection | IHQ | Flagellin | Highly expressed; upregulation in tumor and older patients; association with necrosis and tumor growth in the stomach; overexpression in dysplastic lesions of BE; no association with EAC prognosis | [26,27,32,177] |
| TLR6 | Esophagus | Esophageal carcinoma and human dysplasia | IHQ; IF | Diacyl lipopeptide | Upregulated in tumor tissue | [22,171] |
| TLR7 | Stomach; esophagus | Human ADC and normal tissue | IHQ; WB; RT-qPCR | ssRNA | Downregulated in gastric tumors; high levels correlate with a better outcome in GC; constitutive expression in TE-1 cell line; association between expression and tumor grade in ESCC | [22,32,173,174,176,180,183] |
| TLR9 | Stomach; esophagus | Human ADC | IHQ; RT-qPCR | ssDNA; dsDNA | Upregulated in early tumors; correlation with better prognosis in GC; association with histopathological grade in ESCC and dysplasia; high expression in EAC correlates with advanced tumor stage and metastasis | [24,25,32-34,173,175,176,181,184] |
| TLR10 | Stomach | Human biopsy | RT-qPCR | ssDNA; dsDNA | Upregulated by H. pylori | [35] |
Human gastric epithelial cells and tumor cells were found to express both TLR2 and TLR4 and both receptors are described as responsible for the H. pylori lipopolysaccharides (LPS) recognition. However, the results are contradictory and have not accurately probed the role of those receptors due both to the diversity of the host’s immune system and the pathogenicity of the H. pylori strain[20,21]. TLR2 is the most extensively expressed gene among all the TLRs in gastric tumors and high levels of TLR4 are associated with a higher risk of GC[22-24].
On the other hand, TLR3 and TLR4 have been implicated in several disorders related to the gastroesophageal reflux disease spectrum and largely documented, including the expression of both receptors and expression of their downstream products, such as cyclooxygenase-2, IL-8, nuclear factor-κB (NF-κB), and nitric oxide in human tissue samples and ex vivo cell cultures from the esophagus, the esophageal-gastric junction and the stomach[25].
TLR5 is expressed within the esophageal epithelium and has been shown to increase in a stepwise manner with progression from normal to dysplastic and eventually neoplastic states[26]. In addition, it is well documented that TLR5 is present in both primary gastric epithelial cells and gastric tumor cell lines[22,24,27]; however, the role of TLR5 during the gastric precancerous cascade is not yet clear.
TLR5 is responsible for flagellin recognition. H. pylori flagellin seems to be a less potent stimulator compared with other flagellins[28] but has a significant role in long-term bacterial persistence. The lack of TLR5 activity in response to H. pylori flagellin is caused mainly by the amino acid residues variation R89, L93, and E114 described as hotspots for binding TLR5 which, replaced with threonine (R89T), lysine (L93K), and aspartate (E114D) in H. pylori flagellin, lead to receptor evasion[29]. TLR5 instead recognizes CagL and CagY, two proteins from the type IV secretion system (T4SS) of H. pylori, and both have immunoregulatory properties[30,31]. A high TLR5 expression has been suggested to have a better prognosis amongst young GC patients in an early stage of disease, and this better outcome may be associated with a non-distant metastasis and an intestinal-type cancer[32].
TLR9, the only TLR with both anti- and pro-inflammatory roles, is involved in the recognition of H. pylori DNA, and the promotion or suppression role of TLR9 will depend on the gastric environment[22]. TLR9 expression has been shown to be up-regulated in H. pylori infected gastric tissue compared with non-infected tissue, and it was not related to the presence of tumor cells, suggesting that increased TLR9 expression was specifically associated with H. pylori infection[33]. It is reported that TLR9 interaction with H. pylori and H. pylori DNA, triggers an IL-8 secretion response mediated by the NF-κB pathway[34].
The role of TLR9 in cancer is not absolutely clear, but patients with stage II of GC and a high TLR9 expression had a better prognosis than cases with lower levels[32].
Other TLRs have been described, TLR1, TLR7, TLR8, and TLR10, but further studies are required in order to understand their role in GC and in H. pylori infected individuals, as well as other pathogens[22,23,35]. However, high levels of TLR10 expression have been observed in gastric biopsy samples from subjects with H. pylori and, when NCI-N87 gastric cells were co-cultured with the bacteria, both TLR10 and TLR2 mRNA levels were upregulated[35]. Those results suggest that TLR10 is a functional receptor and that TLR2/TLR10 heterodimer functions in H. pylori LPS recognition[35].
From a cellular perspective, neutrophils are the most abundant white blood cells in human blood and also considered as part of the first line of defense against infections by pathogens[36]. These cells have the ability to capture and destroy invading microorganisms and participate as mediators of inflammation. Phagocytosis and formation of neutrophil extracellular traps are part of the cellular mechanisms for pathogen elimination, as well as granules releasing[36-38].
Neutrophils extracellular traps (NETs) formation, known as NETosis is a process of releasing extracellular web-like structures, and is described as a coat consisting of decondensed chromatin filaments, histones and antimicrobial proteins[39]. NETosis is a mechanism of innate immunity to contain and prevent microbial spread, and eliminate bacteria[40].
H. pylori can activate different cells of innate immunity, including neutrophils, and these activated cells recognize H. pylori infection through different receptors, such as TLR2, TLR4, and TLR9[34,41,42]. TLR5 has not been detected in neutrophils localized in the lamina propria during H. pylori gastritis[24]. The activation and recruitment of neutrophils is stimulated by H. pylori neutrophil-activating protein, or HP-NAP[37,43]. TLR2 interacts with HP-NAP for the secretion of IL-8[41]. However, the interaction between neutrophils and H. pylori appears to be complex and contradictory and shows the development of different mechanisms of immune evasion, including NET degradation, the increase in bacterial resistance mediated by the modification of proteins or surface polysaccharides, or the suppression of NET formation[44].
H. pylori has shown a selective alteration of neutrophils function mediated by the inactivation of NADPH Oxidase and superoxide release[45]. In addition, the bacterium performs lipid A modification mediated by lipid A phosphatases to resist the polymyxin, an antimicrobial peptide[46]. Another study remarked on the presence of an outer membrane-associated nuclease that can degrade extracellular DNA, where the ability to degrade exogenous DNA was originally proposed as a purine source uptake mechanism[47], but it could also have the potential role of degrading NETs[48].
Although NETosis was described as an antimicrobial process, it has been described in other pathologies, including cancer. The first study that provided evidence on NET in cancer was Berger-Achituv et al[49] studying the Ewing sarcoma. The authors proposed NET as a pro-tumor effect and the possibility of using this parameter as a poor prognostic biomarker[49].
Regarding GC, the first study was reported by Yang et al[50]. The authors found the correlation of NET formation with TNM status and a significant increase in the formation of fibrin and thrombin, however, the focus was on peripheral circulation. More recently, the formation of NETs within the gastric tumor microenvironment including immunofluorescent staining of Neutrophil Elastase (NE) and citrullinated-histone 3[51,52] showed that NETs are more abundant in the tumor core than in the adjacent non-tumor tissue[51,52], and the plasma from GC patients revealed the capacity of NET formation in vitro[52]. NETs measured in peripheral blood have been shown to be significantly correlated with GC and staging, and its levels decrease after surgery[50-52].
A very recent report showed that abdominal infectious complications after gastrectomy would stimulate neutrophils to release NETs both in peripheral blood and the abdominal cavity, facilitating GC metastasis in vitro and in vivo dependent on transforming growth factor (TGF)-β signaling[53]. The formation of NETs has an important role in the epithelial-mesenchymal transition and gastric tumor progression, because NETosis may induce proliferation, invasion, migration, and a mesenchymal phenotype, in addition to its immune role, which makes it difficult to be therapeutically targeted.
Additionally, the macrophages have been demonstrated to be important cells for the innate immune system in healthy and tumor tissue. Within the tumor microenvironment (TME), macrophages are known as tumor-associated macrophages (TAMs) and play a key role in the recognition and clearance of foreign and damaged cells, as well as in tumor development and the response to several cancer therapies.
Macrophages can infiltrate solid tumors modulating T cell activity within the TME, and often undergo phenotype polarization in response to stimuli or inhibitory factors, either to pro-inflammatory (M1) or anti-inflammatory (M2) subtypes, which cause immune response or immune escape of the tumors respectively[54-57]. The general consensus is that TAMs are usually pro-tumorigenic. These cells are recruited by tumor-derived chemokines and produce low levels of inflammatory cytokines, promote Th2-T cell response, favor wound healing, and increase angiogenesis and metastases[55,56].
IL-6 and tumor necrosis factor (TNF)-α are both pro-inflammatory cytokines, exerting pro-tumoral functions, including the promotion of angiogenesis and metastasis, and these molecules can be secreted by myeloid cells and leukocytes under different conditions and stimuli[54-56]. In parallel to classic immunomodulators such as cytokines and chemokines, some microRNAs (miR) have shown a significant impact on macrophages activity[57]. miR-125b, miR-127, miR-155, miR-181 and miR-451 are significantly upregulated in M1 macrophages, whereas miR-125a-5p, miR-146a, miR-145-5p, miR-143-3p are highly expressed in M2 macrophages[58,59].
miR-155 directly targets the expression of the IL-13 receptor α1, thereby inhibiting STAT6 activation and promoting M1 polarization[59]. miR-155 knockdown in myeloid cells induces faster tumor growth, reduction of M1-macrophages and enrichment of pro-tumor cytokines within the TME[60]. In addition, miR-125b overexpression enhanced responsiveness to interferon (IFN)-γ, through the targeting of IRF4 and increased expression of pro-inflammatory cytokines[58,61].
miR-187, miR-146a, let-7e, and miR-92a are considered anti-inflammatory miRs because they downregulate IL-6 and TNF-α in human macrophages by targeting the TLRs signaling[57,62]. The NF-κB-dependent miR-146a expression is induced in monocytes and macrophages upon triggering of TLR4 to act as a modulator of the inflammatory response[61]. miR-155 is key to modulating genes related to M2/pro-Th2 phenotype in macrophages, and includes CCL18, SERPINE, CD23 and DC-SIGN[56]. In addition, other microRNAs will modulate directly or indirectly the NF-κB or TLR activity, such as miR9, miR-21, miR-29b, and those can be expressed and released by both TAMs and solid tumors in exosomes[57,62-65]. Due to the inflammatory microenvironment and oncogenic mutations, a significant number of human cancers have constitutive miRs deregulation affecting NF-κB activity, cytokine production and hallmarks of cancer such as apoptosis, proliferation and tumor survival[57,62,65].
Previous studies demonstrated that Gastric Epithelial Cells (GECs) function as antigen presenting cells by constitutively expressing MHC class II[66]. Interestingly, H. pylori infection induces up-regulation of costimulatory molecules (CD86 and CD80) among GECs[66], suggesting its potential to be used as a local bridge between innate and acquired immunity; however, the capacity to play a role secreting cytokines and polarizing macrophages requires further studies.
The inhibition of the polarization of pro-inflammatory macrophages can accelerate the development of precancerous lesions in GC[67]. In addition, when TAMs spread in the peritoneum of GC patients, these cells normally are polarized to an anti-inflammatory subtype (M2), which can promote the growth and progression of GC when the tumor exists[68]. In fact, high densities of TAMs are associated with poor survival in GC patients[68,69].
Currently, there is enough evidence based on epidemiological, molecular and pathological studies that persistent infection with H. pylori is a risk factor for the development of gastric adenocarcinoma[70-73], estimating an increment in the relative risk by 3–6 times in infected people which might represent over 80% of all distal GC cases and some with proximal gastric tumors[1,74,75]. The prevalence of H. pylori infection is extraordinarily high, infecting 50% of the world’s population[1,76-78].
H. pylori is a Gram-negative bacterium which colonizes the human stomach and promotes a full immune response locally and sustainably[19,79,80]. Strains of H. pylori are grouped into two broad families tentatively named type I and type II, which are based on whether they express or not the vacuolating cytotoxin (VacA) and the CagA antigen (cytotoxin-associated gene A)[81].
One of the major determinants of H. pylori’s virulence is the cag pathogenicity island (cag PAI)[82-84]. This cagPAI is a 40-kb DNA region surrounding the cagA gene that contains about 27-31 genes that encode a bacterial type IV secretion system acting as a syringe-like structure, which allows for the delivery of bacterial effector molecules into host gastric epithelial cells[85]. During infection with H. pylori, CagA is translocated into epithelial cells, and it is tyrosine-phosphorylated in the EPIYA motifs by the proto-oncogene tyrosine-protein kinase Src and the members of the Abelson family of non-receptor tyrosine kinases[86,87], which results in the interaction with various intracellular signaling pathways, triggering changes in the cytoskeleton, in the morphology and in the mobility of the host cells[86,87].
Compared with cagA– strains, H. pylori cagA+ strains significantly increase the risk of developing severe gastritis, atrophic gastritis, peptic ulcer disease and distal GC. In fact, people infected with cagA+ strains have higher degrees of gastric inflammation and epithelial cell damage than people from whom cagA- strains have been isolated[88]. People infected with cagA+ H. pylori strains have an enhanced expression of IL-1α, IL-1β, and IL-8 in gastric biopsies compared to uninfected persons or patients infected with cagA- strains[89]. Keeping that consideration in mind, proinflammatory cytokines will be up-regulated by a local infection; however, the magnitude of that systemic response and the profile of released cytokines and chemokines will depend also on host factors.
Regarding host factors, many polymorphisms in genes related to inflammation and innate immunity, such as cytokines and MHC molecules, have been reported to be associated with an increased risk of GC[90-92]. Among the cytokines with polymorphisms that have been associated with GC are IL-1β, IL-1Rβ, IL-4, IL-6, IL-10, TNF-α, and TNF-β[93-97]. Based on ethnic backgrounds, two sets of haplotypes for IL-1β and IL-10 have been related to increased risk for GC[93-95]; specifically, IL-1β-1464G/-511C/-31T and IL-10-1082G/-819C/952C for Asians and IL-1β-1464C/-511T/-31C and IL-10-1082A/-819T/952T for Caucasians[95].
Canedo et al[96] found that IFN-γ receptor 1 -56C/T polymorphism is a relevant host susceptibility factor for GC development associated with H. pylori infection. Polymorphism in the promoter region of the gene coding for IL-10 and TGF-β has been also described in the Mexican population in relation to susceptibility to GC[97]. There is a need for extended studies in different populations and in larger patient groups, particularly in regions of Latin America where the burden of GC is more severe. In recent years, NF-κB has been widely studied in inflammation, immunity and cancer, but its roles are still unclear[98-100]. NF-κB is a master transcription factor activated downstream of the TLR and cytokines such as TNF-α and IL-1β[98]. In contrast to the canonical NF-κB pathway, the noncanonical NF-κB activation responds to specific stimuli, including ligands from the TNFR superfamily members such as LTβR, BAFFR, CD40 and RANK[99].
In GC, H. pylori is associated with increased expression of the proinflammatory NF-κB[101-104]. It has been shown that the induction of NF-κB mediated by H. pylori induces the expression of activation-induced cytidine deaminase, which has been demonstrated to induce nucleotide modifications in the TP53 gene in gastric cell models[105] and suggests that the accumulation of those TP53 gene alterations might contribute to the development of gastric neoplasia.
As an example, in vitro studies showed that programmed death (PD)-1 is increased among gastric epithelial cells after H. pylori infection and its immunosuppressive functions on T-cells may contribute to carcinogenesis[106]. Evidence from small studies observed an up-regulation of PD-1 and programmed cell death-ligand 1 (PD-L1) in human H. pylori-related gastric carcinoma[107].
In addition, H. pylori has shown other effects within the gastrointestinal epithelium not associated with inflammation but compromising the genome stability, for example, reduced levels of transcripts for DNA mismatch repair (MMR) proteins such as MutS, MutL RAD51, FEN1, POLD1, and LIG1[108-110], and this phenotype might be more severe in cases cagA+ H. pylori[110]. However, the deficiency MMR in gastric tumors was recently shown to predict clinical response to pembrolizumab[111], demonstrating also the expansion of antigen-specific T cells reactive to tumor-derived neoantigens, suggesting that further studies are required to understand the interaction between pathogens and genomic features as biomarkers.
The EBV is a ubiquitous virus and member of the subfamily of human Gammaherpesvirinae[112] and can infect several cell types, including B-lymphocytes, epithelial cells, and fibroblasts[113]. EBV is the main pathogenic factor for nasopharyngeal carcinoma. However, studies find that EBV infection is also associated with the development of T-cell lymphoma and EBV-associated GC[114]. Although the infection rate of EBV is extraordinarily high, reaching over 90% of the adult population worldwide[115,116], the incidence rate of EBV-positive GC remains low, representing around 9% of characterized stomach adenocarcinomas[117], these EBV-positive tumors display recurrent PIK3CA mutations, extreme DNA hypermethylation, and amplification of JAK2 and both PD-L1 and PD-L2[5].
The EBV-positive GC has been characterized by an increased expression of PD-L1, and a sustained immune-infiltration, which is indicative of the presence of stable T-cells and supports the use of an immune checkpoint inhibitor for the treatment of this GC subtype[118,119]. In addition, most EBV-positive GCs show MSI which has also been associated with inflammation and local immune activation[120-123]. Previous groups have shown that high density of intra-tumoral or stromal CD8+ T cells with a high percentage of PD-L1 expression seems to be associated with a worse progression-free survival and overall survival[118,120,124]. However, a recent study demonstrated that EBV-positive GC patients treated with immunotherapy showed favorable responses[125], suggesting that viral status represents a potential predictive biomarker for using immune-checkpoint inhibitors; however, the balance between pro-inflammatory and immunosuppressive signals together with a concomitant viral infection requires more studies to clarify its role as a biomarker.
Type I and type II IFN are central to both combating virus infection and modulating the antiviral immune response[126]. The cytokine IFN-γ is mainly produced by T Cell CD4+ and natural killers to activate macrophages. The ligation to its receptor triggers an activation of the Janus-Activated kinases, JAK1 and JAK2, and subsequently the activation of STAT1 and interferon regulatory factor 1 (IRF1). STAT1 and IRF1 are activated by phosphorylation and translocated to the nucleus to regulate the IFN-γ gene expression[127].
Crucial to the induction of type I IFN is the recognition of viral PAMPs by PRRs, among which, the cyclic GMP-AMP synthase-stimulator of interferon genes modulates the antiviral response triggered by DNA viruses and retroviruses[128]. In addition, most viruses, including EBV, stimulate innate immune response during primary infection predominantly by activating the expression of TLRs, such as TLR2, TLR3, TLR4, TLR7, TLR8, and TLR9[129]. TLR2 is likely activated by EBV surface glycoprotein gp350[130] and the nonstructural protein dUTPase[131], while EBV-encoded small RNAs released from EBV-infected cells are detected by TLR3[132]. Furthermore, EBV can activate monocytes and plasmacytoid DCs through cooperative action of TLR9 and TLR2[133].
Activation of PRRs by EBV-PAMPs triggers JAK-STAT-mediated IFN response and different branches of innate immune signaling including NF-κB pathway; inflammasome activation; and programmed cell death such as apoptosis and necroptosis[134]. However, innate immunity is a double-edged sword as the induction of pro-inflammatory responses and activation of programmed cell death might release a burst of virions and may therefore facilitate the spread of infection[135].
Recently, IFN-γ has been the subject of studies due to its immune role in cancer development, especially in GC. There are several signaling pathways of innate immunity during GC development in which interferon is involved, such as in proliferation, metastasis, and advancement of GC through the upregulation of integrin β3-mediated NF-κB signaling[136]. Serum levels of IFN-γ are elevated in GC which may promote systemic and local responses[137] at the same time, for example: peroxisome proliferator-activated receptor delta, a ligand involved in physiologic processes in cell metabolism, proliferation, and inflammation[138] together with IFN-γ signaling creates an inflammatory tumor-promoting microenvironment enabling villin-expressing gastric progenitor cells transformation and gastric tumorigenesis[139]. Furthermore, natural killer (NK) cells play a role in innate immunity against cancer cells. Lee et al[140] reported that IFN-γ produced by the activated NK decreases in GC patients compared with healthy donors. This low level of IFN-γ-NK could be used as a non-invasive biomarker for carcinogenesis in GC[140].
Some reports including Latin American cohorts have shown that concentrations of IFN-γ and IL-10 are significantly higher in GC than in non-oncological cases, and within the GC group, IFN-γ levels are increased at the early stages (I/II) and remain higher in late stage (IV)[141]. Interestingly, increased levels of viral capsid antigen antibodies are significantly associated with elevated serum levels of IFN-γ, particularly in the intestinal type of GC[142]. Therefore, IFN-γ is suggested as a biomarker for assessing GC risk; however, this molecule is known to mediate gastric damage or immune antipathogen responses, as well as the expression of some negative immune checkpoint molecules.
The PD-L1 expression is activated by several cytokines, of which IFN-γ is the strongest[143]. In a melanoma cell line model, PD-L1 has shown to be mainly regulated by the type II interferon receptor signaling pathway through JAK1 and JAK2, several STATs including STAT1/STAT2/STAT3, to converge on the binding of IRF1 to the PD-L1 promoter[144]. Later, Chen et al[145] treated with IFN-γ thirty-four cultured human tumor lines, including 18 melanomas (MEL), 12 renal cell carcinomas (RCC), 3 squamous cell carcinomas of the head and neck (SCCHN), and 1 non-small-cell lung carcinoma, and as wildtype control the authors considered isolated peripheral blood monocytes. The results indicated that PD-L1 was constitutively expressed on 1/17 cultured MELs, 8/11 RCCs, 3/3 SCCHNs, and on monocytes; however, the inhibition of STAT1 but not STAT3 was more critical to reduce IFN-γ-induced PD-L1 protein expression on tumor cells[145]. Other authors have provided evidence of a crosstalk between JAK2-STAT1 and PI3K-AKT pathways in response to IFN-γ in lung adenocarcinoma[146]. Transcriptome analysis demonstrated that tumor tissues expressing IFN-γ display gene expression associated with suppressed cell cycle progression and expansion, which was not observed in PD-L1 negative tumors. In lung adenocarcinoma cells, IFN-γ induces the activation of JAK2-STAT1 and PI3K-AKT pathways, showing that the activation of JAK2-STAT1 is responsible for the anti-proliferative effect of IFN-γ, and the inhibition of PI3K downregulates PD-L1 expression and enhances the anti-proliferative effect of IFN-γ[146]. In addition to the cytokine regulation, a lncRNA (long non-coding RNA) named Interferon-stimulated non-coding RNA 1 (INCR1) has been described as a major regulator of IFN-γ signaling in tumors by post-transcriptional modulation of PD-L1 and JAK2 expression[147]. INCR1 is expressed as an antisense RNA from the PD-L1/PD-L2 locus and has been detected in human samples across multiple tumor types, and its levels increase after IFN-γ stimulation, correlating with PD-L1 but not PD-L2 expression[147].
Regarding GC, PD-L1 has shown a wide and very variable range of expression based on technique and cutoff, however, it seems to be absent in non-tumor gastric tissue[148-151]. Imai et al[152] showed IFN-γ treatments enhanced the expression of intracellular and membranous PD-L1 expression in GC cell lines. This upregulation of PD-L1 induced by IFN-γ was associated with the JAK-STAT but not the MAPK and PI3K-AKT pathway activation[152,153]. PD-L1 overexpression mediated by IFN-γ is also seen in GC with positive EBV[154]. Polymorphism in PD-L1 related to GC has also been described. PD-L1rs2297136 was positively correlated with a higher proportion of PD-L1 protein and could be employed as a tool of prognosis in GC patients[155,156].
A recent study suggested the role of ISG12a as a tumor suppressor in gastrointestinal tissue[157,158]. ISG12 or interferon alpha-inducible protein 27 promotes β-catenin proteasomal degradation by inhibiting the degradation of ubiquitinated Axin, thereby suppressing the canonical Wnt/β-catenin signaling pathway[158]. Reduced levels of ISG12a were observed in gastrointestinal cancer, such as hepatocellular cancer and GC, and it was associated with an immune-suppressive tumor microenvironment. The authors argue that β-catenin is a transcription factor for PD-L1, and the inhibition of the Wnt/β-catenin signaling by ISG12a makes tumor cells more sensitive to NK cell-mediated killing[157]. Therefore, the balance between the induction or suppression of IFN-stimulated genes[159], such as ISG12a, may accelerate the malignant transformation of cancer cells and lead to a poor prognosis in gastrointestinal cancer[157,160].
GC is currently accepted as the consequence of a multifactorial process, involving pathogen infection and the virulence of some strains (as H. pylori), environmental factors, dietary habits, and host intrinsic factors, as we have discussed above; however, socioeconomic factors, such as education level and occupation, have shown to be determinant during the progression of the premalignant cascade of GC and the patient’s outcome after the cancer onset[161-163].
Asia accounts for 71% of GC’s worldwide, in which China’s incidence is 44.1%. The high incidence in China is marked by the rural population, and their exposure to carcinogens through diet, the environment and the H. pylori infection per se[164]. The incidence of GC in Europe is heterogeneous; while the highest incidence is in Central and Eastern Europe, the lowest incidence is in Western and Northern Europe, which correlates with a higher detection of H. pylori in Eastern Europe compared to Western Europe[165], as well as an observed higher consumption of red and processed meat resulting in an increased risk of GC[166].
After Europe, Latin American countries have shown a high incidence of gastric malignancies. The associated risk factors are infection with H. pylori, diet and habits such as smoking, consumption of salt, alcohol and meat, as well as ethnicity and age[167]. Some authors have suggested that Latin America has a close correlation between GC incidence and altitude which is based on the presence of a mountainous geography, such as the Sierra Madre, Cordillera de Centroamerica and the Cordillera de los Andes[168]. However, the mortality and incidence rates for gastric malignancies in the Chilean population is statistically higher than the average rates in the rest of Latin America, becoming the second cause of death from cancer in Chile, affecting 11.6 per 100000 inhabitants and causing around 3000 deaths per year[1]. That incidence seems to correlate mainly with socioeconomic status, Mapuche ethnicity, and age at the primary H. pylori infection[169].
Surprisingly, most of the countries with the highest incidence of GC are not those with low incomes (Table 2). In fact, it seems that the vicious circle between precancerous lesions, inflammation and GC onset is caused by the low level of education within the population. A study performed within the Swedish population, that considered the economically active population, showed an increased risk factor of GC in workers engaged in manual-labor occupations and in industry. The statistics were standardized for categories of occupation and adjusted by age, period and region, and confirmed that overall manual-workers and farmers had the highest risk of GC, including male miners and quarry workers[162]. The European Prospective Investigation into Cancer and Nutrition cohort included about 520000 participants mostly aged 35–70 years and, after an average follow-up of 6.5 years, reported 268 cases with adenocarcinoma of the stomach. Higher education was significantly associated with a reduced risk of GC with a hazard ratio (HR) of 0.64 (95%CI: 0.43–0.98) and, as was expected from other reports, that effect was more pronounced for cancer of the cardia (HR: 0.42) as compared to non-cardia GC (HR: 0.66)[163].
| Country | Estimated cases | Crude rate | ASR, world1 | Cum. risk | HDI classification |
| Japan | 138470 | 109.5 | 31.6 | 9.35 | Very high |
| Korea | 28713 | 56 | 27.9 | 6.51 | Very high |
| Brunei Darussalam | 55 | 12.6 | 13.5 | 5.93 | Very high |
| Russia | 37364 | 25.6 | 13.5 | 3.29 | Very high |
| Chile | 4208 | 22 | 13.1 | 4.17 | Very high |
| Lithuania | 864 | 31.7 | 13 | 3.4 | Very high |
| Estonia | 379 | 28.6 | 12.3 | 3.2 | Very high |
| Latvia | 530 | 28.1 | 12 | 3.01 | Very high |
| Portugal | 2950 | 28.9 | 11 | 2.99 | Very high |
| Slovakia | 1210 | 22.2 | 10.7 | 3.12 | Very high |
| Mongolia | 860 | 26.2 | 32.5 | 7.71 | High |
| China | 478508 | 33.1 | 20.6 | 5.24 | High |
| Iran | 14656 | 17.4 | 17.5 | 6.58 | High |
| Kazakhstan | 3357 | 17.9 | 15.8 | 3.47 | High |
| Belarus | 2739 | 29 | 15.4 | 3.5 | High |
| Peru | 6300 | 19.1 | 15.2 | 5.16 | High |
| Colombia | 8214 | 16.1 | 12.8 | 3.61 | High |
| Costa Rica | 952 | 18.7 | 12.8 | 4.12 | High |
| Samoa | 20 | 10.1 | 12.8 | 3.75 | High |
| Azerbaijan | 1453 | 14.3 | 12.7 | 3.42 | High |
| Tajikistan | 1301 | 13.6 | 23.4 | 6.96 | Medium |
| Kyrgyzstan | 1027 | 15.7 | 19.7 | 5.01 | Medium |
| Cabo Verde | 82 | 14.7 | 18.4 | 5.88 | Medium |
| Bhutan | 118 | 15.3 | 17.7 | 3.88 | Medium |
| Viet Nam | 17906 | 18.4 | 15.5 | 3.57 | Medium |
| Sao Tome and Principe | 18 | 8.2 | 14.7 | 2.06 | Medium |
| Myanmar | 7235 | 13.3 | 13.7 | 3.58 | Medium |
| Lao | 675 | 9.3 | 12.9 | 3.17 | Medium |
| Guatemala | 1637 | 9.1 | 12.2 | 3.93 | Medium |
| Turkmenistan | 583 | 9.7 | 11.8 | 2.37 | Medium |
| Haiti | 1184 | 10.4 | 13.5 | 4.58 | Low |
| Mali | 1097 | 5.4 | 12.8 | 2.96 | Low |
| Afghanistan | 2149 | 5.5 | 12.4 | 3.18 | Low |
| Zimbabwe | 641 | 4.3 | 9.4 | 3.24 | Low |
| Papua New Guinea | 474 | 5.3 | 9.2 | 3.17 | Low |
| Rwanda | 587 | 4.5 | 8.1 | 1.61 | Low |
| Yemen | 966 | 3.2 | 7.1 | 2.68 | Low |
| Senegal | 597 | 3.6 | 7 | 1.66 | Low |
| Benin | 429 | 3.5 | 7 | 2.29 | Low |
| Mauritania | 143 | 3.1 | 5.6 | 1.46 | Low |
A survey to address GC risk factors and endoscopic screening within the North American population showed that ethnicity, cultural habits and immigration patterns are potentially useful to identify high-risk persons from multicultural areas within the United States[170]. The authors identified that dietary habits during teenage years (15-18) and education below high school level may represent signs of risk for GC in older people of foreign birth[170]. Most recently, based on the previous research, a secondary analysis showed that education level was the single most reliable measure of GC risk among three variables of socioeconomic status including, education, income, and occupation, which are the most commonly used for health outcomes such as cancer survival[171]. Similar results were observed in a seroepidemiologic study in Japan where the H. pylori positive rate increased at 1% per year for people born after 1950 but was comparatively constant for people with birth dates before 1950[171]. Based on the authors, the apparent decreased prevalence of H. pylori post-war was accompanied by the Westernization of the country and subsequently by a reduction in the frequency of atrophic gastritis and the incidence of gastric carcinoma during the most modern times[171,172].
The infection status in adults is considered to be influenced by socioeconomic status in childhood; however, given the massive improvement in hygiene and the economic environment around the world, it has contributed to the variation in the trends in incidence and death rates of GC among the countries mentioned above. Based on other authors, the education in H. pylori eradication and gastric malignancies is largely due to unplanned prevention caused by the widespread adoption of technology and improved manufacturing practices of the food industry. In a similar way, the prevalence of H. pylori infection may be reduced owing to improvements in sanitary and housing conditions based on education at early ages by primary schools and in adult life by primary health workers.
Where the intersection between education and immunity is not evident, by intuition a limited knowledge regarding gastrointestinal health and eradication of H. pylori infection might dramatically influence the development of GC. Therefore, the inflammatory process induced by a pathogen or even an incipient neoplasm may not receive enough attention, progressing finally to an advanced disease with limited therapeutic opportunities and uncertain outcome for the patient.
During the transition from premalignant lesions of the gastric epithelium to the final carcinoma, some microorganisms will play a determinant role promoting the neoplastic transformation or contributing with particular tumor phenotype and its heterogeneity, together with different mutagenic agents and genomic aberrations. This review provides an overview on innate immune activation in the context of gastrointestinal malignancies and compiles studies related to TLRs, neutrophils, cytokines and pathogens, as crucial players during the precancerous cascade and the cancer onset, allowing the correlation of those aspects with clinical and socioeconomic variables.
As shown in the graphical abstract, intersections between innate immune response and GC development is driven by common mediators, such as NF-κB and IFN-γ molecular pathways, cellular processes (neutrophil and myeloid cells activation), and activators/inducers (PAMP, damage-associated molecular patterns, tumor-antigens). However, universal risk factors are identified affecting globally any human being which, depending on extrinsic and intrinsic host factors, might facilitate the progression of the precancerous cascade to GC.
Modern therapies such as adoptive cell therapy, vaccines, and especially immunotherapy using checkpoint inhibitors, which have not been mentioned in this work, have shown in clinical trials the role of restoring the balance in favor of the immune system against GC. In the words of Dunn et al[173] the observed benefits in GC may be based on (1) Elimination: NK cells and T lymphocytes (helper and cytotoxic) secrete interferon IFN-γ leading to a reduction of angiogenesis and proliferation of cancerous cells; moreover, macrophages and DC secrete cytokines that activate immune cells to phagocytize dead tumor cells; (2) Equilibrium: Residual cancerous cells remain in a dormant state because DC and cytotoxic T cells secrete IFN-γ and inhibitory cytokines (IL-12) suppress them; and (3) Escape: Tumor cells change their features, up-regulating immune checkpoint pathways, which will be transmitted to the daughter cells, therefore escaping immunosuppression and proliferating, along with the apoptosis of the effector lymphocytes[173]. The success of those treatments relies on an efficient immune system, and of course an innate response able to promote priming and mobilization of newly activated immune cells; the presence of pathogens or their PAMPs, as well as molecules derived from the tumor itself will continuously modulate the tumor immune microenvironment. The shape of the tumor will also depend on the host background (genome stability, driver mutations and other gene variants) but in addition, the external environment shaped by the socioeconomic status of each person will determine the final outcome.
Innate immunity is the beginning, and certainly, will be part of the final response against tumors. All these observations require further investigation but it is clear that pathogens like H. pylori and EBV need to be assessed in GC based on their role as biomarkers and as potential mechanisms of resistance to therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: European Society for Medical Oncology, No. 529044.
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Chile
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin C, China; Kanaoujiya R, India; Luo W, China; Zhao Q, China S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 44230] [Article Influence: 14743.3] [Reference Citation Analysis (47)] |
| 2. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 432] [Cited by in F6Publishing: 682] [Article Influence: 170.5] [Reference Citation Analysis (1)] |
| 3. | Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1317] [Cited by in F6Publishing: 1298] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 4. | Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4230] [Cited by in F6Publishing: 4333] [Article Influence: 433.3] [Reference Citation Analysis (2)] |
| 6. | Jin G, Lv J, Yang M, Wang M, Zhu M, Wang T, Yan C, Yu C, Ding Y, Li G, Ren C, Ni J, Zhang R, Guo Y, Bian Z, Zheng Y, Zhang N, Jiang Y, Chen J, Wang Y, Xu D, Zheng H, Yang L, Chen Y, Walters R, Millwood IY, Dai J, Ma H, Chen K, Chen Z, Hu Z, Wei Q, Shen H, Li L. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020;21:1378-1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 7. | Röcken C. Molecular classification of gastric cancer. Expert Rev Mol Diagn. 2017;17:293-301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Carneiro F, Lauwers G. Epithelial tumours of the stomach. In: Shepherd NA, Warren BF, Williams GT, Greenson JK, Lauwers GY, Novelli MR, editors. Morson and Dawson's Gastrointestinal Pathology. Wiley Online Library, 2013: 180-222. [DOI] [Cited in This Article: ] |
| 9. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1159] [Cited by in F6Publishing: 1163] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 10. | Soto D, Song C, McLaughlin-Drubin ME. Epigenetic Alterations in Human Papillomavirus-Associated Cancers. Viruses. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 12. | Hashemi Goradel N, Heidarzadeh S, Jahangiri S, Farhood B, Mortezaee K, Khanlarkhani N, Negahdari B. Fusobacterium nucleatum and colorectal cancer: A mechanistic overview. J Cell Physiol. 2019;234:2337-2344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Nagy KN, Sonkodi I, Szöke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304-308. [PubMed] [Cited in This Article: ] |
| 14. | Michaud DS. Role of bacterial infections in pancreatic cancer. Carcinogenesis. 2013;34:2193-2197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1953] [Article Influence: 130.2] [Reference Citation Analysis (0)] |
| 16. | Matsushima N, Miyashita H, Mikami T, Kuroki Y. A nested leucine rich repeat (LRR) domain: the precursor of LRRs is a ten or eleven residue motif. BMC Microbiol. 2010;10:235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8061] [Cited by in F6Publishing: 8325] [Article Influence: 462.5] [Reference Citation Analysis (0)] |
| 18. | Behzadi P, García-Perdomo HA, Karpiński TM. Toll-Like Receptors: General Molecular and Structural Biology. J Immunol Res. 2021;2021:9914854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 19. | Smith SM. Role of Toll-like receptors in Helicobacter pylori infection and immunity. World J Gastrointest Pathophysiol. 2014;5:133-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 66] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D'Elios MM, Del Prete G, de Bernard M. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Yokota S, Ohnishi T, Muroi M, Tanamoto K, Fujii N, Amano K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol Med Microbiol. 2007;51:140-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Melit LE, Mărginean CO, Mărginean CD, Mărginean MO. The Relationship between Toll-like Receptors and Helicobacter pylori-Related Gastropathies: Still a Controversial Topic. J Immunol Res. 2019;2019:8197048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Uno K, Kato K, Shimosegawa T. Novel role of toll-like receptors in Helicobacter pylori - induced gastric malignancy. World J Gastroenterol. 2014;20:5244-5251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 35] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Müller-Hermelink HK, Eck M. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Baghdadi J, Chaudhary N, Pei Z, Yang L. Microbiome, innate immunity, and esophageal adenocarcinoma. Clin Lab Med. 2014;34:721-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Helminen O, Huhta H, Takala H, Lehenkari PP, Saarnio J, Kauppila JH, Karttunen TJ. Increased Toll-like receptor 5 expression indicates esophageal columnar dysplasia. Virchows Arch. 2014;464:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Smith MF Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem. 2003;278:32552-32560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 28. | Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005;102:9247-9252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 29. | Kim JH, Namgung B, Jeon YJ, Song WS, Lee J, Kang SG, Yoon SI. Helicobacter pylori flagellin: TLR5 evasion and fusion-based conversion into a TLR5 agonist. Biochem Biophys Res Commun. 2018;505:872-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Pachathundikandi SK, Tegtmeyer N, Arnold IC, Lind J, Neddermann M, Falkeis-Veits C, Chattopadhyay S, Brönstrup M, Tegge W, Hong M, Sticht H, Vieth M, Müller A, Backert S. T4SS-dependent TLR5 activation by Helicobacter pylori infection. Nat Commun. 2019;10:5717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Tegtmeyer N, Neddermann M, Lind J, Pachathundikandi SK, Sharafutdinov I, Gutiérrez-Escobar AJ, Brönstrup M, Tegge W, Hong M, Rohde M, Delahay RM, Vieth M, Sticht H, Backert S. Toll-like Receptor 5 Activation by the CagY Repeat Domains of Helicobacter pylori. Cell Rep. 2020;32:108159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Kasurinen A, Hagström J, Laitinen A, Kokkola A, Böckelman C, Haglund C. Evaluation of toll-like receptors as prognostic biomarkers in gastric cancer: high tissue TLR5 predicts a better outcome. Sci Rep. 2019;9:12553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Tang K, McLeod L, Livis T, West AC, Dawson R, Yu L, Balic JJ, Chonwerawong M, Wray-McCann G, Oshima H, Oshima M, Deswaerte V, Ferrero RL, Jenkins BJ. Toll-like Receptor 9 Promotes Initiation of Gastric Tumorigenesis by Augmenting Inflammation and Cellular Proliferation. Cell Mol Gastroenterol Hepatol. 2022;14:567-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 34. | Alvarez-Arellano L, Cortés-Reynosa P, Sánchez-Zauco N, Salazar E, Torres J, Maldonado-Bernal C. TLR9 and NF-κB are partially involved in activation of human neutrophils by Helicobacter pylori and its purified DNA. PLoS One. 2014;9:e101342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Nagashima H, Iwatani S, Cruz M, Jiménez Abreu JA, Uchida T, Mahachai V, Vilaichone RK, Graham DY, Yamaoka Y. Toll-like Receptor 10 in Helicobacter pylori Infection. J Infect Dis. 2015;212:1666-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol. 2018;9:113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 458] [Cited by in F6Publishing: 696] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 37. | Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 517] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 38. | Stephan A, Fabri M. The NET, the trap and the pathogen: neutrophil extracellular traps in cutaneous immunity. Exp Dermatol. 2015;24:161-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Wang W, Zhang J, Zheng N, Li L, Wang X, Zeng Y. The role of neutrophil extracellular traps in cancer metastasis. Clin Transl Med. 2020;10:e126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5773] [Cited by in F6Publishing: 6452] [Article Influence: 322.6] [Reference Citation Analysis (0)] |
| 41. | Wen SH, Hong ZW, Chen CC, Chang HW, Fu HW. Helicobacter pylori Neutrophil-Activating Protein Directly Interacts with and Activates Toll-like Receptor 2 to Induce the Secretion of Interleukin-8 from Neutrophils and ATRA-Induced Differentiated HL-60 Cells. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Alvarez-Arellano L, Camorlinga-Ponce M, Maldonado-Bernal C, Torres J. Activation of human neutrophils with Helicobacter pylori and the role of Toll-like receptors 2 and 4 in the response. FEMS Immunol Med Microbiol. 2007;51:473-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Nishioka H, Baesso I, Semenzato G, Trentin L, Rappuoli R, Del Giudice G, Montecucco C. The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol. 2003;33:840-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Ríos-López AL, González GM, Hernández-Bello R, Sánchez-González A. Avoiding the trap: Mechanisms developed by pathogens to escape neutrophil extracellular traps. Microbiol Res. 2021;243:126644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658-3667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Tran AX, Whittimore JD, Wyrick PB, McGrath SC, Cotter RJ, Trent MS. The lipid A 1-phosphatase of Helicobacter pylori is required for resistance to the antimicrobial peptide polymyxin. J Bacteriol. 2006;188:4531-4541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 47. | Liechti GW, Goldberg JB. Helicobacter pylori salvages purines from extracellular host cell DNA utilizing the outer membrane-associated nuclease NucT. J Bacteriol. 2013;195:4387-4398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15:206-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, Zychlinsky A. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 50. | Yang C, Sun W, Cui W, Li X, Yao J, Jia X, Li C, Wu H, Hu Z, Zou X. Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int J Clin Exp Pathol. 2015;8:14075-14086. [PubMed] [Cited in This Article: ] |
| 51. | Zhang Y, Hu Y, Ma C, Sun H, Wei X, Li M, Wei W, Zhang F, Yang F, Wang H, Gu K. Diagnostic, Therapeutic Predictive, and Prognostic Value of Neutrophil Extracellular Traps in Patients With Gastric Adenocarcinoma. Front Oncol. 2020;10:1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Zhu T, Zou X, Yang C, Li L, Wang B, Li R, Li H, Xu Z, Huang D, Wu Q. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelialmesenchymal transition. Int J Mol Med. 2021;48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 53. | Xia X, Zhang Z, Zhu C, Ni B, Wang S, Yang S, Yu F, Zhao E, Li Q, Zhao G. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun. 2022;13:1017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 58] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 54. | Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000;6:2573-2581. [PubMed] [Cited in This Article: ] |
| 55. | Arlauckas SP, Garren SB, Garris CS, Kohler RH, Oh J, Pittet MJ, Weissleder R. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics. 2018;8:5842-5854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 56. | Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1778] [Cited by in F6Publishing: 2351] [Article Influence: 335.9] [Reference Citation Analysis (0)] |
| 57. | Curtale G. MiRNAs at the Crossroads between Innate Immunity and Cancer: Focus on Macrophages. Cells. 2018;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Chaudhuri AA, So AY, Sinha N, Gibson WS, Taganov KD, O'Connell RM, Baltimore D. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187:5062-5068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 59. | Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med. 2013;31:797-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 60. | Banerjee S, Xie N, Cui H, Tan Z, Yang S, Icyuz M, Abraham E, Liu G. MicroRNA let-7c regulates macrophage polarization. J Immunol. 2013;190:6542-6549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 61. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481-12486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3121] [Cited by in F6Publishing: 3382] [Article Influence: 187.9] [Reference Citation Analysis (0)] |
| 62. | Yang M, Chen J, Su F, Yu B, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 491] [Cited by in F6Publishing: 531] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 63. | Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 413] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 64. | Bazzoni F, Rossato M, Fabbri M, Gaudiosi D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA, Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci U S A. 2009;106:5282-5287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 459] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 65. | Cekaite L, Rantala JK, Bruun J, Guriby M, Agesen TH, Danielsen SA, Lind GE, Nesbakken A, Kallioniemi O, Lothe RA, Skotheim RI. MiR-9, -31, and -182 deregulation promote proliferation and tumor cell survival in colon cancer. Neoplasia. 2012;14:868-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Ye G, Barrera C, Fan X, Gourley WK, Crowe SE, Ernst PB, Reyes VE. Expression of B7-1 and B7-2 costimulatory molecules by human gastric epithelial cells: potential role in CD4+ T cell activation during Helicobacter pylori infection. J Clin Invest. 1997;99:1628-1636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 729] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 68. | Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, Harashima A, Harada S, Yamamoto H, Ohta T. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19:1052-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 175] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 69. | Rojas A, Delgado-López F, Gonzalez I. Tumor-associated macrophages in gastric cancer: more than bystanders in tumor microenvironment. Gastric Cancer. 2017;20:215-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Asaka M, Kimura T, Kato M, Kudo M, Miki K, Ogoshi K, Kato T, Tatsuta M, Graham DY. Possible role of Helicobacter pylori infection in early gastric cancer development. Cancer. 1994;73:2691-2694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 71. | Blaser MJ, Parsonnet J. Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Sipponen P, Hyvärinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196:3-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Talley NJ, Zinsmeister AR, Weaver A, DiMagno EP, Carpenter HA, Perez-Perez GI, Blaser MJ. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83:1734-1739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 267] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 74. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2805] [Cited by in F6Publishing: 2668] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 75. | An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359-1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 725] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 76. | Piazuelo MB, Correa P. Gastric cáncer: Overview. Colomb Med (Cali). 2013;44:192-201. [PubMed] [Cited in This Article: ] |
| 77. | Forman D, Sitas F, Newell DG, Stacey AR, Boreham J, Peto R, Campbell TC, Li J, Chen J. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990;46:608-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 201] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1302] [Cited by in F6Publishing: 1203] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 79. | Correa P. Is gastric carcinoma an infectious disease? N Engl J Med. 1991;325:1170-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 112] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Solnick JV, Tompkins LS. Helicobacter pylori and gastroduodenal disease: pathogenesis and host-parasite interaction. Infect Agents Dis. 1992;1:294-309. [PubMed] [Cited in This Article: ] |
| 81. | Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995;63:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 442] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 82. | Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 894] [Article Influence: 63.9] [Reference Citation Analysis (1)] |
| 83. | Torres J, Pérez-Pérez GI, Leal-Herrera Y, Muñoz O. Infection with CagA+ Helicobacter pylori strains as a possible predictor of risk in the development of gastric adenocarcinoma in Mexico. Int J Cancer. 1998;78:298-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 84. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci U S A. 1996;93:14648-14653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1375] [Cited by in F6Publishing: 1359] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 85. | Varon C, Mosnier JF, Lehours P, Matysiak-Budnik T, Mégraud F. Gastric carcinogenesis and Helicobacter pylori infection. Methods Mol Biol. 2009;511:237-265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 86. | Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971-980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 87. | Poppe M, Feller SM, Römer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462-3472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 88. | Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991;338:332-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 465] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 89. | Peek RM, Miller GG, Tham KT, Perez-Perez GI, Cover TL, Dunn D, Blaser MJ. Detection of cagA expression in vivo and demonstration of preferential cytokinc expression by cagA+ H. pylori strains in gastric mucosa. Am J Gastroenterol. 1994;89:1334. [Cited in This Article: ] |
| 90. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 91. | Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol. 2010;16:1188-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 80] [Cited by in F6Publishing: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 92. | Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J Gastroenterol Hepatol. 2009;24:1725-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 221] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1690] [Cited by in F6Publishing: 1614] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 95. | Kim J, Kim Y, Lee KA. Ethnic differences in gastric cancer genetic susceptibility: allele flips of interleukin gene. World J Gastroenterol. 2014;20:4558-4565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Canedo P, Corso G, Pereira F, Lunet N, Suriano G, Figueiredo C, Pedrazzani C, Moreira H, Barros H, Carneiro F, Seruca R, Roviello F, Machado JC. The interferon gamma receptor 1 (IFNGR1) -56C/T gene polymorphism is associated with increased risk of early gastric carcinoma. Gut. 2008;57:1504-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Martínez-Campos C, Torres-Poveda K, Camorlinga-Ponce M, Flores-Luna L, Maldonado-Bernal C, Madrid-Marina V, Torres J. Polymorphisms in IL-10 and TGF-β gene promoter are associated with lower risk to gastric cancer in a Mexican population. BMC Cancer. 2019;19:453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023-17023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2830] [Cited by in F6Publishing: 4177] [Article Influence: 596.7] [Reference Citation Analysis (0)] |
| 99. | Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 1042] [Article Influence: 148.9] [Reference Citation Analysis (0)] |
| 100. | Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2:823-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 658] [Cited by in F6Publishing: 777] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 101. | Peek RM Jr. IV. Helicobacter pylori strain-specific activation of signal transduction cascades related to gastric inflammation. Am J Physiol Gastrointest Liver Physiol. 2001;280:G525-G530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Soutto M, Bhat N, Khalafi S, Zhu S, Poveda J, Garcia-Buitrago M, Zaika A, El-Rifai W. NF-kB-dependent activation of STAT3 by H. pylori is suppressed by TFF1. Cancer Cell Int. 2021;21:444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Shibata W, Hirata Y, Yoshida H, Otsuka M, Hoshida Y, Ogura K, Maeda S, Ohmae T, Yanai A, Mitsuno Y, Seki N, Kawabe T, Omata M. NF-kappaB and ERK-signaling pathways contribute to the gene expression induced by cag PAI-positive-Helicobacter pylori infection. World J Gastroenterol. 2005;11:6134-6143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 20] [Cited by in F6Publishing: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 104. | Shen L, Zeng J, Ma L, Li S, Chen C, Jia J, Liang X. Helicobacter pylori Induces a Novel NF-kB/LIN28A/let-7a/hTERT Axis to Promote Gastric Carcinogenesis. Mol Cancer Res. 2021;19:74-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 105. | Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 106. | Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol. 2006;176:3000-3009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Lu B, Chen L, Liu L, Zhu Y, Wu C, Jiang J, Zhang X. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50:269-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 108. | Kim JJ, Tao H, Carloni E, Leung WK, Graham DY, Sepulveda AR. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Hardbower DM, Peek RM Jr, Wilson KT. At the Bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Kontizas E, Tastsoglou S, Karamitros T, Karayiannis Y, Kollia P, Hatzigeorgiou AG, Sgouras DN. Impact of Helicobacter pylori Infection and Its Major Virulence Factor CagA on DNA Damage Repair. Microorganisms. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3799] [Cited by in F6Publishing: 4331] [Article Influence: 618.7] [Reference Citation Analysis (0)] |
| 112. | Lange PT, White MC, Damania B. Activation and Evasion of Innate Immunity by Gammaherpesviruses. J Mol Biol. 2022;434:167214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 113. | Epstein A. Why and How Epstein-Barr Virus Was Discovered 50 Years Ago. Curr Top Microbiol Immunol. 2015;390:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Sun K, Jia K, Lv H, Wang SQ, Wu Y, Lei H, Chen X. EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives. Front Oncol. 2020;10:583463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 115. | Lieberman PM. Virology. Epstein-Barr virus turns 50. Science. 2014;343:1323-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 116. | Bocian J, Januszkiewicz-Lewandowska D. [Epstein-Barr virus infection - life cycle, methods of diagnosis, associated diseases]. Postepy Hig Med Dosw (Online). 2011;65:286-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 117. | Camargo MC, Bowlby R, Chu A, Pedamallu CS, Thorsson V, Elmore S, Mungall AJ, Bass AJ, Gulley ML, Rabkin CS. Validation and calibration of next-generation sequencing to identify Epstein-Barr virus-positive gastric cancer in The Cancer Genome Atlas. Gastric Cancer. 2016;19:676-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 118. | Dong M, Wang HY, Zhao XX, Chen JN, Zhang YW, Huang Y, Xue L, Li HG, Du H, Wu XY, Shao CK. Expression and prognostic roles of PIK3CA, JAK2, PD-L1, and PD-L2 in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2016;53:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Yang L, Wang Y, Wang H. Use of immunotherapy in the treatment of gastric cancer. Oncol Lett. 2019;18:5681-5690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 643] [Cited by in F6Publishing: 935] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 121. | Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704-1711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 232] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 122. | Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO⁺ memory T cells on human gastric cancer. Oncol Rep. 2013;29:1756-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 123. | Coutzac C, Pernot S, Chaput N, Zaanan A. Immunotherapy in advanced gastric cancer, is it the future? Crit Rev Oncol Hematol. 2019;133:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 124. | Thompson ED, Zahurak M, Murphy A, Cornish T, Cuka N, Abdelfatah E, Yang S, Duncan M, Ahuja N, Taube JM, Anders RA, Kelly RJ. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66:794-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 125. | Xie T, Liu Y, Zhang Z, Zhang X, Gong J, Qi C, Li J, Shen L, Peng Z. Positive Status of Epstein-Barr Virus as a Biomarker for Gastric Cancer Immunotherapy: A Prospective Observational Study. J Immunother. 2020;43:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 126. | Lee AJ, Ashkar AA. The Dual Nature of Type I and Type II Interferons. Front Immunol. 2018;9:2061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 127. | Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7:4509-4516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 128. | Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei XL, Liu J, Chen J, Zeng ZL, Ju HQ, Luo HY, Xu RH. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020;10:498-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 129. | Lünemann A, Rowe M, Nadal D. Innate Immune Recognition of EBV. Curr Top Microbiol Immunol. 2015;391:265-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol. 2007;81:8016-8024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 131. | Ariza ME, Glaser R, Kaumaya PT, Jones C, Williams MV. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol. 2009;182:851-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 132. | Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, Imai S, Fujieda M, Kawa K, Takada K. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med. 2009;206:2091-2099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 133. | Fiola S, Gosselin D, Takada K, Gosselin J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J Immunol. 2010;185:3620-3631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 134. | Jangra S, Yuen KS, Botelho MG, Jin DY. Epstein-Barr Virus and Innate Immunity: Friends or Foes? Microorganisms. 2019;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 135. | Pontejo SM, Murphy PM, Pease JE. Chemokine Subversion by Human Herpesviruses. J Innate Immun. 2018;10:465-478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 136. | Xu YH, Li ZL, Qiu SF. IFN-γ Induces Gastric Cancer Cell Proliferation and Metastasis Through Upregulation of Integrin β3-Mediated NF-κB Signaling. Transl Oncol. 2018;11:182-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Wu D, Zhang P, Ma J, Xu J, Yang L, Xu W, Que H, Chen M, Xu H. Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med. 2019;8:1576-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 138. | Neels JG, Grimaldi PA. Physiological functions of peroxisome proliferator-activated receptor β. Physiol Rev. 2014;94:795-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 139. | Zuo X, Deguchi Y, Xu W, Liu Y, Li HS, Wei D, Tian R, Chen W, Xu M, Yang Y, Gao S, Jaoude JC, Liu F, Chrieki SP, Moussalli MJ, Gagea M, Sebastian MM, Zheng X, Tan D, Broaddus R, Wang J, Ajami NJ, Swennes AG, Watowich SS, Shureiqi I. PPARD and Interferon Gamma Promote Transformation of Gastric Progenitor Cells and Tumorigenesis in Mice. Gastroenterology. 2019;157:163-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 140. | Lee J, Park KH, Ryu JH, Bae HJ, Choi A, Lee H, Lim J, Han K, Park CH, Jung ES, Oh EJ. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget. 2017;8:70431-70440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 141. | Sánchez-Zauco N, Torres J, Gómez A, Camorlinga-Ponce M, Muñoz-Pérez L, Herrera-Goepfert R, Medrano-Guzmán R, Giono-Cerezo S, Maldonado-Bernal C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: a controlled study. BMC Cancer. 2017;17:384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 142. | Cárdenas-Mondragón MG, Torres J, Sánchez-Zauco N, Gómez-Delgado A, Camorlinga-Ponce M, Maldonado-Bernal C, Fuentes-Pananá EM. Elevated Levels of Interferon-γ Are Associated with High Levels of Epstein-Barr Virus Reactivation in Patients with the Intestinal Type of Gastric Cancer. J Immunol Res. 2017;2017:7069242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 143. | Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1564] [Cited by in F6Publishing: 1658] [Article Influence: 207.3] [Reference Citation Analysis (0)] |
| 144. | Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, Zaretsky JM, Sun L, Hugo W, Wang X, Parisi G, Saus CP, Torrejon DY, Graeber TG, Comin-Anduix B, Hu-Lieskovan S, Damoiseaux R, Lo RS, Ribas A. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19:1189-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 822] [Cited by in F6Publishing: 1120] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 145. | Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, Pan F, Topalian SL. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunother Cancer. 2019;7:305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 281] [Cited by in F6Publishing: 263] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 146. | Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X, Li L. IFN-γ-mediated inhibition of lung cancer correlates with PD-L1 expression and is regulated by PI3K-AKT signaling. Int J Cancer. 2018;143:931-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 147. | Mineo M, Lyons SM, Zdioruk M, von Spreckelsen N, Ferrer-Luna R, Ito H, Alayo QA, Kharel P, Giantini Larsen A, Fan WY, Auduong S, Grauwet K, Passaro C, Khalsa JK, Shah K, Reardon DA, Ligon KL, Beroukhim R, Nakashima H, Ivanov P, Anderson PJ, Lawler SE, Chiocca EA. Tumor Interferon Signaling Is Regulated by a lncRNA INCR1 Transcribed from the PD-L1 Locus. Mol Cell. 2020;78:1207-1223.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 148. | Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 382] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 149. | Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10:e0131403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 150. | Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J, Lunceford J, Savage MJ, Juco J, Emancipator K. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med. 2019;143:330-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 151. | Ma J, Li J, Qian M, Han W, Tian M, Li Z, Wang Z, He S, Wu K. PD-L1 expression and the prognostic significance in gastric cancer: a retrospective comparison of three PD-L1 antibody clones (SP142, 28-8 and E1L3N). Diagn Pathol. 2018;13:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 152. | Imai Y, Chiba T, Kondo T, Kanzaki H, Kanayama K, Ao J, Kojima R, Kusakabe Y, Nakamura M, Saito T, Nakagawa R, Suzuki E, Nakamoto S, Muroyama R, Tawada A, Matsumura T, Nakagawa T, Kato J, Kotani A, Matsubara H, Kato N. Interferon-γ induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol Lett. 2020;20:2161-2168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 153. | Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, Fazreen Z, Asuncion BR, Shabbir A, Yong WP, So J, Soong R, Kono K. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 154. | Nakano H, Saito M, Nakajima S, Saito K, Nakayama Y, Kase K, Yamada L, Kanke Y, Hanayama H, Onozawa H, Okayama H, Fujita S, Sakamoto W, Saze Z, Momma T, Mimura K, Ohki S, Goto A, Kono K. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci Rep. 2021;11:1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 155. | Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8:64066-64082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 156. | Kim JG, Chae YS, Sohn SK, Cho YY, Moon JH, Park JY, Jeon SW, Lee IT, Choi GS, Jun SH. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with colorectal cancer. Clin Cancer Res. 2008;14:62-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 157. | Deng R, Zuo C, Li Y, Xue B, Xun Z, Guo Y, Wang X, Xu Y, Tian R, Chen S, Liu Q, Chen J, Wang J, Huang X, Li H, Guo M, Yang M, Wu Z, Ma J, Hu J, Li G, Tang S, Tu Z, Ji H, Zhu H. The innate immune effector ISG12a promotes cancer immunity by suppressing the canonical Wnt/β-catenin signaling pathway. Cell Mol Immunol. 2020;17:1163-1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 158. | Rasmussen UB, Wolf C, Mattei MG, Chenard MP, Bellocq JP, Chambon P, Rio MC, Basset P. Identification of a new interferon-alpha-inducible gene (p27) on human chromosome 14q32 and its expression in breast carcinoma. Cancer Res. 1993;53:4096-4101. [PubMed] [Cited in This Article: ] |
| 159. | Borden EC. Interferons α and β in cancer: therapeutic opportunities from new insights. Nat Rev Drug Discov. 2019;18:219-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 160. | Xue B, Yang D, Wang J, Xu Y, Wang X, Qin Y, Tian R, Chen S, Xie Q, Liu N, Zhu H. ISG12a Restricts Hepatitis C Virus Infection through the Ubiquitination-Dependent Degradation Pathway. J Virol. 2016;90:6832-6845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 161. | Sarkar S, Dauer MJ, In H. Socioeconomic Disparities in Gastric Cancer and Identification of a Single SES Variable for Predicting Risk. J Gastrointest Cancer. 2022;53:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 162. | Ji J, Hemminki K. Socio-economic and occupational risk factors for gastric cancer: a cohort study in Sweden. Eur J Cancer Prev. 2006;15:391-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 163. | Nagel G, Linseisen J, Boshuizen HC, Pera G, Del Giudice G, Westert GP, Bueno-de-Mesquita HB, Allen NE, Key TJ, Numans ME, Peeters PH, Sieri S, Siman H, Berglund G, Hallmans G, Stenling R, Martinez C, Arriola L, Barricarte A, Chirlaque MD, Quiros JR, Vineis P, Masala G, Palli D, Panico S, Tumino R, Bingham S, Boeing H, Bergmann MM, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Olsen A, Tjonneland A, Trichopoulou A, Bamia C, Soukara S, Sabourin JC, Carneiro F, Slimani N, Jenab M, Norat T, Riboli E, González CA. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Epidemiol. 2007;36:66-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 164. | Yang L, Ying X, Liu S, Lyu G, Xu Z, Zhang X, Li H, Li Q, Wang N, Ji J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 165. | Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf). 2021;9:91-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 166. | Agudo A, Cayssials V, Bonet C, Tjønneland A, Overvad K, Boutron-Ruault MC, Affret A, Fagherazzi G, Katzke V, Schübel R, Trichopoulou A, Karakatsani A, La Vecchia C, Palli D, Grioni S, Tumino R, Ricceri F, Panico S, Bueno-de-Mesquita B, Peeters PH, Weiderpass E, Skeie G, Nøst TH, Lasheras C, Rodríguez-Barranco M, Amiano P, Chirlaque MD, Ardanaz E, Ohlsson B, Dias JA, Nilsson LM, Myte R, Khaw KT, Perez-Cornago A, Gunter M, Huybrechts I, Cross AJ, Tsilidis K, Riboli E, Jakszyn P. Inflammatory potential of the diet and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2018;107:607-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 167. | Ruíz-García E, Guadarrama-Orozco J, Vidal-Millán S, Lino-Silva LS, López-Camarillo C, Astudillo-de la Vega H. Gastric cancer in Latin America. Scand J Gastroenterol. 2018;53:124-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 168. | Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America. Cancer Causes Control. 2013;24:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 169. | Heise K, Bertran E, Andia ME, Ferreccio C. Incidence and survival of stomach cancer in a high-risk population of Chile. World J Gastroenterol. 2009;15:1854-1862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 170. | In H, Langdon-Embry M, Gordon L, Schechter CB, Wylie-Rosett J, Castle PE, Margaret Kemeny M, Rapkin BD. Can a gastric cancer risk survey identify high-risk patients for endoscopic screening? J Surg Res. 2018;227:246-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 171. | Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 430] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 172. | Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in F6Publishing: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 173. | Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1859] [Cited by in F6Publishing: 1891] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 174. | Huhta H, Helminen O, Lehenkari PP, Saarnio J, Karttunen TJ, Kauppila JH. Toll-like receptors 1, 2, 4 and 6 in esophageal epithelium, Barrett's esophagus, dysplasia and adenocarcinoma. Oncotarget. 2016;7:23658-23667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 175. | Kennedy CL, Najdovska M, Tye H, McLeod L, Yu L, Jarnicki A, Bhathal PS, Putoczki T, Ernst M, Jenkins BJ. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene. 2014;33:2540-2546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 176. | Diakowska D, Nienartowicz M, Grabowski K, Rosińczuk J, Krzystek-Korpacka M. Toll-like receptors TLR-2, TLR-4, TLR-7, and TLR-9 in tumor tissue and serum of the patients with esophageal squamous cell carcinoma and gastro-esophageal junction cancer. Adv Clin Exp Med. 2019;28:515-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 177. | Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100-3111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 178. | Fernandez-Garcia B, Eiró N, González-Reyes S, González L, Aguirre A, González LO, Del Casar JM, García-Muñiz JL, Vizoso FJ. Clinical significance of toll-like receptor 3, 4, and 9 in gastric cancer. J Immunother. 2014;37:77-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 179. | Sheyhidin I, Nabi G, Hasim A, Zhang RP, Ainiwaer J, Ma H, Wang H. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:3745-3751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 87] [Cited by in F6Publishing: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 180. | Pimentel-Nunes P, Afonso L, Lopes P, Roncon-Albuquerque R Jr, Gonçalves N, Henrique R, Moreira-Dias L, Leite-Moreira AF, Dinis-Ribeiro M. Increased expression of toll-like receptors (TLR) 2, 4 and 5 in gastric dysplasia. Pathol Oncol Res. 2011;17:677-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 181. | Chen G, Xu M, Chen J, Hong L, Lin W, Zhao S, Zhang G, Dan G, Liu S. Clinicopathological Features and Increased Expression of Toll-Like Receptor 4 of Gastric Cardia Cancer in a High-Risk Chinese Population. J Immunol Res. 2018;2018:7132868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Verbeek RE, Siersema PD, Ten Kate FJ, Fluiter K, Souza RF, Vleggaar FP, Bus P, van Baal JW. Toll-like receptor 4 activation in Barrett's esophagus results in a strong increase in COX-2 expression. J Gastroenterol. 2014;49:1121-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 183. | Jiang J, Dong L, Qin B, Shi H, Guo X, Wang Y. Decreased expression of TLR7 in gastric cancer tissues and the effects of TLR7 activation on gastric cancer cells. Oncol Lett. 2016;12:631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 184. | Takala H, Kauppila JH, Soini Y, Selander KS, Vuopala KS, Lehenkari PP, Saarnio J, Karttunen TJ. Toll-like receptor 9 is a novel biomarker for esophageal squamous cell dysplasia and squamous cell carcinoma progression. J Innate Immun. 2011;3:631-638. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |