Published online Dec 21, 2021. doi: 10.3748/wjg.v27.i47.8182
Peer-review started: June 17, 2021
First decision: July 2, 2021
Revised: July 15, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: December 21, 2021
Cold polypectomy (CP) is a simple and safe procedure for polyps less than 10 mm in size; however, there is concern about local recurrence following CP because of unidentified margins of excised specimens and the lack of tumor suppression effect by coagulation. Some clinical trials have evaluated local persistent recurrence; their results suggest that a higher rate of local recurrence has not been documented so far. There were few reports that observed the course over long periods of time after CP in clinical practice.
To evaluate the presence of local recurrence following CP and hot polypectomy (HP) using propensity score matching.
We analyzed 275 patients who underwent polypectomy for non-pedunculated colorectal polyps less than 10 mm (959 Lesions) between October 2016 and 2017 and underwent follow-up endoscopy subsequently. We divided them into the CP group (706 Lesions), wherein CP was performed, and the HP group (253 Lesions), wherein HP was performed. Using propensity score matching, we extracted 215 Lesions in each group and evaluated the local recurrence and content of CP in the real clinic and adverse events using medical records.
After propensity score matching, there were no significant differences in the patients’ and their endoscopic background (age, use of antithrombotics, indications, size, morphology, location of polyps, and polypectomy device) between the groups. The mean duration between colorectal polypectomy and the next follow-up colonoscopy was 17.5 ± 7.1 (range, 6-39) mo in the CP group and 15.7 ± 6.0 (range, 6-35) mo in the HP group, which was significantly longer in the CP group (P = 0.005). The local recurrence rate was 0.93% in the CP group and 0.93% in the HP group, without a significant difference (P = 0.688). Additionally, no differences were observed in the macroscopic en bloc resection rate, histopathological complete resection rate, and pathological results between the groups. Adverse events did not occur in either group.
Local recurrence after CP was equivalent to that following HP in clinical practice. CP is useful and safe in the treatment of non-pedunculated polyps of less than 10 mm.
Core Tip: In this study, the recurrence rate after cold polypectomy (CP) was evaluated with colonoscopy at long intervals in real clinical practice for non-pedunculated colorectal polyps smaller than 10 mm, and compared with hot polypectomy (HP). Although it is a retrospective study, we used propensity score matching to correct the bias of both groups and compared them. The recurrence rates of both procedures were similar, and it was considered that CP, which is easier in clinical practice, is more useful for small polyps than HP.
- Citation: Saito M, Yamamura T, Nakamura M, Maeda K, Sawada T, Ishikawa E, Mizutani Y, Ishikawa T, Kakushima N, Furukawa K, Ohno E, Kawashima H, Ishigami M, Fujishiro M. Real-world local recurrence rate after cold polypectomy in colorectal polyps less than 10 mm using propensity score matching. World J Gastroenterol 2021; 27(47): 8182-8193
- URL: https://www.wjgnet.com/1007-9327/full/v27/i47/8182.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i47.8182
It has been reported that the mortality of patients with colorectal cancer decreases with the resection of all adenomatous polyps (clean colon)[1]; therefore, it has become desirable to resect even diminutive polyps. There are reports which state that ≥ 90% of polyps are less than 10 mm on colonoscopic examination, with 70%-80% being less than 5 mm[2-4]. According to these reports, it is important to decide how to efficiently and safely remove such diminutive polyps for a clean colon. Cold polypectomy (CP) has been used in the treatment of small colorectal polyps because of the suitable efficiency and safe outcomes[5-8]. It has been reported that CP is an easy-to-perform technique. The frequency of adverse events such as delayed bleeding and perforation is lower with CP than those with hot polypectomy (HP) because it avoids electrocoagulation; furthermore, it has been considered useful in the endoscopic resection of sub-centimeter polyps. However, the presence of the lesions with unknown margins in resection specimens, has been reported in as high as 40% of the cases in cold snare polypectomy (CSP); therefore, there is concern about local recurrence after the treatment[9]. Another concern is the increased risk of local recurrence after CP compared to that after HP because the tumor suppression effect may not be expected by electrocoagulation in the resection sites following CP. One randomized trial reported that the ratio of local recurrence after treatment was equivalent between CSP and hot snare polypectomy (HSP)[10]. Another report indicated that the ratio immediately after CSP is significantly higher than that after endoscopic mucosal resection (EMR)[11]. However, these reports were all evaluations of local recurrence just after endoscopic resection and did not confirm it after a certain period of time. Therefore, in this study, we assessed the presence of local recurrence following CP and HP after a long period following polypectomy in real clinical practice.
This real-world retrospective study was conducted at the Nagoya University Hospital. The inclusion criteria of the CP procedure in Nagoya University Hospital is for non-pedunculated polyps only, less than 10 mm, and diagnosed as Type 2A in the Japan Narrow band imaging Expert Team (JNET) classification[12] using imaged-enhanced endoscopy with magnification, in short, suspected adenomatous lesions. As the size of the polyp increases, the rate of advanced neoplasia (with villous or tubulovillous adenoma components, size ≥ 10 mm, and high-grade dysplasia) also increases[3]. Therefore, a polyp > 10 mm is an indication for EMR. In pedunculated lesions, a large blood vessel is often found in the stem, which may be difficult to resect using CP; this might result in a very high risk of bleeding after resection. Therefore, we excluded them in the inclusion criteria of CP[13-15].
Using clinical records, we extracted data of 612 patients (2619 Lesions) who had undergone polypectomy at the Nagoya University Hospital between October 2016 and October 2017. Of them, data from 313 patients (1449 Lesions) who underwent follow-up colonoscopy more than half a year after the first polypectomy were extracted. We excluded data from 16 patients (303 Lesions) who were diagnosed with polyposis (familial adenomatous polyposis, Peutz–Jeghers syndrome, or other hereditary polyposis syndromes) or inflammatory bowel disease and 187 Lesions (22 patients) which were diagnosed with JNET Type 2B and lesions out of the inclusion criteria for CP such as size ≥ 10 mm and pedunculated or depressed lesions. Finally, we identified 959 Lesions (275 patients), which were divided into the HP (253 Lesions) and CP groups (706 Lesions).
The mean size of the lesions was significantly smaller in the CP group (CP group: 3.82 ± 1.49 mm; HP group: 5.35 ± 1.77 mm; P < 0.001), the ratio of flat lesions was significantly higher in the CP group (CP group: 62.0%; HP group: 43.5%; P < 0.001), and the resection ratio with the snare was significantly higher in the HP group (CP group: 86.5%; HP group: 99.2%; P < 0.001) (Table 1). It has been reported that the recurrence rate after polypectomy increases as the size of the lesion increases. Regarding the morphology of the lesion, the morphological difference might affect the treatment method (CP or HP). For polypectomy devices, the biopsy forceps has been reported to have a lower complete resection rate than the snare, which may affect recurrence rates. Therefore, to adjust for the bias between both groups, we performed propensity score matching based on the size and morphology (sessile or flat) of the lesions and the polypectomy device (biopsy forceps or snare), which could have an influence on the local recurrence. The CP (215 Lesions) and HP groups (215 Lesions) (total 206 patients) were compared after propensity score matching (Figure 1).
| CP, mean ± SD or n (%) | HP, mean ± SD or n (%) | P-value | |
| Number of polyps resected | 706 | 253 | |
| Size, mm | 3.82 ± 1.49 | 5.35 ± 1.77 | < 0.001a |
| Morphology | < 0.001b | ||
| Sessile | 268 (38.0) | 143 (56.5) | |
| Flat | 438 (62.0) | 110 (43.5) | |
| Location | 0.439b | ||
| Cecum | 46 (6.5) | 15 (5.9) | |
| Ascending colon | 182 (25.8) | 59 (23.3) | |
| Transverse colon | 220 (31.2) | 58 (22.9) | |
| Descending colon | 92 (13.0) | 33 (13.0) | |
| Sigmoid colon | 128 (18.1) | 67 (26.5) | |
| Rectum | 38 (5.4) | 21 (8.3) | |
| Polypectomy device | < 0.001b | ||
| Snare | 611 (86.5) | 251 (99.2) | |
| Biopsy forceps | 95 (13.5) | 2 (0.8) |
The evaluation items included local recurrence, histological complete resection rate, delayed bleeding, and perforation. We defined local recurrence as a polyp on the post-polypectomy scar and delayed bleeding as bleeding requiring endoscopic hemostasis treatment within two weeks of the polypectomy. For identification of the resected lesion, we referred to the post-polypectomy scar and the scope insertion length from the anal verge to the lesion as described in the patient’s previous colonoscopy report.
The instruments used in this study included XL-4450/LL-4450 (light source), VP-4450HD (processor), EC-L590ZW/EC-600ZP (scope) (Fujifilm Co., Tokyo, Japan) and CLV-290SL (light source), CV-290 (processor), and CF-H260AZI/CF-HQ290I (scope) (Olympus Co., Tokyo, Japan). The participating physicians were 22 expert endoscopists who had each performed > 1000 colonoscopies, including polypectomies. The snare used in this study included Snare Master 15 mm (Olympus Co., Tokyo, Japan), Profile 11 mm/13 mm and Captivator II 10 mm (Boston Scientific Co., Boston, MA, United States) in both groups. Additionally, as the biopsy forceps, Radial Jaw 4 JUMBO in the CP group and Radial Jaw 4 in the HP group (Boston Scientific Co., Boston, MA, United States) were used. In principle, the biopsy forceps were used for lesions < 4 mm, while the snare was used for lesions ≥ 4 mm because the histological complete resection rate for lesions ≥ 4 mm is lower with cold forceps polypectomy (CFP)[16]. The physicians decided whether to use the biopsy forceps or the snare. We determined the size of the lesion based on the outer diameter of the tip cup diameter of the biopsy forceps or the snare.
JMP v15 (SAS Institute, Cary NC, United States) was used for propensity score matching, and SPSS v24.0 (IBM, Armonk, New York, United States) was used for statistical analysis in this study. We used the chi-square test to compare the morphology, location of lesions, polypectomy device (before propensity score matching), pathological diagnosis, histopathology results, and histological complete resection rate between the groups. Student’s t-test was used to compare the mean size of lesions (after propensity score matching) and the mean follow-up period. Welch test was used to compare the mean size of lesions (before propensity score matching), and Fisher’s exact test was used to compare the polypectomy device (after propensity score matching), tissue retrieval rate, macroscopic en bloc resection rate, acute bleeding rate, and local recurrence. Statistical significance was set at P < 0.05.
In the 206 patients (139 men and 67 women) included in the study, the mean age was 68.7 ± 8.63 years, the use of antithrombotics was 19.4%, and the indications for colonoscopy included screening (n = 186), constipation (n = 9), abdominal pain (n = 6), anemia (n = 2), and bloody stools (n = 2) (Table 2).
| mean ± SD, (%) | |
| n = 206 | |
| Male/female | 139 (67.5)/67 (32.5) |
| Age, yr | 68.7 ± 8.6 |
| Range | 46-85 |
| Antithrombotic agent users | 40 (19.4) |
| Indication for colonoscopy | |
| Screening | 186 (90.3) |
| Constipation | 9 (4.3) |
| Abdominal pain | 6 (2.9) |
| Bloody stools | 2 (1.0) |
| Anemia | 2 (1.0) |
| Others | 1 (0.5) |
Regarding the excised polyps, the mean size was 4.95 ± 1.60 mm in the CP group and 4.94 ± 1.58 mm in the HP group. The morphology included 111 sessile lesions (51.6%) and 104 flat lesions (48.4%) in the CP group, and 113 sessile lesions (52.6%) and 102 flat lesions (47.4%) in the HP group. The lesion locations in the CP and HP groups included the cecum (6.0% and 7.0%, respectively), ascending colon (25.1% and 23.7%, respectively), transverse colon (33.0% and 22.3%, respectively), descending colon (12.6% and 12.1%, respectively), sigmoid colon (19.1% and 25.6%, respectively), and the rectum (4.2% and 9.3%, respectively). In both groups, 99.1% of the procedures were performed using a snare and 0.9% using biopsy forceps. There were no significant differences in the mean size, morphology, location, or polypectomy device between the groups.
Regarding the subsequent pathological diagnosis of the lesions, the difference was not statistically significant between the groups (P = 0.117): 186 Lesions with low-grade adenoma (88.6%), seven lesions with advanced neoplasia (high-grade dysplasia or lesions including villous or tubulovillous adenoma components) (3.3%) in the CP group vs 168 Lesions with low-grade adenoma (79.2%), 14 Lesions with advanced neoplasia (6.6%) in the HP group (Table 3).
| CP, mean ± SD or n (%) | HP, mean ± SD or n (%) | P-value | |
| Number of polyps excised | 215 | 215 | |
| Size, mm | 4.95 ± 1.60 | 4.94 ± 1.58 | 0.952a |
| Morphology | 0.847b | ||
| Sessile | 111 (51.6) | 113 (52.6) | |
| Flat | 104 (48.4) | 102 (47.4) | |
| Location | 0.736b | ||
| Cecum | 13 (6.0) | 15 (7.0) | |
| Ascending colon | 54 (25.1) | 51 (23.7) | |
| Transverse colon | 71 (33.0) | 48 (22.3) | |
| Descending colon | 27 (12.6) | 26 (12.1) | |
| Sigmoid colon | 41 (19.1) | 55 (25.6) | |
| Rectum | 9 (4.2) | 20 (9.3) | |
| Polypectomy device | 0.688c | ||
| Snare | 213 (99.1) | 213 (99.1) | |
| Biopsy forceps | 2 (0.9) | 2 (0.9) | |
| Pathologic diagnosis1 | 210 lesions | 212 lesions | 0.117b |
| Low-grade adenoma | 186 (88.6) | 168 (79.2) | |
| Advanced neoplasia2 | 7 (3.3) | 14 (6.6) | |
| Hyperplastic polyp and SSL | 14 (6.7) | 28 (13.2) | |
| Others | 3 (1.4) | 2 (0.9) | |
| Failure of tissue retrieval | 5 (2.3) | 3 (1.4) | 0.362c |
The macroscopic en bloc resection rate was 99.1% (213 Lesions) in the CP group and 98.1% (211 Lesions) in the HP group; however, the difference was not significant (P = 0.343). The number of lesions with low-grade adenoma and advanced neoplasia whose margins were evaluated pathologically was 183 in the CP group and 181 in the HP group. The rate of histological complete resection was 82.5% (151 Lesions) in the CP group and 84.0% (152 Lesions) in the HP group, and no significant difference was identified between the groups (P = 0.708). Acute bleeding was observed in six (2.8%) lesions in the CP group and three (1.4%) lesions in the HP group; it tended to be more common in the CP group, but the difference was not statistically significant (P = 0.252). Delayed bleeding or perforation was not observed in either group (Table 4).
| CP, n (%) | HP, n (%) | P value | |
| n = 215 | n = 215 | ||
| Macroscopic en bloc resection | 213 (99.1) | 211 (98.1) | 0.343a |
| Tissue retrieval successfully | 210 (97.7) | 212 (98.6) | 0.362a |
| Snare polypectomy | 208/213 (97.7) | 210/213 (98.6) | |
| Biopsy forceps polypectomy | 2/2 (100) | 2/2 (100) | |
| Lesions diagnosed with low-grade adenoma or advanced lesion and evaluated for histological margin | n = 183 | n = 181 | |
| Histological complete resection | 151 (82.5) | 152 (84.0) | 0.708b |
| Complications (%) | |||
| Acute bleeding1 | 6 (2.8) | 3 (1.4) | 0.252a |
| Delayed bleeding2 | 0 (0) | 0 (0) | - |
| Perforation | 0 (0) | 0 (0) | - |
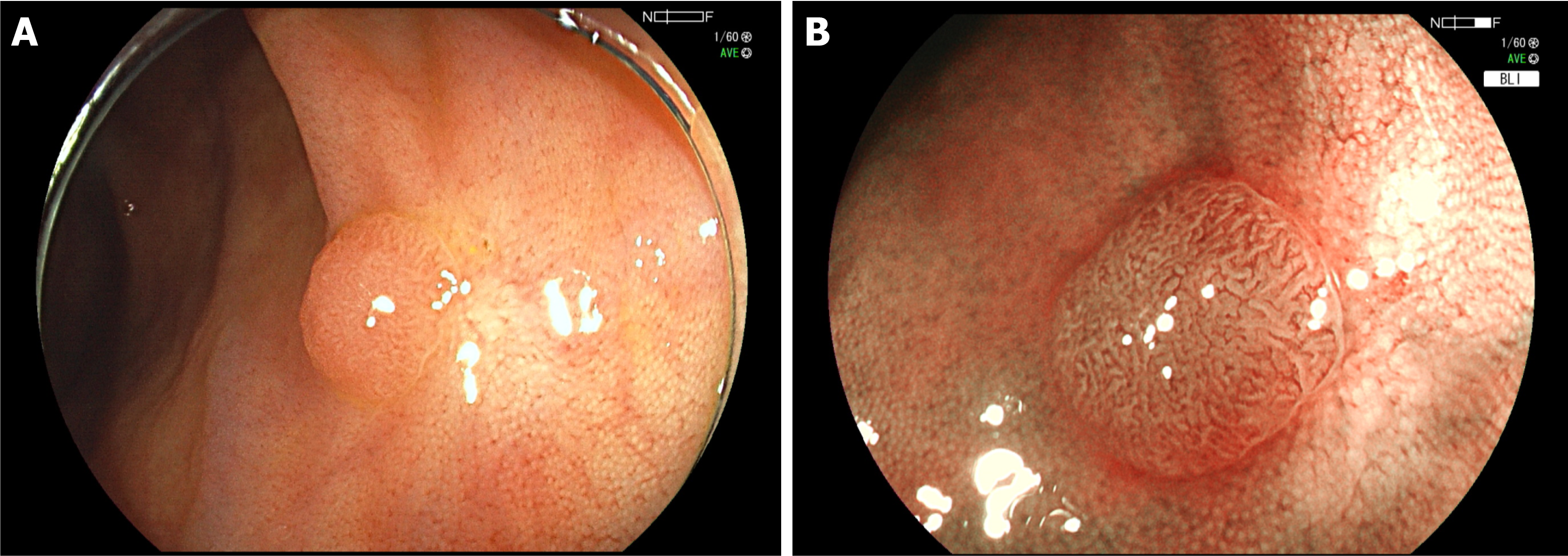
The mean duration between colorectal polypectomy and the next follow-up colonoscopy was 17.5 ± 7.1 (range, 6-39) mo in the CP group and 15.7 ± 6.0 (range, 6-35) mo in the HP group, which was significantly longer in the CP group (P = 0.005). Local recurrence was observed in two (0.93%) lesions in both groups with no significant difference between them (P = 0.688) (Table 5; Figures 2 and 3).
CP is a safe treatment with a simple procedure and few complications. However, compared to HP, it may be difficult to evaluate whether or not complete resection is possible pathologically, and there is a concern that the risk of local recurrence may increase because there is no tumor suppression effect by electrocoagulation. Some facilities are cautious about its adaptation. In this study, we focused on the local recurrence rate and retrospectively analyzed lesions that could be followed up with an endoscope for a relatively long period of time in real clinical practice. From the results of this study, it was considered that there is no difference in recurrence rate between CP and HP in non-pedunculated colorectal polyps smaller than 10 mm, and CP can be selected as one of the useful treatment methods for small colorectal polyps.
Endoscopic resection of colorectal polyps is one of the common treatments in digestive endoscopy, and resection of all adenomatous polyps, including diminutive lesions, is expected to become increasingly important to decrease the morbidity and mortality of colorectal cancer. Delayed bleeding or perforation, as complications of polypectomy, may require re-admission to the hospital along with additional endoscopy to stop the bleeding, blood transfusion, or surgery if necessary, which is not only a burden to the patient but also to the medical staff and the economy. The incidence of delayed bleeding and perforation in conventional HP has been reported to be 0.26%-1.4% and 0.017%-0.091%[17]. However, polypectomy is routinely performed in many patients, and the adverse events should never be ignored, even if their frequency is low. It has been reported that CP has a lower risk of complications compared with HP[6,16,18,19]. However, the long-term risk of residual recurrence after CP in clinical practice has not yet been sufficiently investigated.
It is important to visually confirm that there are no residual lesions following polypectomy; however, since CP does not have a burn effect like HP does, there is a risk of recurrence if there are residual lesions that cannot be detected visually after the treatment. There are three methods for examining the presence of remnants after polypectomy. First, histopathological evaluation of the resected specimen is performed to confirm whether complete resection was achieved. In CP, specimen damage due to aspiration and collection of specimens is more likely to occur than in HP; it has been reported that pathological resection margins are more frequently unknown in CP[18,19]. Second, biopsy of the resected ulcer margins or mucosal resection is performed to confirm any remnants histologically. It has been reported that the resected region is resected again with a snare or biopsied with forceps immediately after polypectomy and histopathologically evaluated for the presence of remnants. The residual rate has been reported to be 3.4% in CSP and 17.4% in CFP by Kim et al[20], 10% in CSP and 11% in CFP by Gómez et al[21], 3.9% in CSP by Matsuura et al[22], and 1.8% in CSP and 2.6% in HSP by Kawamura et al[10]. The rates varied slightly between these reports. However, lesions left at the margins immediately after resection may fall off later (especially in HP, due to the effects of electrical coagulation). It remains unclear whether they will eventually become residual recurrent lesions. Third, endoscopic confirmation of the polyp resection site is repeated after a certain duration. This is a reliable assessment of residual recurrence but includes some hurdles. First, the patient must undergo a follow-up colonoscopy, which can be physically burdensome. Additionally, because of the sufficient follow-up period, the resection sites become scars, and the scar after CP is more obscured than that after HP, which may make it difficult to identify the regions of post-polypectomy. Lee et al[23] reported that the overall recurrence over 59.7 mo was 17% (4% definite recurrence and 13% probable recurrence) after CFP in 1111 diminutive polyps. This recurrence rate is much higher than that reported in other studies. Probable recurrence was defined as recurrence at a similar distance from the anal verge (± 3 cm) in the same colorectal segment as a previous polyp and accounted for the majority of all recurrences. These lesions may be indistinguishable from newly formed polyps or previously overlooked polyps.
Murakami et al[24] reported that recurrence was observed in 1.4% of lesions less than 10 mm and 5.4% of lesions of 10-14 mm in follow-up colonoscopy more than 10 mo after CSP. If the scar was unclear, they were observed by going back and forth multiple times across segments estimated by the distance from the anal verge. When there were no new polyps after such cautious colonoscopy, the patient was considered to have no recurrence. The frequency of detection of scars was not reported; however, it appears to be an acceptable method in actual clinical practice. Maruoka et al[25] reported that clipping was performed in the vicinity of the ulcer after CSP, and colonoscopy was repeated three weeks later. After the scar was identified using the clip as a guide, the scar area was biopsied to evaluate the remnants. They indicated that the recurrence rate using the above method was 0.98%. Since the clip would naturally drop off after a certain period, it appeared to be the limit of the period that can be evaluated using this method.
In this study, follow-up colonoscopy was performed after 6-39 mo (average of 17.5 mo in the CP group and 16.2 mo in the HP group) after the treatment. We adopted an evaluation method similar to that used by Murakami et al[24] to assess the presence of residual recurrence. That is, we first looked for a scar after treatment, and if it was unclear, observed the excision site estimated from the distance from the anal margin multiple times, and judged that there was no recurrence if there was no new polyp. The number of lesions that led to recurrence was 2 (0.93%) in both groups, which was not significantly different (P = 0.688). The local recurrence rate in the CP group in this study was 0.93%. It was similar to 1.4% in CSP for < 10-mm colorectal polyps that Murakami et al[24] reported or 0.98% in CSP that Maruoka et al[25] reported, and it was expected to be lower than the residual rates reported by the second method of confirming the presence of remnants after polypectomy, that is, pathologically evaluated by biopsy or snare immediately after polypectomy[10,20-22]. The result may be due to the fall-off of small residual lesions at the margins of the excision and overlooking recurrent lesions in actual clinical practice. Additionally, no significant difference was observed in the residual rate compared with the HP group, as previously reported.
Regarding the safety, the acute bleeding rate immediately after the procedure was 2.8% in the CP group and 1.4% in the HP group (P = 0.252), with no significant difference between the two groups. In all cases, the bleeding was stopped by clipping hemostasis. Acute bleeding in CP often stops spontaneously. In contrast, delayed bleeding and perforation were not observed in either group, thus, confirming the safety of CP as reported previously[5-8].
It has been reported that CP has a higher rate of pathologically positive or unknown resection margins than HP[18,19]. In this study, the histopathological complete resection rate was 82.5% in the CP group vs 84.0% in the HP group (P = 0.708), with no significant difference between the two groups.
This study was a retrospective examination at a single institution, and the sample size was not very large. The follow-up period was not long and averaged a little over a year. The mean follow-up period was significantly longer in the CP group than that in the HP group. However, the results emphasize that the local recurrence rate in the CP group did not become higher compared with the HP group because the local recurrence rate was equivalent in both groups.
Colonoscopy was performed by several different endoscopists who might not have detected all recurrences because of differences in individual skills and the possibility of missing residual or recurrent lesions. Although the endoscopists in this study were experts, a new study should be conducted, including colonoscopy trainees.
Of the lesions selected in this study using propensity score matching, only two lesions were resected using forceps in each of the groups, and biopsy polypectomy was not fully evaluated because of the small sample size. Future prospective studies with a larger number of patients and longer follow-up periods are needed.
CP for non-pedunculated polyps of less than 10 mm is equivalent to HP in terms of the local recurrence rate. There were no complications of delayed bleeding or perforation, and CP was considered a safe and useful procedure for the treatment of non-pedunculated colorectal polyps less than 10 mm.
Cold polypectomy (CP) is widely used as a simple and safe procedure for small colorectal polyps. However, there is concern that recurrence rate following CP may be higher than Hot polypectomy (HP) because of unidentified margins of excised specimens and the lack of tumor suppression effect by coagulation.
There were few reports that observed the course over long periods of time after CP in clinical practice. It is important to compare and evaluate the recurrence rate following CP and HP.
The aim of this study was to evaluate the presence of local recurrence following CP and HP using propensity score matching.
We analyzed 275 patients who underwent polypectomy for non-pedunculated colorectal polyps less than 10 mm (959 Lesions) and follow-up endoscopy subsequently. We divided them into the CP group (706 Lesions) and the HP group (253 Lesions). Using propensity score matching, we extracted 215 Lesions in each group and evaluated the local recurrence of CP in the real clinic using medical records.
The local recurrence rate was 0.93% in the CP group and 0.93% in the HP group, without a significant difference (P = 0.688).
Local recurrence after CP was equivalent to that following HP in clinical practice. CP is useful and safe in the treatment of non-pedunculated polyps of less than 10 mm.
Future prospective studies with a larger number of patients and longer follow-up periods are needed in clinical practice.
The authors would like to thank Nagura A, Yoshimura T, Nakano A, Oshima H, Sato J, Ueno Y, Matsuura R, and Mizutani Y for performing the colonoscopies and polypectomies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Z S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2085] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 2. | Gupta N, Bansal A, Rao D, Early DS, Jonnalagadda S, Wani SB, Edmundowicz SA, Sharma P, Rastogi A. Prevalence of advanced histological features in diminutive and small colon polyps. Gastrointest Endosc. 2012;75:1022-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Hassan C, Pickhardt PJ, Kim DH, Di Giulio E, Zullo A, Laghi A, Repici A, Iafrate F, Osborn J, Annibale B. Systematic review: distribution of advanced neoplasia according to polyp size at screening colonoscopy. Aliment Pharmacol Ther. 2010;31:210-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, Nowacki MP, Butruk E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 522] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 5. | Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E, Pagano N, Rando G, Strangio G, Carlino A, Romeo F, de Paula Pessoa Ferreira D, Zullo A, Ridola L, Malesci A. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Ichise Y, Horiuchi A, Nakayama Y, Tanaka N. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion. 2011;84:78-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Paspatis GA, Tribonias G, Konstantinidis K, Theodoropoulou A, Vardas E, Voudoukis E, Manolaraki MM, Chainaki I, Chlouverakis G. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis. 2011;13:e345-e348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Uraoka T, Ramberan H, Matsuda T, Fujii T, Yahagi N. Cold polypectomy techniques for diminutive polyps in the colorectum. Dig Endosc. 2014;26 Suppl 2:98-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 9. | Takeuchi Y, Yamashina T, Matsuura N, Ito T, Fujii M, Nagai K, Matsui F, Akasaka T, Hanaoka N, Higashino K, Iishi H, Ishihara R, Thorlacius H, Uedo N. Feasibility of cold snare polypectomy in Japan: A pilot study. World J Gastrointest Endosc. 2015;7:1250-1256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 59] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Kawamura T, Takeuchi Y, Asai S, Yokota I, Akamine E, Kato M, Akamatsu T, Tada K, Komeda Y, Iwatate M, Kawakami K, Nishikawa M, Watanabe D, Yamauchi A, Fukata N, Shimatani M, Ooi M, Fujita K, Sano Y, Kashida H, Hirose S, Iwagami H, Uedo N, Teramukai S, Tanaka K. A comparison of the resection rate for cold and hot snare polypectomy for 4-9 mm colorectal polyps: a multicentre randomised controlled trial (CRESCENT study). Gut. 2018;67:1950-1957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 11. | Zhang Q, Gao P, Han B, Xu J, Shen Y. Polypectomy for complete endoscopic resection of small colorectal polyps. Gastrointest Endosc. 2018;87:733-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 316] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 13. | Kim JH, Lee HJ, Ahn JW, Cheung DY, Kim JI, Park SH, Kim JK. Risk factors for delayed post-polypectomy hemorrhage: a case-control study. J Gastroenterol Hepatol. 2013;28:645-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Rosen L, Bub DS, Reed JF 3rd, Nastasee SA. Hemorrhage following colonoscopic polypectomy. Dis Colon Rectum. 1993;36:1126-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Kim DH, Lim SW. Analysis of delayed postpolypectomy bleeding in a colorectal clinic. J Korean Soc Coloproctol. 2011;27:13-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Lee CK, Shim JJ, Jang JY. Cold snare polypectomy vs. Cold forceps polypectomy using double-biopsy technique for removal of diminutive colorectal polyps: a prospective randomized study. Am J Gastroenterol. 2013;108:1593-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M, Saito Y, Ikematsu H, Kobayashi K, Inoue Y, Yahagi N, Tsuda S, Simizu S, Iishi H, Yamano H, Kudo SE, Tsuruta O, Tamura S, Cho E, Fujii T, Sano Y, Nakamura H, Sugihara K, Muto T. Current status in the occurrence of postoperative bleeding, perforation and residual/Local recurrence during colonoscopic treatment in Japan. Dig Endosc. 2010;22:376-380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Shimodate Y, Itakura J, Mizuno M, Takezawa R, Kobayashi M, Yamazaki T, Doi A, Nishimura N, Mouri H, Matsueda K, Yamamoto H. Factors Associated with possibly Inappropriate Histological Evaluation of Excised Specimens in Cold-snare Polypectomy for Small Colorectal Polyps. J Gastrointestin Liver Dis. 2018;27:25-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ito A, Suga T, Ota H, Tateiwa N, Matsumoto A, Tanaka E. Resection depth and layer of cold snare polypectomy vs endoscopic mucosal resection. J Gastroenterol. 2018;53:1171-1178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Kim JS, Lee BI, Choi H, Jun SY, Park ES, Park JM, Lee IS, Kim BW, Kim SW, Choi MG. Cold snare polypectomy vs cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 21. | Gómez V, Badillo RJ, Crook JE, Krishna M, Diehl NN, Wallace MB. Diminutive colorectal polyp resection comparing hot and cold snare and cold biopsy forceps polypectomy. Results of a pilot randomized, single-center study (with videos). Endosc Int Open. 2015;3:E76-E80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Matsuura N, Takeuchi Y, Yamashina T, Ito T, Aoi K, Nagai K, Kanesaka T, Matsui F, Fujii M, Akasaka T, Hanaoka N, Higashino K, Tomita Y, Ito Y, Ishihara R, Iishi H, Uedo N. Incomplete resection rate of cold snare polypectomy: a prospective single-arm observational study. Endoscopy. 2017;49:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Lee HS, Park HW, Lee JS, Kim JC, Choe J, Soh JS, Lee S, Bae JH, Lee HJ, Yang DH, Myung SJ, Yang SK, Chang HS, Byeon JS. Treatment outcomes and recurrence following standard cold forceps polypectomy for diminutive polyps. Surg Endosc. 2017;31:159-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Murakami T, Yoshida N, Yasuda R, Hirose R, Inoue K, Dohi O, Kamada K, Uchiyama K, Konishi H, Naito Y, Morinaga Y, Kishimoto M, Konishi E, Ogiso K, Inada Y, Itoh Y. Local recurrence and its risk factors after cold snare polypectomy of colorectal polyps. Surg Endosc. 2020;34:2918-2925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Maruoka D, Arai M, Akizue N, Ishikawa K, Kasamatsu S, Taida T, Ishigami H, Okimoto K, Saito K, Matsumura T, Nakagawa T, Katsuno T, Kato N. Residual adenoma after cold snare polypectomy for small colorectal adenomas: a prospective clinical study. Endoscopy. 2018;50:693-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |