Copyright
©The Author(s) 2021.
World J Gastroenterol. Oct 7, 2021; 27(37): 6277-6289
Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6277
Published online Oct 7, 2021. doi: 10.3748/wjg.v27.i37.6277
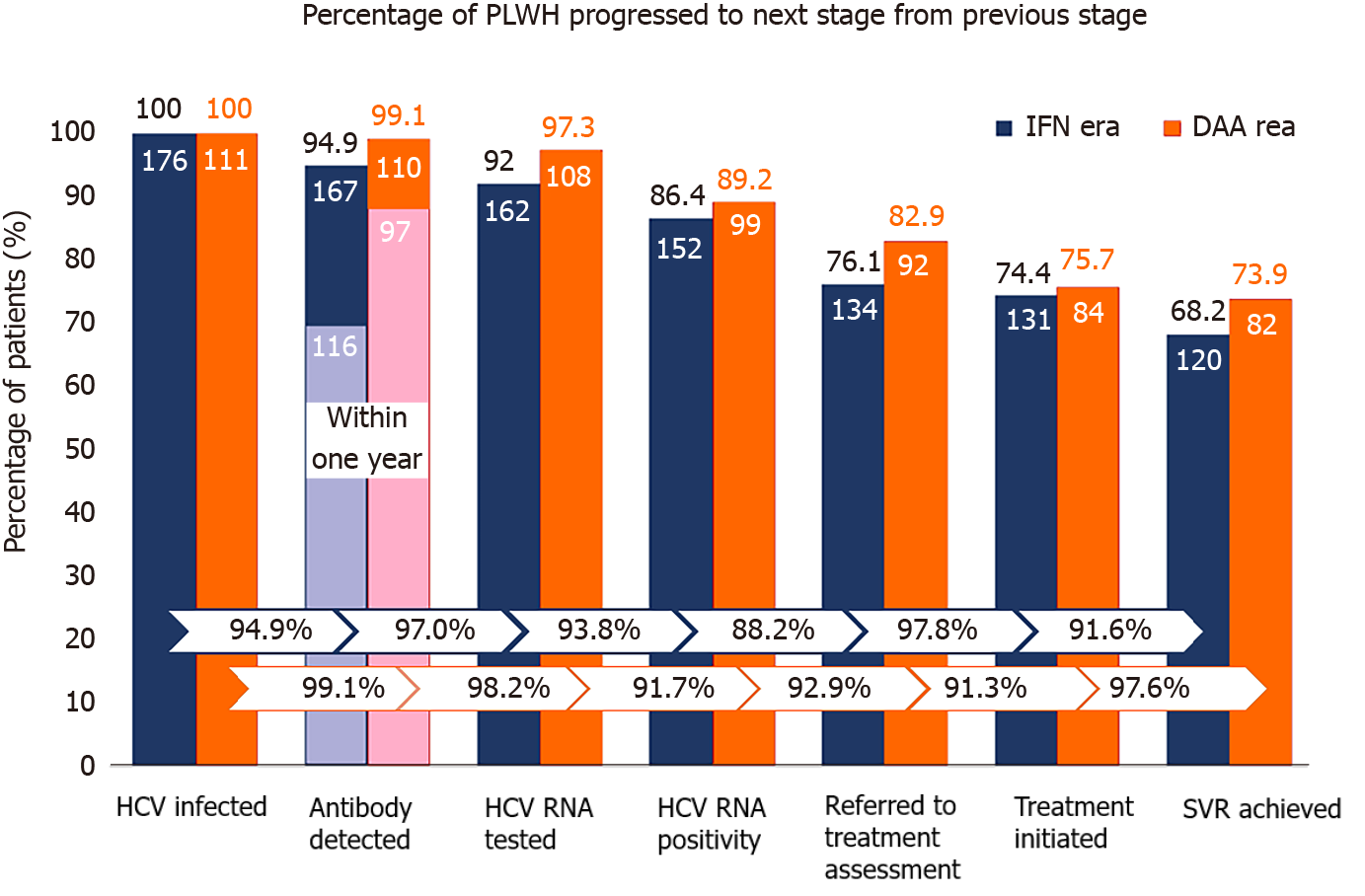
Figure 2 Care cascade of incident hepatitis C virus infections in the interferon and direct-acting antiviral eras.
In the interferon era (blue columns), 176 people living with human immunodeficiency virus (HIV) (PLWH) had incident hepatitis C virus (HCV) infections by retrospective testing of all archive blood samples; 167 (94.9%) were found to have HCV seroconversion by HIV-treating physicians; and the diagnostic rate within one year after seroconversion was 69.4% (116 out of 167). Plasma HCV RNA was tested in 162 (97.0%) PLWH after seroconversion, of which 152 (93.8%) were viremic. A total of 134 (88.2%) viremic PLWH were referred to hepatology clinics; anti-HCV treatments were initiated in 131 (97.8%), and 120 (91.6% of all treated PLWH) achieved sustained virologic response (SVR). In the direct-acting antiviral era (orange columns), 111 PLWH had incident HCV infections; 110 (99.1%) were found to have HCV seroconversion by HIV-treating physicians; and the diagnostic rate within one year after seroconversion was 88.2% (97 out of 110). Plasma HCV RNA was detected in 108 (98.2%) PLWH after seroconversion and 99 (91.7%) were viremic. A total of 92 (92.9%) viremic PLWH received treatment assessment, anti-HCV treatments were initiated in 84 (91.3%), and 82 (97.6% of all treated patients) achieved SVR. IFN: Interferon; DAA: Direct-acting antiviral; HCV: Hepatitis C virus; PLWH: People living with human immunodeficiency virus.
- Citation: Huang MH, Sun HY, Ho SY, Chang SY, Hsieh SM, Sheng WH, Chuang YC, Huang YS, Su LH, Liu WC, Su YC, Hung CC. Recently acquired hepatitis C virus infection among people living with human immunodeficiency virus at a university hospital in Taiwan. World J Gastroenterol 2021; 27(37): 6277-6289
- URL: https://www.wjgnet.com/1007-9327/full/v27/i37/6277.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i37.6277