Published online Aug 28, 2021. doi: 10.3748/wjg.v27.i32.5341
Peer-review started: January 28, 2021
First decision: March 29, 2021
Revised: April 15, 2021
Accepted: July 27, 2021
Article in press: July 27, 2021
Published online: August 28, 2021
Hepatocellular carcinoma (HCC) is the most common primary malignant liver tumor in China. Preoperative diagnosis of HCC is challenging because of atypical imaging manifestations and the diversity of focal liver lesions. Artificial intelligence (AI), such as machine learning (ML) and deep learning, has recently gained attention for its capability to reveal quantitative information on images. Currently, AI is used throughout the entire radiomics process and plays a critical role in multiple fields of medicine. This review summarizes the applications of AI in various aspects of preoperative imaging of HCC, including segmentation, differential diagnosis, prediction of histo
Core Tip: Hepatocellular carcinoma (HCC) threatens human health because of its high morbidity and recurrence rates. Patients with HCC may benefit from early diagnosis, timely treatment, and appropriate follow-up strategies. In the era of big data, artificial intelligence (AI) provides critical information regarding the diagnosis, treatment, and prognosis of HCC. We herein discuss the role of AI in the following aspects of preope
- Citation: Feng B, Ma XH, Wang S, Cai W, Liu XB, Zhao XM. Application of artificial intelligence in preoperative imaging of hepatocellular carcinoma: Current status and future perspectives. World J Gastroenterol 2021; 27(32): 5341-5350
- URL: https://www.wjgnet.com/1007-9327/full/v27/i32/5341.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i32.5341
Hepatocellular carcinoma (HCC), which most often arises from chronic hepatitis B virus infection, is one of the main causes of cancer-related deaths worldwide, particularly in China[1]. HCC presents with characteristic radiological features and can be diagnosed without biopsy. Imaging is therefore crucial for diagnosis and management. Computed tomography (CT) is the most widely used method for HCC diagnosis, although magnetic resonance imaging (MRI) is the optimal diagnostic modality, owing to its multi-parameter imaging techniques. However, even with the application of dynamic contrast-enhanced MRI (DCE-MRI), the imaging diagnosis of HCC is challenging because of atypical imaging manifestations and liver tumor diversity.
A single clinical image contains a large amount of quantitative information that can provide crucial data for diagnostic and treatment purposes. This information can be processed using innovative methods. Radiomics has recently gained attention for its potential to further analyze images. It allows for the extraction of a large amount of quantitative objective data included in radiological images that could be explored for determining underlying biological processes[2]. The workflow of a radiomics study generally includes five stages: Image acquisition, segmentation, feature extraction, exploratory analysis, and modeling[3] (Figure 1). Every stage is closely related to artificial intelligence (AI). The concept of AI was first advocated in 1955 by McCarthy et al[4], who described AI as a computer program that attempts to simulate human cognitive functions. AI can learn and solve problems to improve itself.
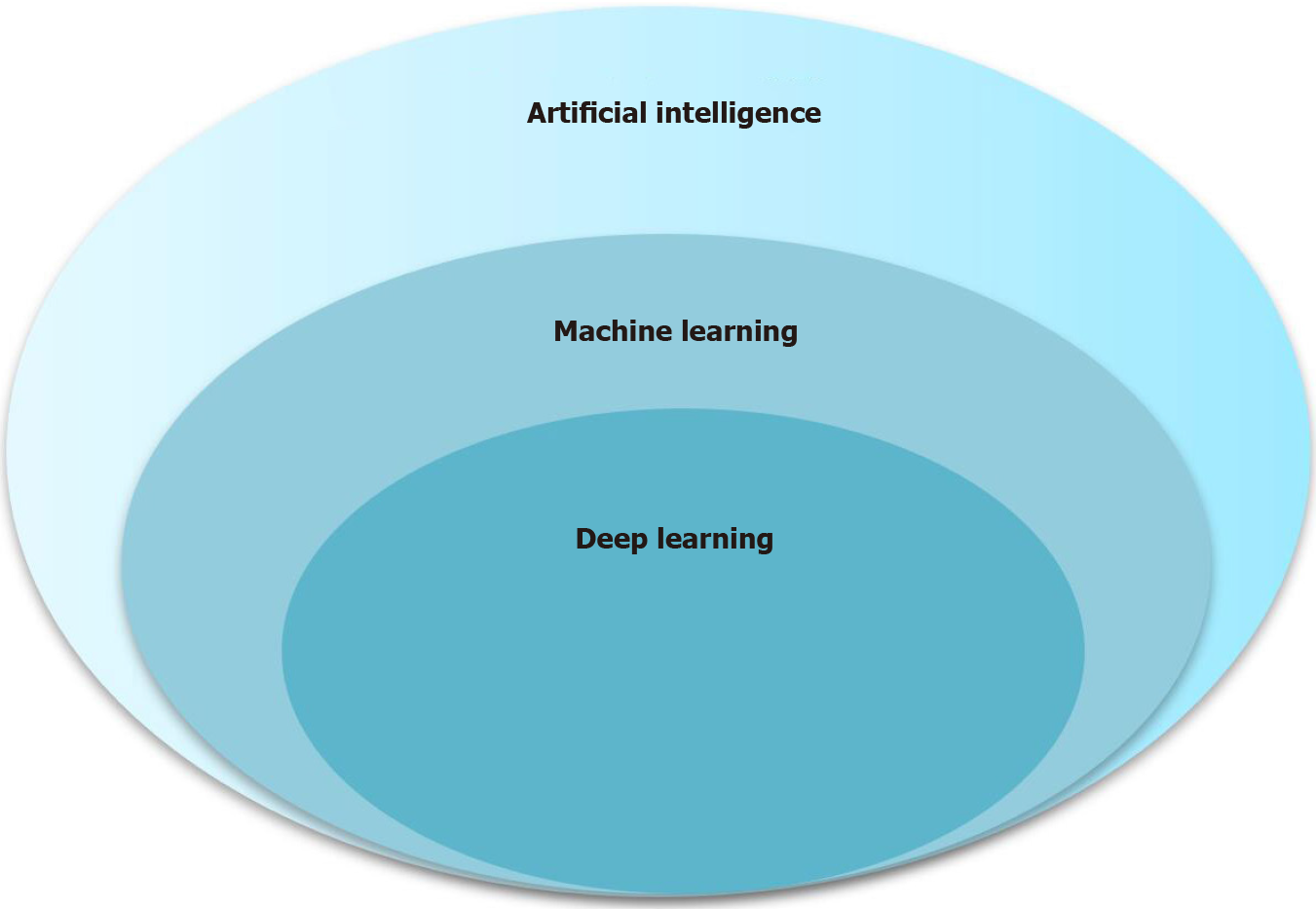
Machine learning (ML) is at the core of AI and involves various techniques, such as artificial neural networks, support vector machines (SVM), and random forest (RF). Deep learning (DL) is an important branch of ML, and convolutional neural networks (CNNs) are a type of DL algorithm. The relationships among AI, ML, and DL are described in Figure 2. With technological advances and the use of AI, radiomics has rapidly developed in recent years, and all radiomics techniques have now been utilized in studies within the medical field. This review focuses on the application of AI for HCC imaging, including segmentation, differential diagnosis, prediction of histopathology, early detection of recurrence after curative treatment, and evaluation of treatment response.
Although HCC segmentation is challenging, it is crucial for various medical imaging analyses. Manual and semi-manual segmentation are time-consuming and susceptible to interobserver variability, which might in turn lead to the biased results. In contrast, automatic segmentation based on an AI algorithm is more repeatable and efficient. CNNs have been successfully applied for the automatic segmentation of hepatic lesions in both CT and MRI. Chlebus et al[5] figured out a CNN model for the automatic segmentation of liver tumors on CT; however, with respect to tumor detection, the model performed poorly compared with human performance. Bousabarah et al[6] established a DL algorithm to automatically delineate the liver and HCC lesions on MRI. The model was a combination of a deep CNN, RF, and thresholding. The mean dice similarity coefficient between the DL model and manual segmentation was 0.64/0.68 (validation/test) for tumor segmentation and 0.91/0.91 for liver segmentations. Current methods do not achieve a comparably high level of performance in liver imaging for automatic tumor segmentation. This may be due to the heterogeneity of the liver parenchyma and the tumor itself. Larger datasets will be required to improve the accuracy of DL algorithms and will ensure optimal sensitivity and specificity.
Imaging-based differential diagnosis improves the accuracy of diagnosis. For HCC, the primary diagnostic modalities are CT and MRI. Radiomics with AI has been gradually applied in clinical research, as it can provide quantitative information, including additional differential characteristics not yet recognized in current radiological diagnosis. This could, in turn, help less-experienced radiologists diagnose focal liver lesions more accurately. Table 1 summarizes the studies on the differential diagnosis of HCC with AI.
| Ref. | Aim of the study | Modality | Patients | Method | AUC of the final model |
| Nie et al[7] | Differentiation between HCC and HCA | CT | 131 | ML | T: 0.96, V: 0.94 |
| Nie et al[8] | Differentiation between HCC and FNH | CT | 156 | ML | T: 0.979, V: 0.917 |
| Mokrane et al[9] | Differentiation between HCC and non-HCC nodules | CT | 178 | ML | T: 0.70, V: 0.66 |
| Ponnoprat et al[10] | Differentiation between HCC and ICC | CT | 257 | ML | NA |
| Shi et al[11] | Differentiation of HCC from other FLLs | CT | 342 | DL | 0.925 |
| Yasaka et al[12] | Liver mass classification | CT | 560 | DL | 0.92 |
| Cao et al[13] | FLL classification | CT | 375 | DL | V: 0.88-0.99 |
| Jiang et al[14] | Comparing the diagnostic accuracies of EASL (v2018), LI-RADS criteria, and radiomics models for HCC | MRI | 211 | ML | T: 0.861, V: 0.810 |
| Zhen et al[15] | Classification of liver tumors | MRI | 1411 | DL | 0.963-0.998 |
| Liu et al[16] | Differentiation of cHCC-CC from CC and HCC | MRI | 85 | ML | 0.77 |
| Huang et al[17] | Diagnosis of DPHCC | MRI | 100 | ML | 0.784 |
| Jian et al[18] | Characterization of HCC | MRI | 112 | DL | NA |
| Wu et al[19] | Classification of HCC and hepatic hemangioma | MRI | 369 | ML | T: 0.86, V: 0.89 |
Some studies have applied conventional ML algorithms for the differentiation of liver masses. In the non-cirrhotic liver, Nie et al[7,8] reported CT-based radiomics nomograms for the preoperative differentiation of HCC from hepatocellular adenoma (HCA) and focal nodular hyperplasia (FNH). It showed good discrimination capability, with an area under the curve (AUC) of 0.96/0.94 and 0.979/0.917 (tra
MRI can provide more comprehensive information for differential diagnosis than CT because of its multi-parameter techniques and various tissue contrast mechanisms. Accurate diagnosis of HCC remains a challenge because of liver tumor diversity and relies on the experience of radiologists. Jiang et al[14] established a radiomics signature from multi-sequence MRI using the least absolute shrinkage and selection operator (LASSO) model and multivariate logistic regression analysis. The AUC of the radiomics signature was 0.810. It showed a comparable accuracy with the LI-RADS (0.841) and EASL criteria (0.811) for HCC diagnosis. Zhen et al[15] used CNNs to develop a DL model to classify liver tumors based on MR images. The CNN model combined with clinical data performed well in identifying HCC (AUC, 0.985; 95%CI: 0.960-1.000), metastatic tumors (AUC, 0.998; 95%CI: 0.989-1.000), and other primary malignancies (AUC, 0.963; 95%CI: 0.896-1.000). Some researchers have also applied ML algorithms to the differential diagnosis of primary liver cancer subtypes. Liu et al[16] reported an ML analysis of MRI radiomics features for the differentiation of combined hepatocellular cholangiocarcinoma (cHCC-CC) from HCC and cholangiocarcinoma. The model showed acceptable performance, with an AUC of 0.77. Huang et al[17] created a radiomics signature from Gd-EOB-DTPA-enhanced MRI for the diagnosis of dual-phenotype HCC (DPHCC). They used LASSO and four classifiers: Multi-layer perceptron, SVM, logistic regression, and K-nearest neighbor. The combination of different phases and classifiers achieved the best performance in the preoperative diagnosis of DPHCC. ML and DL algorithms can also be used in non-contrast MRI to increase the diagnostic accuracy of HCC[18,19]. This is beneficial for patients who cannot receive contrast injections.
CT- or MRI-based ML and DL models have demonstrated promising predictive performance and have reached a high level of accuracy similar to that of experienced radiologists. Accurate preoperative diagnosis of focal liver lesions can help clinicians make proper decisions, optimize patient management, and improve patient prognosis.
Histological grade represents the biological behavior and aggressiveness of tumors. The high recurrence rate of HCC is associated with its pathological grade. The pathological grade of HCC determined using imaging may provide valuable prognostic information. Mao et al[20] developed an ML model based on contrast-enhanced CT for preoperative prediction of the pathological grade of HCC. The model achieved an AUC of 0.8014 when combined with clinical factors. The radiomics signature based on T1WI, T2WI, and DCE-MRI was also found to be significantly related to the histopathological grade of HCC[21,22]. Yang et al[22] proposed a DL model with a multichannel fusion three-dimensional CNN (3D-CNN) based on DCE-MR to differentiate among pathological grades. The model reached an average accuracy of 0.7396 ± 0.0104. Thus, further improvements are needed to achieve better diagnostic performance of radiomics models for histological grade.
In addition to predicting the HCC grade, the prediction of microvascular invasion (MVI) has also become a common topic of study. MVI status is a crucial factor influencing treatment selection and follow-up planning; thus, it must be determined preoperatively[23,24]. Some studies have tried to predict MVI before surgery using radiological images and clinical factors. They found that MVI was closely related to several factors. Some studies used LASSO to construct radiomics signatures from CT and MRI and developed models with various conventional ML algorithms for the preoperative prediction of MVI[25-27]. The results showed favorable predictive accuracy for MVI status in HCC patients, especially when combined with clinical factors[28,29]. Jiang et al[30] figured out a CT-based model using eXtreme Gradient Boosting and DL algorithm with 3D-CNN to predict MVI preoperatively. The 3D-CNN model demonstrated high diagnostic capability, with an AUC of 0.980/0.906 (training/validation). However, different dimensionality reduction and modeling methods affect the diagnostic performance of the final models. Ni et al[31] retrospectively analyzed 206 HCC cases to explore the best radiomic-based diagnostic model. The LASSO + GBDT method showed better performance than the other methods, when the threshold probability was more than 0.22.
MVI usually occurs in the peritumoral region, but it is unclear whether including the features of the peritumoral region could improve predictive capability. Nebbia et al[32] developed an ML model for preoperative prediction of MVI status using multiparametric MRI. They found that radiomics features extracted from the tumor region had good diagnostic performance, with an AUC of 0.867. Radiomics features from the peritumoral region also showed an association with MVI; however, the AUC was slightly lower than that of intratumoral radiomics features. Although peritumoral enhancement pattern is reported to be a good predictor of MVI status[33], the usefulness of the features extracted from peritumoral regions for predicting MVI status needs further evaluation in larger populations.
HCC originates from hepatocytes and/or hepatic progenitor cells and can express various molecular phenotypes[34]. As such, prognosis varies even among patients with the same pathological grade. Molecular profiling is an important modality that may reflect the biological behavior and invasiveness of tumors. Several studies have used ML methods to identify the molecular phenotypes of HCC preoperatively. CK19 is a biliary-specific marker, and CK19 positivity is associated with a poor posto
Glypican 3 (GPC3), a type of heparan sulfate proteoglycan, is located on the cell surface. Previous studies have found that GPC3 is closely associated with postoperative metastasis and recurrence in patients with HCC[38]. Furthermore, GPC3 is considered a potential immunotherapeutic target for HCC therapy, especially in patients with unresectable HCC. Gu et al[39] constructed a radiomics signature from MRI and achieved good predictive efficacy, with an AUC of 0.879/0.871 (tra
Individualized medical care depends on accurate risk stratification systems. These systems help select the proper treatment and evaluate treatment response. Pretreatment imaging acts as an important role in predicting the effects of treatment, helping clinicians choose the best individualized treatment strategy for patients.
Early recurrence, that is, recurrence within 1-2 years after resection or ablation, is a strong influencing factor of poor prognosis in patients with HCC. A radiomics nomogram derived from preoperative CT and MRI was established to predict early recurrence after surgery or curative ablation in HCC patients[42,43]. The radiomics nomogram comprising both the radiomics score and clinicoradiological risk factors demonstrated good performance, with an AUC of 0.785-0.844[44,45]. Prediction of early recurrence is critical for planning follow-up surveillance strategies and determining the necessity of further interventions after curative treatment. Wang et al[46] proposed a DL-based radiomics approach from multi-phase CT images to predict the early recurrence of HCC; it achieved an AUC of 0.825. Unfortunately, image-based radiomics models for early recurrent HCC have poor reproducibility among medical centers[47], limiting their application in clinical practice.
Patients with unresectable HCC can undergo trans-arterial chemoembolization (TACE), local radiofrequency ablation, and systemic treatment with sorafenib. ML or DL models based on pretreatment CT or MRI have been recently considered as potential tools for predicting treatment response to TACE for HCC[48,49]. Morshid et al[50] used pretreatment quantitative CT image features and clinical factors to develop an ML model to predict treatment response to TACE; the model had an accuracy of 74.2%. Abajian et al[51] used MR imaging and clinical data to create an AI model for the prediction of TACE treatment response; the model had an overall accuracy of 78%. Similar results were obtained in other studies, confirming the usefulness of radiomics features extracted from pretreatment CT or MRI for the prediction of tumor response to TACE[52-54]. Peng et al[55] described a DL model of a residual CNN to predict the treatment response to TACE for HCC. The final model had a high accuracy in predicting four different therapy response types (complete response, partial response, stable disease, and progressive disease). Thus, it could help clinicians identify patients who will optimally benefit from TACE.
Immunotherapy has been shown to be a promising treatment for HCC; however, treatment response to immunotherapy remains low[56-58]. Therefore, it is necessary for clinicians to identify which patients will respond to immunotherapy. Treatment response to immunotherapy is highly dependent on the immune status of the tumor[59]. Tumor immunoprofiling is thus important in predicting its effect. A contrast-enhanced CT-based Rad score developed using the ML algorithm showed high predictive power for CD8+ T-cell infiltration, which is associated with the immunotherapy response[60]. Hectors et al[61] reported that MRI radiomics features were correlated with various immunohistochemical cell markers and the expression of certain immunotherapy targets. Chen et al[62] used clinical data and intratumoral and peritumoral radiomics features to build an ML model from Gd-EOB-DTPA-enhanced MRI. The model showed excellent performance in predicting the immunoscore, with an AUC of 0.926 (95%CI: 0.884-0.967). Collectively, these findings indicated that radiomics features extracted by ML methods might serve as noninvasive predictors of the immune characteristics of HCC and assist physicians in identifying patients who will benefit from immunotherapy.
The application of AI in different fields of medical research has demonstrated promising results; however, there are some limitations. First, nearly all the research was retrospective and included a relatively small sample size. Therefore, the performance of these predictive models should be validated in larger, multicenter, and prospective studies. Second, the predictive models had limited reproducibility for application in clinical practice, and image heterogeneity might be a significant influencing factor. Third, the AI calculation algorithm requires specialized software packages, leading to increased medical costs. The workflow includes imaging acqui
While AI and radiomics have proven useful in various aspects of HCC, the underlying mechanisms have not been clearly stated, such as pathological correlation and relationship between radiomics and genomics. More research is needed to explore the relationships among imaging, pathophysiology, and prognosis.
AI has been applied in many studies on preoperative imaging of HCC. It can extract a large amount of quantitative information from images and reflect pathophysiological processes. Diagnostic and predictive models using AI algorithms have demonstrated promising results in the fields of segmentation, differential diagnosis, prediction of histology, and guidance for treatment selection. However, considering the limitations and complexity of AI, additional research is needed before it can be widely used in clinical practice. Some specific issues, such as reproducibility, heterogeneity of imaging acquisition, and lack of external multicenter validation, need to be considered. Further research will be crucial in improving the accuracy and reproducibility of diagnostic and predictive models, enabling their application for individualized treatment in patients with HCC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang SS S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2066] [Cited by in F6Publishing: 2600] [Article Influence: 520.0] [Reference Citation Analysis (0)] |
| 2. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4541] [Cited by in F6Publishing: 4516] [Article Influence: 564.5] [Reference Citation Analysis (2)] |
| 3. | Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY). 2021;46:111-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | McCarthy J, Minsky ML, Rochester N, Shannon CE. A Proposal for the Dartmouth Summer Research Project on Artificial Intelligence. AI Mag. 1955;27:12-14. [Cited in This Article: ] |
| 5. | Chlebus G, Schenk A, Moltz JH, van Ginneken B, Hahn HK, Meine H. Automatic liver tumor segmentation in CT with fully convolutional neural networks and object-based postprocessing. Sci Rep. 2018;8:15497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 6. | Bousabarah K, Letzen B, Tefera J, Savic L, Schobert I, Schlachter T, Staib LH, Kocher M, Chapiro J, Lin M. Automated detection and delineation of hepatocellular carcinoma on multiphasic contrast-enhanced MRI using deep learning. Abdom Radiol (NY). 2021;46:216-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Nie P, Wang N, Pang J, Yang G, Duan S, Chen J, Xu W. CT-Based Radiomics Nomogram: A Potential Tool for Differentiating Hepatocellular Adenoma From Hepatocellular Carcinoma in the Noncirrhotic Liver. Acad Radiol. 2021;28:799-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Nie P, Yang G, Guo J, Chen J, Li X, Ji Q, Wu J, Cui J, Xu W. A CT-based radiomics nomogram for differentiation of focal nodular hyperplasia from hepatocellular carcinoma in the non-cirrhotic liver. Cancer Imaging. 2020;20:20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Mokrane FZ, Lu L, Vavasseur A, Otal P, Peron JM, Luk L, Yang H, Ammari S, Saenger Y, Rousseau H, Zhao B, Schwartz LH, Dercle L. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol. 2020;30:558-570. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 10. | Ponnoprat D, Inkeaw P, Chaijaruwanich J, Traisathit P, Sripan P, Inmutto N, Na Chiangmai W, Pongnikorn D, Chitapanarux I. Classification of hepatocellular carcinoma and intrahepatic cholangiocarcinoma based on multi-phase CT scans. Med Biol Eng Comput. 2020;58:2497-2515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Shi W, Kuang S, Cao S, Hu B, Xie S, Chen S, Chen Y, Gao D, Zhu Y, Zhang H, Liu H, Ye M, Sirlin CB, Wang J. Deep learning assisted differentiation of hepatocellular carcinoma from focal liver lesions: choice of four-phase and three-phase CT imaging protocol. Abdom Radiol (NY). 2020;45:2688-2697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 13. | Cao SE, Zhang LQ, Kuang SC, Shi WQ, Hu B, Xie SD, Chen YN, Liu H, Chen SM, Jiang T, Ye M, Zhang HX, Wang J. Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrast-enhanced computed tomography. World J Gastroenterol. 2020;26:3660-3672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 14. | Jiang H, Liu X, Chen J, Wei Y, Lee JM, Cao L, Wu Y, Duan T, Li X, Ma L, Song B. Man or machine? Cancer Imaging. 2019;19:84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Zhen SH, Cheng M, Tao YB, Wang YF, Juengpanich S, Jiang ZY, Jiang YK, Yan YY, Lu W, Lue JM, Qian JH, Wu ZY, Sun JH, Lin H, Cai XJ. Deep Learning for Accurate Diagnosis of Liver Tumor Based on Magnetic Resonance Imaging and Clinical Data. Front Oncol. 2020;10:680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Liu X, Khalvati F, Namdar K, Fischer S, Lewis S, Taouli B, Haider MA, Jhaveri KS. Can machine learning radiomics provide pre-operative differentiation of combined hepatocellular cholangiocarcinoma from hepatocellular carcinoma and cholangiocarcinoma to inform optimal treatment planning? Eur Radiol. 2021;31:244-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Huang X, Long L, Wei J, Li Y, Xia Y, Zuo P, Chai X. Radiomics for diagnosis of dual-phenotype hepatocellular carcinoma using Gd-EOB-DTPA-enhanced MRI and patient prognosis. J Cancer Res Clin Oncol. 2019;145:2995-3003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Jian W, Ju H, Cen X, Cui M, Zhang H, Zhang L, Wang G, Gu L, Zhou W. Improving the malignancy characterization of hepatocellular carcinoma using deeply supervised cross modal transfer learning for non-enhanced MR. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:853-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Wu J, Liu A, Cui J, Chen A, Song Q, Xie L. Radiomics-based classification of hepatocellular carcinoma and hepatic haemangioma on precontrast magnetic resonance images. BMC Med Imaging. 2019;19:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Mao B, Zhang L, Ning P, Ding F, Wu F, Lu G, Geng Y, Ma J. Preoperative prediction for pathological grade of hepatocellular carcinoma via machine learning-based radiomics. Eur Radiol. 2020;30:6924-6932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Wu M, Tan H, Gao F, Hai J, Ning P, Chen J, Zhu S, Wang M, Dou S, Shi D. Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol. 2019;29:2802-2811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 22. | Yang DW, Jia XB, Xiao YJ, Wang XP, Wang ZC, Yang ZH. Noninvasive Evaluation of the Pathologic Grade of Hepatocellular Carcinoma Using MCF-3DCNN: A Pilot Study. Biomed Res Int. 2019;2019:9783106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Peng Z, Chen S, Xiao H, Wang Y, Li J, Mei J, Chen Z, Zhou Q, Feng S, Chen M, Qian G, Peng S, Kuang M. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology. 2019;292:237-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Tang A. Using MRI to Assess Microvascular Invasion in Hepatocellular Carcinoma. Radiology. 2020;297:582-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Ruan S, Xiao W, Shao J, Tian W, Liu W, Zhang Z, Wan D, Huang J, Huang Q, Yang Y, Yang H, Ding Y, Liang W, Bai X, Liang T. Contrast-enhanced CT radiomics for preoperative evaluation of microvascular invasion in hepatocellular carcinoma: A two-center study. Clin Transl Med. 2020;10:e111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Peng J, Zhang J, Zhang Q, Xu Y, Zhou J, Liu L. A radiomics nomogram for preoperative prediction of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma. Diagn Interv Radiol. 2018;24:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, Wang S, Zhao X, Tian J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595-3605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 28. | Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, Chen C, Ding Y, Tian J, Zeng M. A Radiomics Nomogram for Preoperative Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2019;8:373-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 29. | Zhang R, Xu L, Wen X, Zhang J, Yang P, Zhang L, Xue X, Wang X, Huang Q, Guo C, Shi Y, Niu T, Chen F. A nomogram based on bi-regional radiomics features from multimodal magnetic resonance imaging for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Quant Imaging Med Surg. 2019;9:1503-1515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Jiang YQ, Cao SE, Cao S, Chen JN, Wang GY, Shi WQ, Deng YN, Cheng N, Ma K, Zeng KN, Yan XJ, Yang HZ, Huan WJ, Tang WM, Zheng Y, Shao CK, Wang J, Yang Y, Chen GH. Preoperative identification of microvascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J Cancer Res Clin Oncol. 2021;147:821-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 31. | Ni M, Zhou X, Lv Q, Li Z, Gao Y, Tan Y, Liu J, Liu F, Yu H, Jiao L, Wang G. Radiomics models for diagnosing microvascular invasion in hepatocellular carcinoma: which model is the best model? Cancer Imaging. 2019;19:60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Nebbia G, Zhang Q, Arefan D, Zhao X, Wu S. Pre-operative Microvascular Invasion Prediction Using Multi-parametric Liver MRI Radiomics. J Digit Imaging. 2020;33:1376-1386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). 2019;44:539-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Lee SH, Lee JS, Na GH, You YK, Kim DG. Immunohistochemical markers for hepatocellular carcinoma prognosis after liver resection and liver transplantation. Clin Transplant. 2017;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Roncalli M, Park YN, Di Tommaso L. Histopathological classification of hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S228-S234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Wang W, Gu D, Wei J, Ding Y, Yang L, Zhu K, Luo R, Rao SX, Tian J, Zeng M. A radiomics-based biomarker for cytokeratin 19 status of hepatocellular carcinoma with gadoxetic acid-enhanced MRI. Eur Radiol. 2020;30:3004-3014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 38. | Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, Fang-yin Z, Da-yong Z, Rong-cheng L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39:351-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Gu D, Xie Y, Wei J, Li W, Ye Z, Zhu Z, Tian J, Li X. MRI-Based Radiomics Signature: A Potential Biomarker for Identifying Glypican 3-Positive Hepatocellular Carcinoma. J Magn Reson Imaging. 2020;52:1679-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Ye Z, Jiang H, Chen J, Liu X, Wei Y, Xia C, Duan T, Cao L, Zhang Z, Song B. Texture analysis on gadoxetic acid enhanced-MRI for predicting Ki-67 status in hepatocellular carcinoma: A prospective study. Chin J Cancer Res. 2019;31:806-817. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 41. | Wu H, Han X, Wang Z, Mo L, Liu W, Guo Y, Wei X, Jiang X. Prediction of the Ki-67 marker index in hepatocellular carcinoma based on CT radiomics features. Phys Med Biol. 2020;65:235048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 42. | Zhou Y, He L, Huang Y, Chen S, Wu P, Ye W, Liu Z, Liang C. CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol (NY). 2017;42:1695-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 43. | Yuan C, Wang Z, Gu D, Tian J, Zhao P, Wei J, Yang X, Hao X, Dong D, He N, Sun Y, Gao W, Feng J. Prediction early recurrence of hepatocellular carcinoma eligible for curative ablation using a Radiomics nomogram. Cancer Imaging. 2019;19:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Zhu HB, Zheng ZY, Zhao H, Zhang J, Zhu H, Li YH, Dong ZY, Xiao LS, Kuang JJ, Zhang XL, Liu L. Radiomics-based nomogram using CT imaging for noninvasive preoperative prediction of early recurrence in patients with hepatocellular carcinoma. Diagn Interv Radiol. 2020;26:411-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Zhang Z, Jiang H, Chen J, Wei Y, Cao L, Ye Z, Li X, Ma L, Song B. Hepatocellular carcinoma: radiomics nomogram on gadoxetic acid-enhanced MR imaging for early postoperative recurrence prediction. Cancer Imaging. 2019;19:22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 46. | Wang W, Chen Q, Iwamoto Y, Han X, Zhang Q, Hu H, Lin L, Chen YW. Deep Learning-Based Radiomics Models for Early Recurrence Prediction of Hepatocellular Carcinoma with Multi-phase CT Images and Clinical Data. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:4881-4884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Hu HT, Shan QY, Chen SL, Li B, Feng ST, Xu EJ, Li X, Long JY, Xie XY, Lu MD, Kuang M, Shen JX, Wang W. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: technical reproducibility of acquisition and scanners. Radiol Med. 2020;125:697-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 48. | Kim J, Choi SJ, Lee SH, Lee HY, Park H. Predicting Survival Using Pretreatment CT for Patients With Hepatocellular Carcinoma Treated With Transarterial Chemoembolization: Comparison of Models Using Radiomics. AJR Am J Roentgenol. 2018;211:1026-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 49. | Liu QP, Xu X, Zhu FP, Zhang YD, Liu XS. Prediction of prognostic risk factors in hepatocellular carcinoma with transarterial chemoembolization using multi-modal multi-task deep learning. EClinicalMedicine. 2020;23:100379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Morshid A, Elsayes KM, Khalaf AM, Elmohr MM, Yu J, Kaseb AO, Hassan M, Mahvash A, Wang Z, Hazle JD, Fuentes D. A machine learning model to predict hepatocellular carcinoma response to transcatheter arterial chemoembolization. Radiol Artif Intell. 2019;1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 51. | Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Intra-arterial Therapies for Hepatocellular Carcinoma with the Use of Supervised Machine Learning-An Artificial Intelligence Concept. J Vasc Interv Radiol. 2018;29:850-857.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 52. | Song W, Yu X, Guo D, Liu H, Tang Z, Liu X, Zhou J, Zhang H, Liu Y. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging. 2020;52:461-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 53. | Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Image-Guided Therapies Using Machine Learning: An Example for Trans-Arterial Treatment of Hepatocellular Carcinoma. J Vis Exp. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Meng XP, Wang YC, Ju S, Lu CQ, Zhong BY, Ni CF, Zhang Q, Yu Q, Xu J, Ji J, Zhang XM, Tang TY, Yang G, Zhao Z. Radiomics Analysis on Multiphase Contrast-Enhanced CT: A Survival Prediction Tool in Patients With Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front Oncol. 2020;10:1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Peng J, Kang S, Ning Z, Deng H, Shen J, Xu Y, Zhang J, Zhao W, Li X, Gong W, Huang J, Liu L. Residual convolutional neural network for predicting response of transarterial chemoembolization in hepatocellular carcinoma from CT imaging. Eur Radiol. 2020;30:413-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 56. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 505] [Cited by in F6Publishing: 703] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 57. | Lee MS, Ryoo BY, Hsu CH, Numata K, Stein S, Verret W, Hack SP, Spahn J, Liu B, Abdullah H, Wang Y, He AR, Lee KH; GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21:808-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 320] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 58. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2542] [Cited by in F6Publishing: 3400] [Article Influence: 850.0] [Reference Citation Analysis (1)] |
| 59. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2373] [Cited by in F6Publishing: 3068] [Article Influence: 438.3] [Reference Citation Analysis (0)] |
| 60. | Liao H, Zhang Z, Chen J, Liao M, Xu L, Wu Z, Yuan K, Song B, Zeng Y. Preoperative Radiomic Approach to Evaluate Tumor-Infiltrating CD8+ T Cells in Hepatocellular Carcinoma Patients Using Contrast-Enhanced Computed Tomography. Ann Surg Oncol. 2019;26:4537-4547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Hectors SJ, Lewis S, Besa C, King MJ, Said D, Putra J, Ward S, Higashi T, Thung S, Yao S, Laface I, Schwartz M, Gnjatic S, Merad M, Hoshida Y, Taouli B. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. 2020;30:3759-3769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 62. | Chen S, Feng S, Wei J, Liu F, Li B, Li X, Hou Y, Gu D, Tang M, Xiao H, Jia Y, Peng S, Tian J, Kuang M. Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol. 2019;29:4177-4187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |