Copyright
©The Author(s) 2021.
World J Gastroenterol. May 14, 2021; 27(18): 2177-2192
Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2177
Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2177
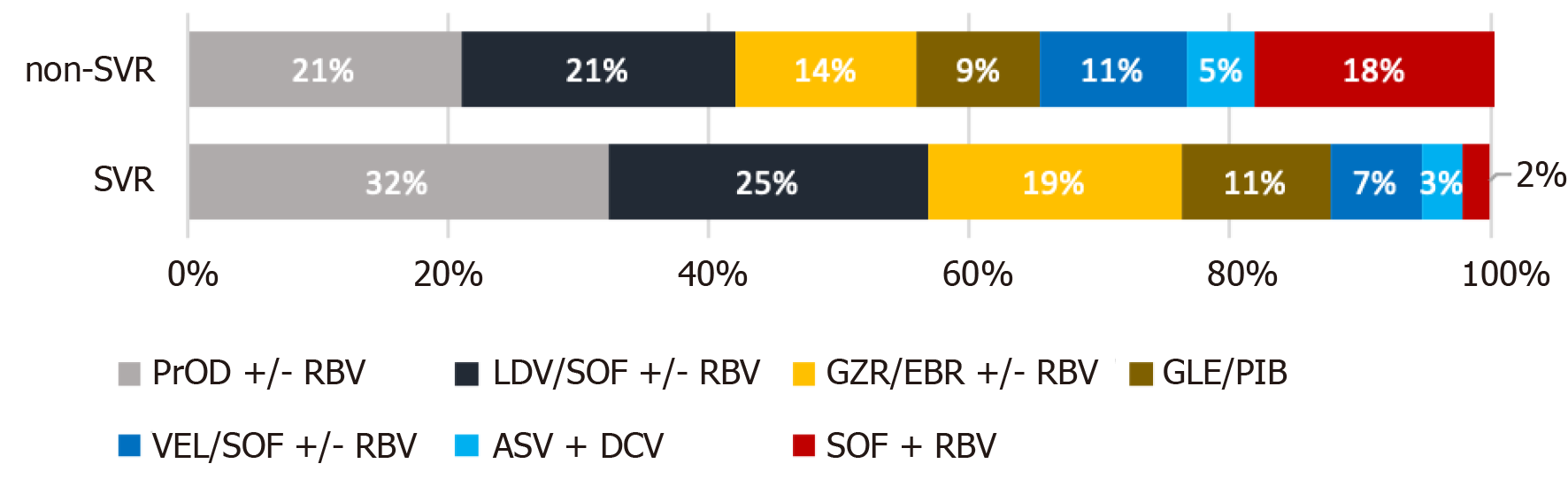
Figure 2 Distribution of treatment regimens among patients who did and did not achieve sustained virologic response.
SVR: Sustained virologic response; RBV: Ribavirin; SOF: Sofosbuvir; PrOD: Paritaprevir/ritonavir +/- dasabuvir; LDV: Ledipasvir; GZR: Grazoprevir; EBR: Elbasvir; GLE: Glecaprevir; PIB: Pibrentasvir; VEL: Velpatasvir; ASV: Asunaprevir; DCV: Daclatasvir.
- Citation: Janczewska E, Kołek MF, Lorenc B, Klapaczyński J, Tudrujek-Zdunek M, Sitko M, Mazur W, Zarębska-Michaluk D, Buczyńska I, Dybowska D, Czauż-Andrzejuk A, Berak H, Krygier R, Jaroszewicz J, Citko J, Piekarska A, Dobracka B, Socha Ł, Deroń Z, Laurans Ł, Białkowska-Warzecha J, Tronina O, Adamek B, Tomasiewicz K, Simon K, Pawłowska M, Halota W, Flisiak R. Factors influencing the failure of interferon-free therapy for chronic hepatitis C: Data from the Polish EpiTer-2 cohort study. World J Gastroenterol 2021; 27(18): 2177-2192
- URL: https://www.wjgnet.com/1007-9327/full/v27/i18/2177.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i18.2177