Copyright
©The Author(s) 2020.
World J Gastroenterol. Aug 21, 2020; 26(31): 4589-4606
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
Published online Aug 21, 2020. doi: 10.3748/wjg.v26.i31.4589
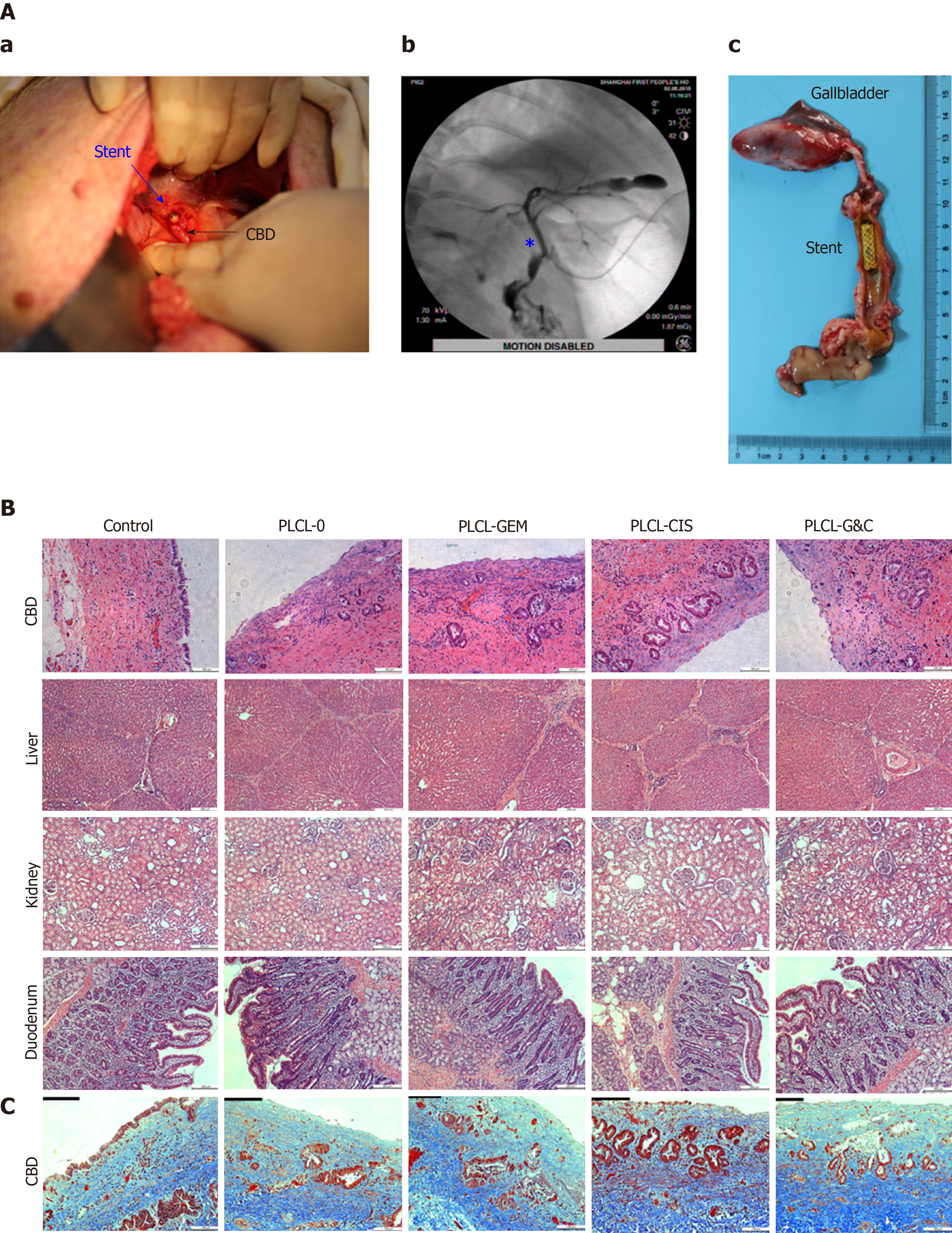
Figure 5 Safety evaluation of different drug-eluting biliary stents in a porcine model.
A: (a) Critical surgical step necessary to place a stent into the porcine CBD; the stent was sutured on the CBD wall carefully when closing the CBD to avoid stent migration; (b) cholangiogram after stent placement; the pentacle marks the location of the stent; (c) macroscopic appearance of the CBD after animals were sacrificed; all the stents were still in the CBD and had not migrated; B: HE staining of the CBD (magnification ×200, scale bar 100 µm), liver, kidney, and duodenum (× 100, scale bar 200 µm) 30 d after placement of different drug-eluting biliary stents; C: Masson trichrome staining of the CBD 30 d after placement of different drug-eluting biliary stents (× 200, scale bar 100 µm). CBD: Common bile duct; CIS: Cisplatin; GEM: Gemcitabine; PLCL: Poly-L-lactide-caprolactone; PLCL-0: Non-drug-loaded PLCL nanofilm; PLCL-CIS: PLCL nanofilm loaded with CIS; PLCL-GEM: PLCL nanofilm loaded with GEM; PLCL-GC: PLCL nanofilm loaded with both GEM and CIS.
- Citation: Xiao JB, Weng JY, Hu YY, Deng GL, Wan XJ. Feasibility and efficacy evaluation of metallic biliary stents eluting gemcitabine and cisplatin for extrahepatic cholangiocarcinoma. World J Gastroenterol 2020; 26(31): 4589-4606
- URL: https://www.wjgnet.com/1007-9327/full/v26/i31/4589.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i31.4589