Copyright
©The Author(s) 2019.
World J Gastroenterol. Oct 7, 2019; 25(37): 5604-5618
Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5604
Published online Oct 7, 2019. doi: 10.3748/wjg.v25.i37.5604
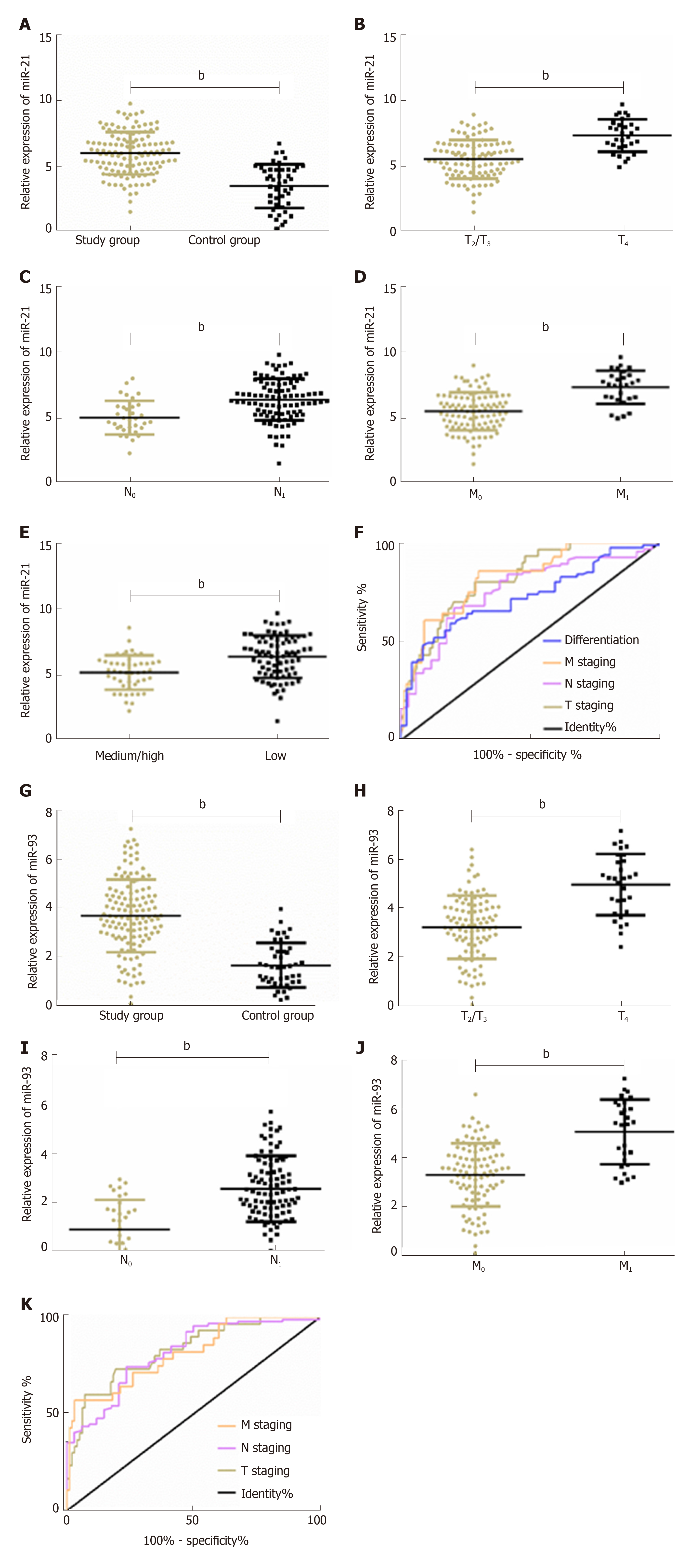
Figure 1 Clinical value of plasma miR-21 and miR-93 for esophageal squamous cell carcinoma patients.
A: Expression of plasma miR-21 in esophageal squamous cell carcinoma (ESCC) patients; B: Expression of plasma miR-21 in patients with different T stages of ESCC ; C: Expression of plasma miR-21 in patients with different N stages of ESCC; D: Expression of plasma miR-21 in patients with different M stages of ESCC; E: Expression of plasma miR-21 in patients with different degrees of pathological differentiation of ESCC; F: Receiver operating characteristic (ROC) curves of plasma miR-21 for diagnosing T stage, N stage, M stage, and pathological differentiation of ESCC patients; G: Expression of plasma miR-93 in ESCC patients; H: Expression of plasma miR-93 in patients with different T stages of ESCC; I: Expression of plasma miR-93 in patients with different N stages of ESCC ; J: Expression of plasma miR-93 in patients with different M stages of ESCC; K: ROC curves of plasma miR-93 for diagnosing T stage, N stage, and M stage of ESCC patients. bP < 0.01 vs control group.
- Citation: Wang WT, Guo CQ, Cui GH, Zhao S. Correlation of plasma miR-21 and miR-93 with radiotherapy and chemotherapy efficacy and prognosis in patients with esophageal squamous cell carcinoma. World J Gastroenterol 2019; 25(37): 5604-5618
- URL: https://www.wjgnet.com/1007-9327/full/v25/i37/5604.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i37.5604