Published online Sep 7, 2019. doi: 10.3748/wjg.v25.i33.4835
Peer-review started: July 12, 2019
First decision: July 22, 2019
Revised: July 24, 2019
Accepted: August 7, 2019
Article in press: August 7, 2019
Published online: September 7, 2019
Liver fibrosis is the common pathological basis of all chronic liver diseases, and is the necessary stage for the progression of chronic liver disease to cirrhosis. As one of pathogenic factors, inflammation plays a predominant role in liver fibrosis via communication and interaction between inflammatory cells, cytokines, and the related signaling pathways. Damaged hepatocytes induce an increase in pro-inflammatory factors, thereby inducing the development of inflammation. In addition, it has been reported that inflammatory response related signaling pathway is the main signal transduction pathway for the development of liver fibrosis. The crosstalk regulatory network leads to hepatic stellate cell activation and proinflammatory cytokine production, which in turn initiate the fibrotic response. Compared with the past, the research on the pathogenesis of liver fibrosis has been greatly developed. However, the liver fibrosis mechanism is complex and many pathways involved need to be further studied. This review mainly focuses on the crosstalk regulatory network among inflammatory cells, cytokines, and the related signaling pathways in the pathogenesis of chronic inflammatory liver diseases. Moreover, we also summarize the recent studies on the mechanisms underlying liver fibrosis and clinical efforts on the targeted therapies against the fibrotic response.
Core tip: Liver fibrosis is a chronic liver lesion with inflammation. Reciprocally, increased inflammatory response exacerbates the severity of liver disease. Clinical data reveal that an aberrant increase of inflammatory cytokines is highly correlated with poor outcome of patients with liver fibrosis. However, the mechanism underlying liver fibrosis is not completely understood. It is urgently needed to enrich the knowledge of liver fibrosis. This review focuses on the role of inflammation in liver fibrosis and discusses the crosstalk network involving immune cells, cytokines, and the related signaling pathways.
- Citation: Zhangdi HJ, Su SB, Wang F, Liang ZY, Yan YD, Qin SY, Jiang HX. Crosstalk network among multiple inflammatory mediators in liver fibrosis. World J Gastroenterol 2019; 25(33): 4835-4849
- URL: https://www.wjgnet.com/1007-9327/full/v25/i33/4835.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i33.4835
Chronic inflammatory lesions results in extracellular matrix accumulation and hepatic fibrosis, eventually leading to cirrhosis[1]. Liver cirrhosis is a life-threatening factor for human health in the world. Sustained stimulations by a series of pathogenic mediators impair the regeneration capacity of the liver and thus result in the development of liver fibrosis. Among many pathogenic factors, inflammation is a key inducer for liver fibrosis progression. Cross activation of hepatic stellate cells (HSCs), Kupffer cells, and other immune cells is a hallmark for the pathogenesis of liver fibrosis. Furthermore, critical cell signal pathway-related apoptosis, autophagy, collagen and inflammatory cytokine production are involved in the development of liver fibrosis by crosstalk with immune cells. Chronic pathogenic factors activated abundant hepacytes to generate inflammatory cytokines and chemokine mediators, which subsequently form a crosstalk network in liver fibrosis. Until now, liver fibrosis is still a serious unsolved problem in chronic liver disease. This review focuses on this crosstalk network in liver fibrosis and discusses the detailed mechanism by which the process of liver fibrosis is modulated.
As the precursor of myofibroblasts, HSCs differentiate into an activated myofibroblastic phenotype with the assistance of Kupffer cells and cytokine-cytokine receptor signaling pathways. HSCs comprise 15% of total resident cells in the normal human liver. Through secretion of interleukins and chemokines, HSCs communicate with Kupffer cells and other liver cells in quiescent conditions[2]. However, deregulation of HSC activation can initiate inflammation and enhance the susceptibility to liver fibrosis. Activated HSCs produce endothelin-1 to promote fibrogenesis[3]. A homologous protein of YB1 (a negative mediator for liver fibrosis) mediated anti-fibrotic activity by suppressing the expression of collagen type I in HSCs[4]. Moreover, Wnt signaling can also enhance HSC activation and promote liver fibrosis[5]. Some data showed that loss of interleukin (IL)-1Ra in mice decreased the number of HSCs and Kupffer cells in the liver compared to the other groups, which suggested that IL-1 signaling is also involved in this process[6]. Additionally, mature HSCs have been reported to stimulate allogeneic regulatory T cell proliferation in a cell-cell contact-dependent manner[7]. Mast cells might crosstalk with HSCs to inhibit liver fibrosis via the HLA-G-mediated decrease of collagen I, and IL-10 also mediates crosstalk between mast cells and HSCs[8]. Endothelial progenitor cells dramatically inhibit the proliferation, adhesion, and migration of HSCs, promote the apoptosis of HSCs, and down-regulate the mRNA and protein expression of collagen I and collagen III in HSCs[9]. Epigenetic crosstalk between histone acetylation and miRNAs inhibited HSC activation[10]. Researchers have explored drugs targeting HSCs. A number of protein markers were found to be overexpressed in activated HSCs, and their ligands have been utilized to specifically deliver various anti-fibrotic agents[11]. Natural killer (NK) cells are important in regulating hepatic fibrosis, and their cytotoxic killing of HSCs has been reported. Activated NK cells lead HSCs to death in a TRAIL-involved mechanism via the p38/PI3K/AKT pathway, which suggested that the p38/PI3K/AKT pathway in NK cells may be a novel drug target to inhibit liver fibrosis[12]. It has been confirmed that activation of HSCs could be inhibited by reducing the production of transforming growth factor-β1 (TGF-β1) in HSCs via inhibition of the NF-κB pathway through downregulation of the TGF-β1/Smad3 pathway[13].
Kupffer cells as resident macrophages are one of important liver inflammatory cell types, and account for 30% of sinusoidal cells[14]. Activated Kupffer cells secrete abundant cytokines and signaling molecules, which enhance liver immunopathology. Activated Kupffer cells participate in the initial injury/fibrogenic response to TGF-β1 and methotrexate, which results in upregulated production of cytokines, including IL-10, IL-4, IL-6, and IL-13[15]. CXCL6 stimulates the phosphorylation of epidermal growth factor receptor (EGFR) and the expression of TGF-β in cultured Kupffer cells, thereby resulting in activation of HSCs[16]. In response to liver injury induced by endotoxin, IL-35 can promote Kupffer cells to secrete IL-10 and reduce acute liver injury[17]. A crosstalk network including Ly6C+ monocytes, CCL2-CCR2, and Kupffer cells determines HBV clearance/tolerance, and manipulation of these two cell types may be a potential strategy for immunotherapy of HBV-related liver diseases[18]. Activation of Kupffer cells by pathogens and the CCL2/CCR2 axis can be the key factor to recruit innate effector cells to the injured liver[19]. In alcoholic liver disease mice, a crosstalk network including Kupffer cells, T cells, CCL2/CCR2, and CCL5/CCR5 sensitizes hepatocytes[20]. NLRP3 inflammasome from Kupffer cells is involved in the occurrence of schistosomiasis-induced liver fibrosis (SSLF) via NF-κB signaling and IL-1β in serum increased strongly[21]. An effective method of isolating Kupffer cells was explored to eliminate endothelial cell contamination, which could be meaningful for illuminating Kupffer cell function and mechanism in diseases[22]. RAMP 1 in Kupffer cells mediates a crosstalk network involving infiltration of immune cells and pro-inflammatory cytokines secreted by Kupffer cells and splenic T cells, and such crosstalk network can regulate the immune response[23]. ATG5-dependent autophagy involved in crosstalk between Kupffer cells and cytokines (IL-6 and IL-10) mediated acute liver injury response[24]. The cross communication of Sphk1 with HSCs and Kupffer cells regulated the CCL2-CCR2 axis in liver fibrosis[25]. Fas ligand stimulated Fas-expressing Kupffer cells or macrophages to secrete active IL-18 in a caspase-1-independent manner and finally resulted in acute liver injury in mice[26]. Kupffer cells with high expression of CD1d only presented lipid antigen to NKT cells for activation of the pro-inflammatory cytokine pathway[27]. Huangqi decoction activated Kupffer cells to promote liver fibrosis[28]. The crosstalk between Th2 microenvironment and Kupffer cells promoted liver fibrodsis[29]. The interaction between NK cells and Kupffer cells mediated by the CD205-TLR9-IL-12 axis promoted liver injury[30]. MM9 from Kupffer cell can remodel the matrix and repair the architecture during liver fibrosis regression[31]. Taken together, multiple functions of Kupffer cells modified by different molecules, signal pathways, inflammatory cytokines, and immune cells are essential in the development of liver fibrosis.
NKT cells are activated in an NKG2D-dependent manner, and the crosstalk of IL-30 with NKG2D activates NKT cells to remove collagen-produced HSCs[32]. Regulatory CD4 T cells modulate the crosstalk network between NK cells and HSCs[33]. Neutrophils are the source of many inflammation cytokines and important inflammatory cells for acute liver injury and chronic fibrosis. Neutrophil-to-lymphocyte ratio is determined to be related with inflammatory activity and fibrosis in non-alcohol fatty liver disease[34]. A latest report shows that Th22 cells are closely associated with chronic liver fibrosis; moreover, the close crosstalk in the cell number of CD4+ T cells and Th22 cells suggests that Th22 plays an important role in chronic liver fibrosis[35]. One report demonstrates that NK cells migrate into the fibrosis scar and play a role in immune surveillance by clearing senescent activated HSC cells[36]. However, the chemokine CXCL-10 reverses NK cell-mediated HSC inactivation function and promotes liver fibrosis[37]. Therefore, liver fibrosis progresses in the inflammatory mediator crosstalk network microenvironment.
Proinflammatory cytokines: IL-17A in combination with TGF-βRI can phosphorylate SMAD2/3 in HSCs to activate liver fibrosis[38]. A cross communication involving BM-MSCs and IL-6/STAT3 can down-regulate IL-17 and affect liver fibrosis[39]. In a new mouse model with a pre-injured liver (Abcb4/Mdr2-/-), IL-6-driven inflammatory response may determine the outcome of acute liver injury[40]. IL-6 is a primary regulator of both acute and chronic inflammation, which exhibits two contrasting functions. It acts as a pro-inflammatory cytokine in models of chronic inflammatory diseases[41], and contrarily shows anti-inflammatory effects in acute inflammation. Therefore, as a classic pro-inflammatory cytokine biomarker, IL-6 is used to clinically diagnose chronic liver fibrosis[42]. A crosstalk axis involving IL-6 and polymorphism of its gene (C174G) accelerates progression of chronic liver fibrosis[43]. As a potent chemoattractant for neutrophils, IL-8 and its receptor CXCR1 are involved in inflammation activation and liver fibrosis[44]. As potent predictors of liver injury, IL-8, MCP-1, and OPN are associated with advanced liver fibrosis in nonalcoholic fatty liver disease[45]. CXCL-6 can phosphorylate EGFR and activate the TGF-β pathway in Kupffer cells in liver fibrosis[16]. A latest report shows that IL-9-derived interaction between Raf/MEK/ERK and CXCL-10 can promote liver fibrosis[46]. As a profibrogenic factor, IL-34 may become a diagnostic biomarker for liver fibrosis[47]. In a mouse model, the crosstalk between IL-13 and STAT6 signaling pathways activates schistosomiasis-induced liver fibrosis[48]. In non-alcoholic fatty liver disease, fibroblast-derived marker IL-34 is developed as a feasible diagnostic marker[49]. IL-34, together with macrophage colony-stimulating factor, activates HSCs to promote collagen synthesis[50]. Plasma IL-18 in children with nonalcoholic fatty liver disease has been proposed as a novel biomarker for liver fibrosis[51]. CCL2-dependent monocytes may promote angiogenesis induced by inflammation in the progression of liver fibrosis[52]. The communication of TGF-β with JAK1-STAT3 may promote HSC proliferation as well as collagen I and α-SMA up-regulation in CCL4-derived live fibrosis[53]. In fibrotic liver, activated HSC-derived CTGF may respond to TGF-β stimulation in order to form a crosstalk regulatory network, and this crosstalk contributes to extracellular matrix production in a STAT3-dependent mode[54]. Alternatively, the interaction of TGF-β with long non-coding RNA-21 may promote hepatocyte apoptosis in liver fibrosis[55]. Neutralizing of IL-1α and IL-1 can inhibit the progression of liver fibrosis, which suggests that IL-1α and IL-1β promote inflammatory liver fibrosis[56]. Higher IL-9-derived Th9 cell expression was investigated in patients with HBV associated liver cirrhosis, and the result suggested that IL-9 may relate closely to the liver fibrosis. IL-9 is reported to promote hepatic dysfunction in CCL4-mediated liver fibrosis[57].
Anti-fibrosis cytokines: As an autophagy inhibitor, IL-10 crosstalks with STAT3 to exert an anti-fibrogenic function in liver injury[58]. IL-10 producing regulatory B cells can enhance regulatory T cell function in chronic liver fibrosis mediated by HBV[59]. Through restriction fragment length polymorphism (RFLP) analysis, IL-10 gene promoter (rs1800896) polymorphism was correlated with an increased risk of chronic liver fibrosis, especially that mediated by HBV[60]. IL-22 belongs to the IL-10 family and is produced by Th17 cells, Th22 cells, and NKT cells. IL-22 crosstalks with the microRNA (miRNA) and inflammatory cytokine pathways to attenuate HSC activation and inhibit liver fibrosis[61,62]. Crosstalk of IL-22 with p53-p21 in a STAT3 dependent way may induce the senescence of activated HSCs in liver fibrosis[63]. Crosstalk of IL-22 with Nrf2-keap1-ARE inhibits acetaldehyde-induced HSC activation and proliferation[64]. As a liver protector, IL-22 may activate liver cell STAT3 to inhibit liver injury[65]. Moreover, IL-22 inhibits ConA-induced acute liver inflammation[66]. Crosstalk of IL-22 with STAT3 exerts an anti-apoptotic and mitogenic activity[67]. IL-22 is up-regulated strongly in patients with HCV infection, and administration of IL-22 promotes α-SMA expression and collagen production from HSCs[68]. However, crosstalk between IL-22 and HSC-derived IL-22-R1 may induce up-regulation of HSC-derived chemokines (CXCL10 and CCL20) to recruit Th17 cells to migrate into the inflammatory liver in response to chronic liver inflammation and fibrosis mediated by HBV. Therefore, the ultimate effect of IL-22 in liver fibrosis needs to be determined by the balance between induction of HSC apoptosis and promotion of liver inflammation[69]. Crosstalk between IL-22 and the TGF-β1/Notch signaling pathway may induce HSC inactivation and inhibit liver fibrosis[70]. Therefore, liver fibrosis progresses gradually via a crosstalk regulatory network involving multiple cytokines and their related downstream signaling pathways. IL-23 produced by Th2 cells down-regulates proinflammatory cytokines and inhibits liver fibrosis[71]. High expression of IL-23R on the Th17 cell surface in acute-on-chronic liver injury patients suggests that it strongly correlates with liver disease severity[72]. High expression of IL-23 in monocyte-derived dendritic cells presents in a TRAF6/NF-κB dependent manner and is closely associated with HBV-mediated acute-on-chronic liver injury[73]. Besides, IL-23 on the basis of IL-17A-producing γδT cells has a protective effect against ConA-mediated liver injury[74].
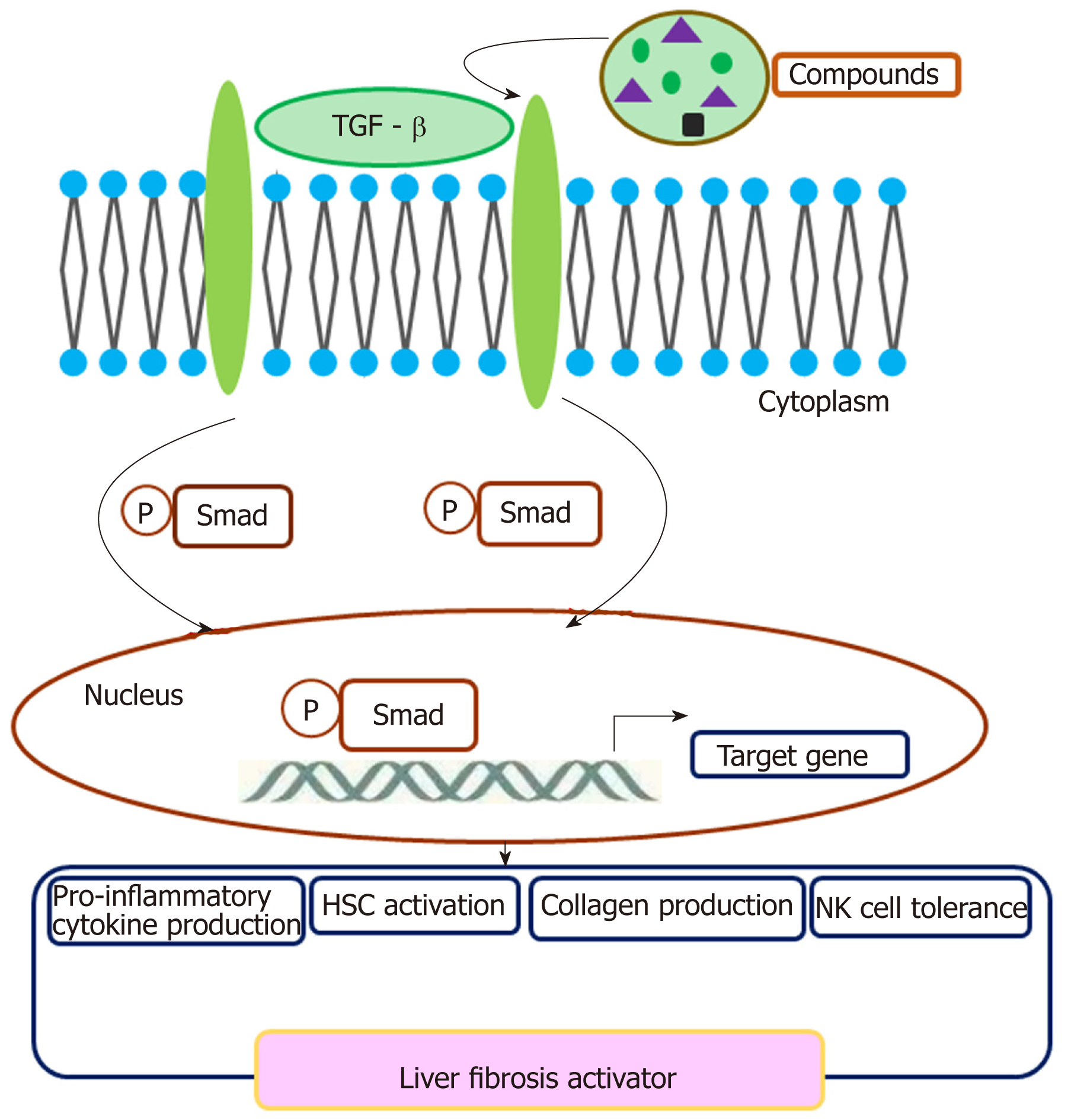
A crosstalk involving TGF-β and TGF-β R exerts a regulatory effect on cell plasticity in liver fibrosis (Figure 1). In CCL4 induced acute liver injury mice, CCL2/CCR2 recruits monocytes to infiltrate to the injury liver, then monocytes differentiate preferentially into inducible nitric oxide synthase-producing macrophages exerting pro-inflammatory and pro-fibrogenic actions, e.g., promoting HSC activation via the TGF-β pathway[75]. Collagen triple helix repeat containing 1 (CTHRC1) promotes HSC proliferation, migration, and contractility for supporting liver fibrosis via crosstalk with the TGF-β signal pathway[76]. IL-13 activates the TGF-β signaling pathway to promote HSC proliferation and cell viability[77]. M2 Kupffer cells produce TGF-β and IL-10, which mediate immune tolerance in mouse liver injury by down-regulating the production of TNF-α and IL-12. In addition, M2 polarization of Kupffer cells contributes to the apoptosis of M1 Kupffer cells in fatty liver disease[78]. Therefore, TGF-β is critical for the activation of HSCs to transdifferentiate into fibrogenic myofibroblasts. Crosstalk between TGF-β and SMAD3 contributes to CCL4-induced liver fibrosis[79]. Activated HSCs may impair NK cell-mediated anti-fibrosis function through crosstalk with TGF-β in HBV-induced chronic liver fibrosis[80]. Some small compounds may crosstalk with the TGF-β pathway and exert an effect on liver fibrosis. Crosstalk of paeoniflorin with the TGF-β pathway may exert a protective role in radiation-induced liver fibrosis[81]. Sauchinone also reduces activation of HSCs and liver fibrosis through crosstalk with the TGF-β1 pathway[82]. Isorhamnetin may control liver fibrosis progression through inhibitive crosstalk with TGF-β1 and relieving oxidative stress[83]. Synthetic oligodeoxynucleotide may prevent fibrogenesis and deposition of collagen by targeting the TGF-β1/Smad pathway[84]. Platelets are a rich source of TGF-β1 and platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of live injury[85]. TGF-β mediates the transformation of mesothelial cells to myofibroblast[86].
MiRNAs as an important regulatory element are involved in liver fibrosis. Crosstalk between miR-101 and the PI3K/Akt/mTOR signaling pathway presents an anti-fibrotic effect in a CCL4 induced mouse model[87]. MiRNA-29b can target the PI3K/AKT pathway to prevent liver fibrosis by attenuating HSC activation and inducing apoptosis[88]. MiRNA-29b and its crosstalk with the TGF-β1/Smad3 may suppress HSC activation[89]. MiRNA-34a-5p inhibits liver fibrosis by regulating the TGF-β1/Smad3 pathway in HSCs[90]. A cross-communication between miR-130a-3p and its down-regulatory TGFBR1 and TGFBR2 induces HSC apoptosis[91]. MiR-19b can down-regulate CCL2 in HSCs and further inhibit liver fibrosis[25]. A crosstalk involving miRNA-21 and the NLRP3 inflammasome/IL-1β axis mediates angiotensin II-induced liver fibrosis[92]. As a Wnt/β-catenin activator, miR-17-5p contributes to progression of liver fibrosis via activating HSCs[93]. Much evidence suggests that miR-17-5p promotes HSC proliferation and activation, on the contrary, down-regulation of miR-17-5p expression contributes to the suppression of activated HSCs[94]. MiRNA-142-3p inhibits TGF-β-induced fibrosis by targeting the TGF-RI pathway and was found to decrease the plasma of chronic liver fibrosis patients[95]. A considerable amount of evidence has shown that miRNA-200 participates in fibrosis[96]. As a PI3K/Akt pathway activator, interaction of miR-200c with its related FOG2 results in HSC activation and liver fibrosis[97]. MiRNA-181b-3p and its target importin α5 may regulate sensitivity of TLR4 in Kupffer cells[98]. MiRNA-193a/b-3p relives liver fibrosis by inhibiting the activation and proliferation of HSCs[99]. MiRNA-26b-5p inhibits mouse liver fibrosis by targeting platelet-derived growth factor receptor-β[100]. MiRNA-219 plays a protective role in liver fibrosis by targeting TGF-βRII[101]. MiRNA-145 promotes HSC activation by targeting Krüppel-like factor 4[102]. The effects of alcohol on DNA methylation in hepatocytes in liver fibrosis and miRNA regulation have been elucidated[103]. Therefore, the core miRNAs and the related downstream targets form a complicate regulatory miRNA-mRNA communication network in liver fibrosis, and this provides a basis for the development of more effective therapy for liver fibrosis.
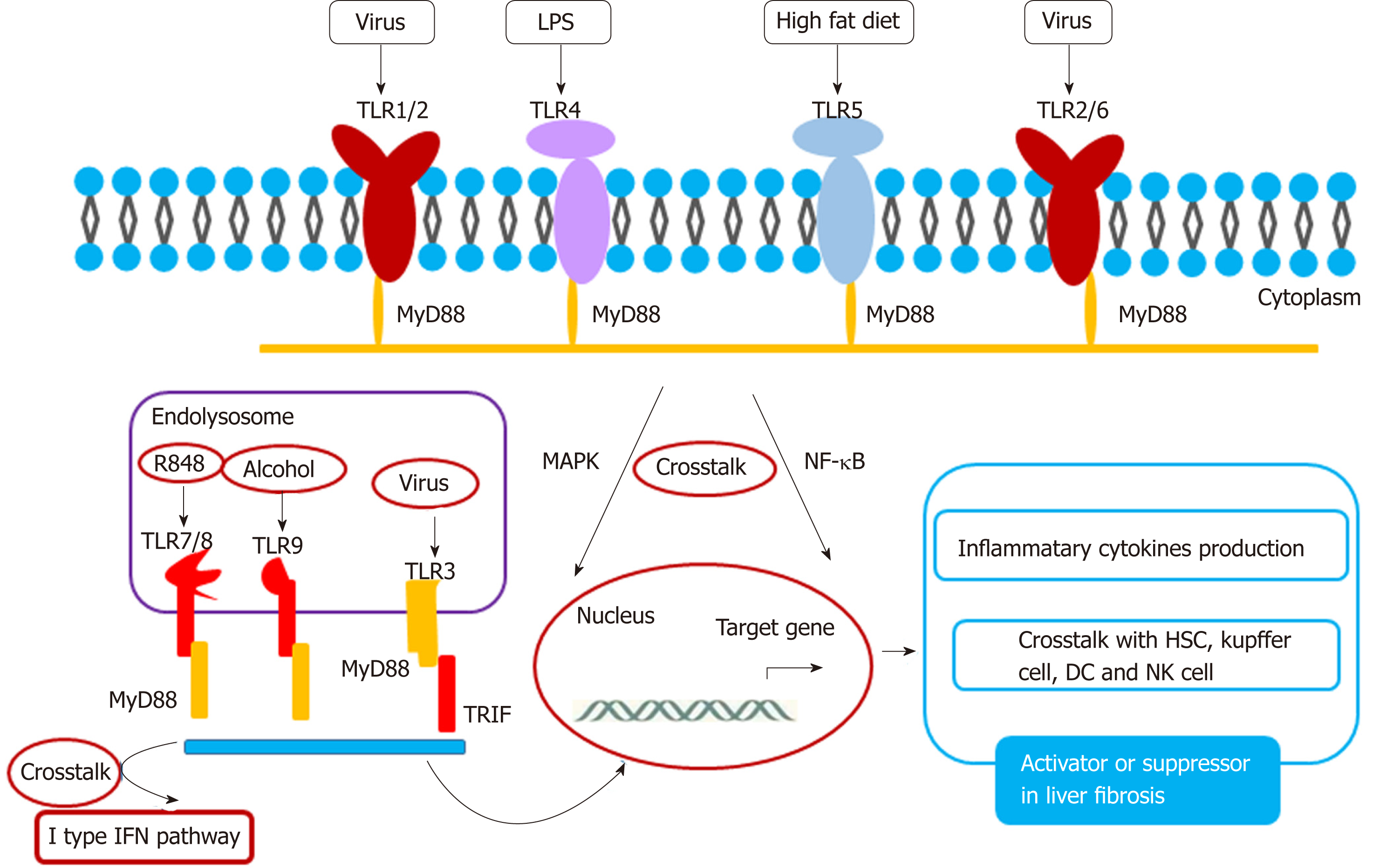
TLR has the ability to recognize pathogens and contains ten members: TLR1-10. Among the TLR family, TLR3, 7, 8, 9, and 10 are located in the endolysosome[104,105], and TLR1, 2, 4, 5, and 6 are located on the membrane. A crosstalk between TLR and their ligands activates the liver fibrosis pathway (Figure 2). TLR2 and its ligand stimulate Kupffer cells to secret IL-10 in HBV-dependent liver fibrosis[106,107]. In HBV-induced chronic liver fibrosis, TLR2 acts in a homodimer form or in a heterodimer form with TLR1 or TLR6 and activates NF-kB in a MyD-88 dependent manner[108]. TLR3 silencing induces HSC and Kupffer cell activation, suggesting that TLR3 is related closely to liver injury. This supports the basis for TLR3-targeted therapy of liver disease[109]. Crosstalk between TLR3 and CCL5 plays a key role in HCV- mediated liver fibrosis[110]. Exosome-mediated TLR3 promotes liver fibrosis by enhancing IL-17A production from γδT cells[111]. In a non-alcoholic steatohepatitis rat model, TLR4-p38 MAPK signaling may induce Kupffer cell activation, suggesting that TLR4 is closely associated with steatofibrosis[112]. Ethyl pyruvate may protect the liver from CCL4-mediated fibrosis by inhibition of TLR4[113]. TLR5 promotes liver bacterial clearance and protects from liver injury and fibrosis[114]. Bioactive compound luteolin may protect the liver from fibrosis through up-regulation of TLR5, and knockdown of TLR5 induces metabolic syndrome[115]. These data suggest that TLR5 is a possible key transcription factor for preventing lipotoxicity. TLR2, together with the TLR9-dependent myD88-dependent pathway, may activate HSCs to secret CXCL1, and the CXCL1/CXCR2 axis recruits neutrophils to the liver, which contributes to the development of alcohol-mediated liver injury[116]. TLR7 may activate dendritic cells to secret type I interferon (IFN) to activate Kupffer cells to produce profibrogenic IL-1ra. The TLR7/type I IFN/IL-1ra axis opens a selective target therapy for liver fibrosis[117]. Besides TLR3, other TLR family members are dependent on the MyD88 pathway. Curcumin promotes apoptosis of activated HSCs by inhibiting the MyD-88 pathway[118].
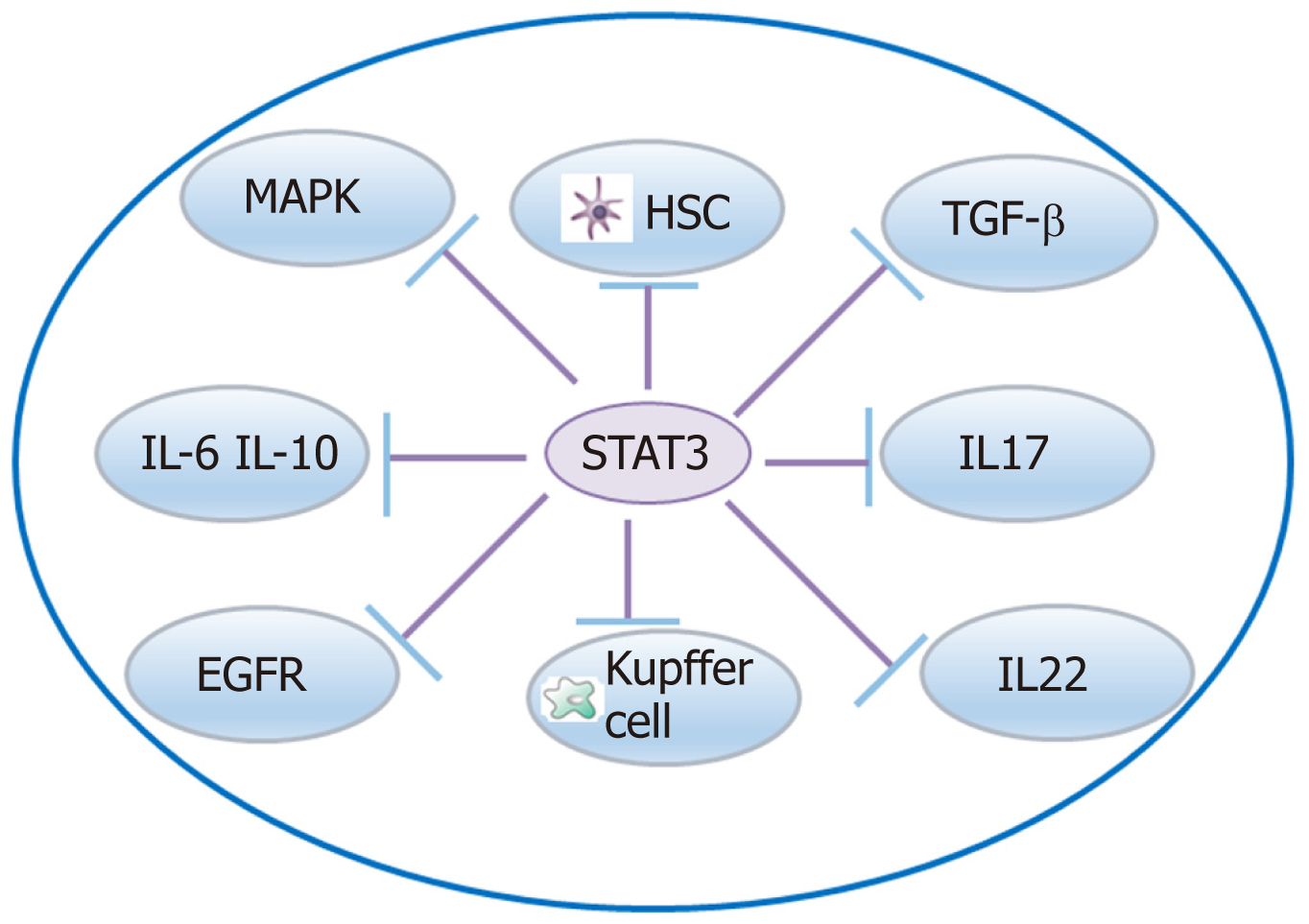
There are other signaling pathways, such as STAT-3, Wnt/β-catenin, and NF-кB signaling pathways, involved in liver fibrosis (Figure 3). A crosstalk involving IL-17 and the STAT3 signaling pathway activates HSCs to produce collagen I[119]. A crosstalk network involving IL-6 and IL-10 with STAT3 may protect the liver against alcohol-mediated inflammation and injury[120]. STAT3/IL-10/IL-6 signaling regulates hepatocyte proliferation and is a key factor associated with acute injury and chronic liver fibrosis[121]. Moreover, crosstalk of IL-22 with STAT3 induces senescence of HSCs in liver fibrosis[53]. STAT3 is required to for TGF-β-induced proliferation and fibrosis in LX-2 cells, and this supports that there is a close crosstalk between the TGF-β and STAT3 pathways[122]. STAT3-EGFR signaling promotes liver protective function in cholestatic liver injury and fibrosis[123]. STAT3 and MAPK are necessary for IL-6-mediated liver fibrosis[63]. STX-0119 reduces liver fibrosis by inhibition of STAT3 and inactivation of HSCs in mice[124]. Crosstalk of FGF21 with the NF-кB and JNK signaling pathways protects the liver from inflammation and fibrosis[125]. Crosstalk between NF-κB and type I IFN signaling promotes liver inflammation and fibrosis, while crosstalk of ADAR1 with this pathway restrains this function[126]. The Wnt/β-catenin pathway exerts a function in HSC activation induced collagen I formation and liver fibrosis, and crosstalk of hBM-MSC with this pathway may inhibit liver fibrosis[127]. HGF activation promotes HSC apoptosis through the Rho pathway[128].
There are currently some drugs available for the therapy of liver fibrosis, however, their efficacy is limited (Table 1). It is the time to explore promising drugs to improve the treatment of liver fibrosis by developing promising therapeutic strategies, such as inhibition of HSC activation and anti-inflammation. Following molecular targeted therapy increasingly development, protein marker on HSC, signal pathway molecule may be potential marker to be selected for improving liver fibrosis. Many anti-fibrotic compounds are being on road. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been evaluated to improve liver fibrosis. TRAIL can reverse liver fibrosis by promoting apoptosis of primary HSCs and inhibiting Kupffer cells in a CCL4-mediated liver fibrosis model. Therefore, TRAIL-based therapy is a useful direction for exploring new anti-fibrotic drugs[129]. Wnt/β-based ICG-001 has been assessed to selectively induce target cell apoptosis, with encouraging results obtained in terms of reversing fibrosis and improving survival rate of model animals[130]. 24-nor-ursodeoxycholic acid (norUDCA) has been found to have anti-fibrotic effects and improve inflammation-mediated liver fibrosis[131]. Cenicriviroc, an inhibitor of CCR2/CCR5, is on a phase III clinical trial, which presents an anti-liver fibrosis effect[132]. Accumulating experiments of tyrosine kinase inhibitors make it possible to exploit their beneficial effects on fibrotic disease, although it should not also neglect the side effects of TK inhibitors for liver fibrosis, such as rash and gastrointestinal symptoms[133]. Taken together, these new drug therapies will provide a new avenue for the treatment of liver fibrosis.
| Crosstalk family member | Mechanism | Function in liver fibrosis | Biological basis as therapeutic target |
| TGF-β | Proliferation Migration Collagen production Crosstalk with small compounds Induces NK cell tolerance | Fibrosis activator | Deficiency of TGF-β inhibits liver fibrosis |
| Wnt/β-catenin | Promotes activation of HSC Collagen I production | Fibrosis activator | |
| TLR-2 TLR1/2 TLR2/6 | Activates NF-kB pathway Pro-inflammatory cytokines Activates Kupffer cell and IL-10 production | Inducer or suppressor in liver fibrosis | |
| TLR-3 | Crosstalk with IL-17A and γδT cell Crosstalk with CCL5 | Inducer or suppressor in liver fibrosis | Loss of TLR3 aggravates liver inflammation |
| TLR-4 | Pro-inflammatory cytokine production | Fibrosis activator | Inhibition of TLR4 promotes liver protection |
| TLR-5 | Crosstalk other pathway Regulates metabolism Anti-inflammatory cytokine production | Fibrosis inhibitor | Activation of TLR5 reduces liver fibrosis |
| TLR7 | Pro-inflammatory cytokine production Activates DCs Crosstalk with IFN signaling pathway | Fibrosis inhibitor | |
| TLR-9 | CXCL1 production Neutrophil infiltration | Fibrosis activator | |
| STAT3 | Crosstalk with IL-17, IL-10, and IL-6 Crosstalk with other signal pathways | Fibrosis activator or suppressor | Inhibition of STAT3 may inactivate HSCs and prevent liver fibrosis |
| miR-29b | Crosstalk with PI3K/AKT pathway Crosstalk with TGF-β1/SMAD3 pathway Induces HSC apoptosis | Fibrosis inhibitor | |
| miR-34a-5p | Crosstalk with TGF-β1/SMAD3 | Fibrosis inhibitor | |
| miR-130a-3p | Crosstalk with TGFBR1 and TGFBR2 Induces HSC apoptosis | Fibrosis inhibitor | |
| miR-19b | Crosstalk with HSC CCL2 | Fibrosis inhibitor | |
| miR-21 | Crosstalk with NLRP3 inflammasome/IL-1β axis | Fibrosis regulator | |
| miR-17-5p | Crosstalk with Wnt/β-catenin Activation of HSCs | Fibrosis promoter | |
| miR-142-3p | Crosstalk with TGF-β | Fibrosis inhibitor | |
| miR-200c | Crosstalk with PI3K/Akt | Fibrosis promoter | |
| miR-181b-3p | Crosstalk with TLR4 Kupffer cells | Fibrosis regulator | |
| miR-193a/b-3p | Inhibits activation of HSCs | Fibrosis regulator | |
| miR-26b-5p | Crosstalk with platelet-derived growth factor receptor-β | Fibrosis inhibition | |
| miR-219 | Crosstalk with TGF-βRII | Fibrosis inhibition | |
| miR-145 | Crosstalk with Krüppel-like factor 4 Promotes activation of HSCs | Fibrosis inhibition |
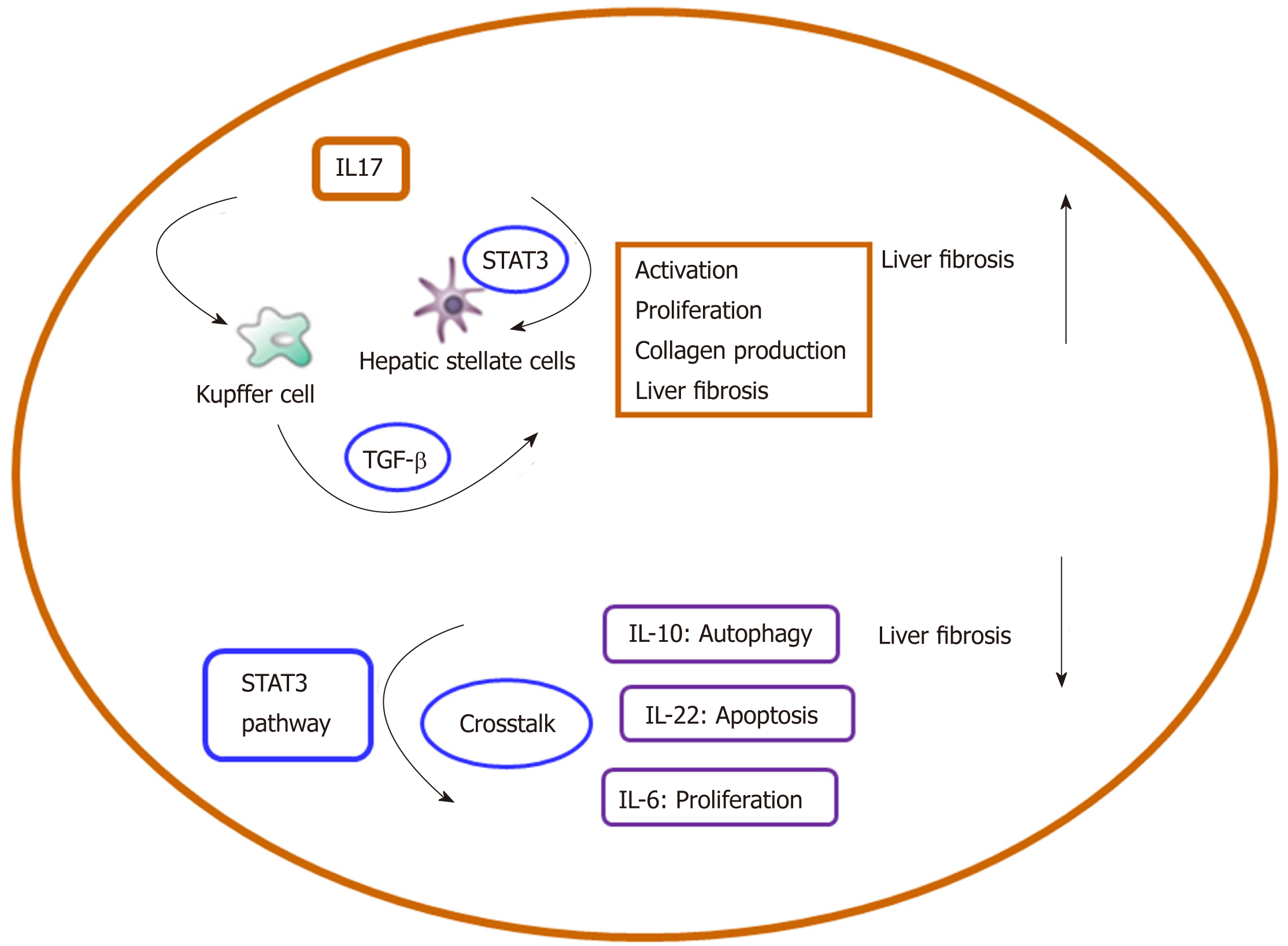
A better understanding of the crosstalk among inflammation-related cells, cytokines, and signaling pathways in liver fibrosis could help clarify the pathogenesis of liver fibrosis. The aim of this review is to describe the present knowledge about inflammation-related crosstalk networks, which effectively perform regulatory functions in HSC activation and liver fibrosis. Moreover, we discuss different interactions among crosstalk-related members in liver fibrosis. The crosstalk-related complex regulatory network modulates several important aspects of cell function, including proliferation, activation, and differentiation (Table 1, Figure 4). Targeting each node of the crosstalk network can be a promising direction for liver fibrosis treatment. Interaction of IL-34 with the PI3K/Akt signal pathway promotes the M2 polarization of Kupffer cells to inhibit acute rejection in rat liver transplantation[134]. IL-17 stimulates Kupffer cells to secret TGF-β and activates HSCs to form myofibroblasts by stimulating collagen synthesis via the STAT3 signal pathway. In the future, we will focus on the function of IL-22 in the crosstalk between Kupffer cells and the CCL2-CCR2 pathway in order to enrich our knowledge on inflammatory cytokines in liver fibrosis. This will provide a basis for the therapy of liver fibrosis[118]. In addition, it should be noted that impaired macroautophagy/autophagy is involved in the pathogenesis of hepatic fibrosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Bendary M, Tanabe S S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | Chen L, Brenner DA, Kisseleva T. Combatting Fibrosis: Exosome-Based Therapies in the Regression of Liver Fibrosis. Hepatol Commun. 2018;3:180-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2084] [Cited by in F6Publishing: 2041] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 3. | Li T, Shi Z, Rockey DC. Preproendothelin-1 expression is negatively regulated by IFNγ during hepatic stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G948-G957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chen L, Ji Z, Duan L, Zhu D, Chen J, Sun X, Yu Y, Duan Y. rSJYB1 inhibits collagen type I protein expression in hepatic stellate cells via down-regulating activity of collagen α1 (I) promoter. J Cell Mol Med. 2019;23:3676-3682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Miao CG, Yang YY, He X, Huang C, Huang Y, Zhang L, Lv XW, Jin Y, Li J. Wnt signaling in liver fibrosis: progress, challenges and potential directions. Biochimie. 2013;95:2326-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Meier RPH, Meyer J, Montanari E, Lacotte S, Balaphas A, Muller YD, Clément S, Negro F, Toso C, Morel P, Buhler LH. Interleukin-1 Receptor Antagonist Modulates Liver Inflammation and Fibrosis in Mice in a Model-Dependent Manner. Int J Mol Sci. 2019;20:pii: E1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Huang H, Deng Z. Adoptive transfer of regulatory T cells stimulated by Allogeneic Hepatic Stellate Cells mitigates liver injury in mice with concanavalin A-induced autoimmune hepatitis. Biochem Biophys Res Commun. 2019;512:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Amiot L, Vu N, Drenou B, Scrofani M, Chalin A, Devisme C, Samson M. The anti-fibrotic role of mast cells in the liver is mediated by HLA-G and interaction with hepatic stellate cells. Cytokine. 2019;117:50-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Chen XX, Zhang XY, Ding YZ, Li X, Guan XM, Li H, Cheng M, Cui XD. [Effects of endothelial progenitor cells on proliferation and biological function of hepatic stellate cells under shear stress]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2018;34:404-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 10. | Lu P, Yan M, He L, Li J, Ji Y, Ji J. Crosstalk between Epigenetic Modulations in Valproic Acid Deactivated Hepatic Stellate Cells: An Integrated Protein and miRNA Profiling Study. Int J Biol Sci. 2019;15:93-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | D'Ippolito D, Pisano M. Dupilumab (Dupixent): An Interleukin-4 Receptor Antagonist for Atopic Dermatitis. P T. 2018;43:532-535. [PubMed] [Cited in This Article: ] |
| 12. | Li T, Yang Y, Song H, Li H, Cui A, Liu Y, Su L, Crispe IN, Tu Z. Activated NK cells kill hepatic stellate cells via p38/PI3K signaling in a TRAIL-involved degranulation manner. J Leukoc Biol. 2019;105:695-704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Feng J, Chen K, Xia Y, Wu L, Li J, Li S, Wang W, Lu X, Liu T, Guo C. Salidroside ameliorates autophagy and activation of hepatic stellate cells in mice via NF-κB and TGF-β1/Smad3 pathways. Drug Des Devel Ther. 2018;12:1837-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718-722. [PubMed] [Cited in This Article: ] |
| 15. | Norona LM, Nguyen DG, Gerber DA, Presnell SC, Mosedale M, Watkins PB. Bioprinted liver provides early insight into the role of Kupffer cells in TGF-β1 and methotrexate-induced fibrogenesis. PLoS One. 2019;14:e0208958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Cai X, Li Z, Zhang Q, Qu Y, Xu M, Wan X, Lu L. CXCL6-EGFR-induced Kupffer cells secrete TGF-β1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2 pathway in liver fibrosis. J Cell Mol Med. 2018;22:5050-5061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Zheng XF, Hu XY, Ma B, Fang H, Zhang F, Mao YF, Yang FY, Xiao SC, Xia ZF. Interleukin-35 Attenuates D-Galactosamine/Lipopolysaccharide-Induced Liver Injury via Enhancing Interleukin-10 Production in Kupffer Cells. Front Pharmacol. 2018;9:959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Wu LL, Peng WH, Wu HL, Miaw SC, Yeh SH, Yang HC, Liao PH, Lin JS, Chen YR, Hong YT, Wang HY, Chen PJ, Chen DS. Lymphocyte Antigen 6 Complex, Locus C<sup>+</sup> Monocytes and Kupffer Cells Orchestrate Liver Immune Responses Against Hepatitis B Virus in Mice. Hepatology. 2019;69:2364-2380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front Immunol. 2018;9:2948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 20. | Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, Iracheta-Vellve A, Adejumo A, Saha B, Calenda C, Mehta J, Lefebvre E, Vig P, Szabo G. Pharmacological Inhibition of CCR2/5 Signaling Prevents and Reverses Alcohol-Induced Liver Damage, Steatosis, and Inflammation in Mice. Hepatology. 2019;69:1105-1121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 21. | Daghestani MH, Daghestani MH, Daghistani MH, Bjørklund G, Chirumbolo S, Warsy A. The influence of the rs1137101 genotypes of leptin receptor gene on the demographic and metabolic profile of normal Saudi females and those suffering from polycystic ovarian syndrome. BMC Womens Health. 2019;19:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Lynch RW, Hawley CA, Pellicoro A, Bain CC, Iredale JP, Jenkins SJ. An efficient method to isolate Kupffer cells eliminating endothelial cell contamination and selective bias. J Leukoc Biol. 2018;104:579-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Inoue T, Ito Y, Nishizawa N, Eshima K, Kojo K, Otaka F, Betto T, Yamane S, Tsujikawa K, Koizumi W, Majima M. RAMP1 in Kupffer cells is a critical regulator in immune-mediated hepatitis. PLoS One. 2018;13:e0200432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhou S, Gu J, Liu R, Wei S, Wang Q, Shen H, Dai Y, Zhou H, Zhang F, Lu L. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response Through ATG5-Dependent Autophagy. Front Immunol. 2018;9:948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Lan T, Li C, Yang G, Sun Y, Zhuang L, Ou Y, Li H, Wang G, Kisseleva T, Brenner D, Guo J. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018;68:1070-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Nakanishi K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front Immunol. 2018;9:763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 27. | Tang T, Sui Y, Lian M, Li Z, Hua J. Pro-inflammatory activated Kupffer cells by lipids induce hepatic NKT cells deficiency through activation-induced cell death. PLoS One. 2013;8:e81949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, Sun M, Liu C, Liu P. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Altern Med. 2012;12:51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | López-Navarrete G, Ramos-Martínez E, Suárez-Álvarez K, Aguirre-García J, Ledezma-Soto Y, León-Cabrera S, Gudiño-Zayas M, Guzmán C, Gutiérrez-Reyes G, Hernández-Ruíz J, Camacho-Arroyo I, Robles-Díaz G, Kershenobich D, Terrazas LI, Escobedo G. Th2-associated alternative Kupffer cell activation promotes liver fibrosis without inducing local inflammation. Int J Biol Sci. 2011;7:1273-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Hou X, Hao X, Zheng M, Xu C, Wang J, Zhou R, Tian Z. CD205-TLR9-IL-12 axis contributes to CpG-induced oversensitive liver injury in HBsAg transgenic mice by promoting the interaction of NKT cells with Kupffer cells. Cell Mol Immunol. 2017;14:675-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Feng M, Ding J, Wang M, Zhang J, Zhu X, Guan W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int J Biol Sci. 2018;14:1033-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Mitra A, Satelli A, Yan J, Xueqing X, Gagea M, Hunter CA, Mishra L, Li S. IL-30 (IL27p28) attenuates liver fibrosis through inducing NKG2D-rae1 interaction between NKT and activated hepatic stellate cells in mice. Hepatology. 2014;60:2027-2039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Langhans B, Alwan AW, Krämer B, Glässner A, Lutz P, Strassburg CP, Nattermann J, Spengler U. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J Hepatol. 2015;62:398-404. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Khoury T, Mari A, Nseir W, Kadah A, Sbeit W, Mahamid M. Neutrophil-to-lymphocyte ratio is independently associated with inflammatory activity and fibrosis grade in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2019;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Qin S, Chen M, Guo X, Luo W, Wang J, Jiang H. The clinical significance of intrahepatic Th22 cells in liver cirrhosis. Adv Clin Exp Med. 2019;28:765-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1263] [Cited by in F6Publishing: 1362] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 37. | Hintermann E, Bayer M, Pfeilschifter JM, Luster AD, Christen U. CXCL10 promotes liver fibrosis by prevention of NK cell mediated hepatic stellate cell inactivation. J Autoimmun. 2010;35:424-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Fabre T, Kared H, Friedman SL, Shoukry NH. IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-β receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol. 2014;193:3925-3933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Farouk S, Sabet S, Abu Zahra FA, El-Ghor AA. Bone marrow derived-mesenchymal stem cells downregulate IL17A dependent IL6/STAT3 signaling pathway in CCl4-induced rat liver fibrosis. PLoS One. 2018;13:e0206130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Tang LX, He RH, Yang G, Tan JJ, Zhou L, Meng XM, Huang XR, Lan HY. Asiatic acid inhibits liver fibrosis by blocking TGF-beta/Smad signaling in vivo and in vitro. PLoS One. 2012;7:e31350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Pradere JP, Kluwe J, De Minicis S, Jiao JJ, Gwak GY, Dapito DH, Jang MK, Guenther ND, Mederacke I, Friedman R, Dragomir AC, Aloman C, Schwabe RF. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461-1473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 375] [Cited by in F6Publishing: 403] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 42. | Yakut M, Özkan H, F Karakaya M, Erdal H. Diagnostic and Prognostic Role of Serum Interleukin-6 in Malignant Transformation of Liver Cirrhosis. Euroasian J Hepatogastroenterol. 2018;8:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Bulatova IA, Schekotova AP, Paducheva SV, Dolgikh OV, Krivtsov AV, Tretyakova YI. [The significance of interleukin-6 and polymorphism of its gene (C174G) under viral, alcoholic and mixed cirrhosis of liver]. Klin Lab Diagn. 2017;62:100-103. [PubMed] [Cited in This Article: ] |
| 44. | Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One. 2011;6:e21381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Glass O, Henao R, Patel K, Guy CD, Gruss HJ, Syn WK, Moylan CA, Streilein R, Hall R, Mae Diehl A, Abdelmalek MF. Serum Interleukin-8, Osteopontin, and Monocyte Chemoattractant Protein 1 Are Associated With Hepatic Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2018;2:1344-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Guo X, Cen Y, Wang J, Jiang H. CXCL10-induced IL-9 promotes liver fibrosis via Raf/MEK/ERK signaling pathway. Biomed Pharmacother. 2018;105:282-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Wang YQ, Cao WJ, Gao YF, Ye J, Zou GZ. Serum interleukin-34 level can be an indicator of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2018;24:1312-1320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Du P, Ma Q, Zhu ZD, Li G, Wang Y, Li QQ, Chen YF, Shang ZZ, Zhang J, Zhao L. Mechanism of Corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model. Eur J Pharmacol. 2016;793:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Shoji H, Yoshio S, Mano Y, Kumagai E, Sugiyama M, Korenaga M, Arai T, Itokawa N, Atsukawa M, Aikata H, Hyogo H, Chayama K, Ohashi T, Ito K, Yoneda M, Nozaki Y, Kawaguchi T, Torimura T, Abe M, Hiasa Y, Fukai M, Kamiyama T, Taketomi A, Mizokami M, Kanto T. Interleukin-34 as a fibroblast-derived marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6:28814. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 50. | Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, Blanchard S, Frémaux I, Croué A, Fouchard I, Lunel-Fabiani F, Boursier J, Roingeard P, Calès P, Delneste Y, Jeannin P. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879-1890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Mandelia C, Collyer E, Mansoor S, Lopez R, Lappe S, Nobili V, Alkhouri N. Plasma Cytokeratin-18 Level As a Novel Biomarker for Liver Fibrosis in Children With Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2016;63:181-187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Möckel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T, Tacke F. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960-1971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 53. | Tang LY, Heller M, Meng Z, Yu LR, Tang Y, Zhou M, Zhang YE. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J Biol Chem. 2017;292:4302-4312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 54. | Liu Y, Liu H, Meyer C, Li J, Nadalin S, Königsrainer A, Weng H, Dooley S, ten Dijke P. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem. 2013;288:30708-30719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 55. | Tu X, Zhang Y, Zheng X, Deng J, Li H, Kang Z, Cao Z, Huang Z, Ding Z, Dong L, Chen J, Zang Y, Zhang J. TGF-β-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice. Sci Rep. 2017;7:2957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, Olteanu S, Barshack I, Dotan S, Voronov E, Dinarello CA, Apte RN, Harats D. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55:1086-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 57. | de Lira Silva NS, Borges BC, da Silva AA, de Castilhos P, Teixeira TL, Teixeira SC, Dos Santos MA, Servato JPS, Justino AB, Caixeta DC, Tomiosso TC, Espindola FS, da Silva CV. The Deleterious Impact of Interleukin 9 to Hepatorenal Physiology. Inflammation. 2019;42:1360-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Hu BL, Shi C, Lei RE, Lu DH, Luo W, Qin SY, Zhou Y, Jiang HX. Interleukin-22 ameliorates liver fibrosis through miR-200a/beta-catenin. Sci Rep. 2016;6:36436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Liu Y, Cheng LS, Wu SD, Wang SQ, Li L, She WM, Li J, Wang JY, Jiang W. IL-10-producing regulatory B-cells suppressed effector T-cells but enhanced regulatory T-cells in chronic HBV infection. Clin Sci (Lond). 2016;130:907-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Yao L, Xing S, Fu X, Song H, Wang Z, Tang J, Zhao Y. Association between interleukin-10 gene promoter polymorphisms and susceptibility to liver cirrhosis. Int J Clin Exp Pathol. 2015;8:11680-11684. [PubMed] [Cited in This Article: ] |
| 61. | Zhang XW, Mi S, Li Z, Zhou JC, Xie J, Hua F, Li K, Cui B, Lv XX, Yu JJ, Hu ZW. Antagonism of Interleukin-17A ameliorates experimental hepatic fibrosis by restoring the IL-10/STAT3-suppressed autophagy in hepatocytes. Oncotarget. 2017;8:9922-9934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Lu DH, Guo XY, Qin SY, Luo W, Huang XL, Chen M, Wang JX, Ma SJ, Yang XW, Jiang HX. Interleukin-22 ameliorates liver fibrogenesis by attenuating hepatic stellate cell activation and downregulating the levels of inflammatory cytokines. World J Gastroenterol. 2015;21:1531-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 48] [Cited by in F6Publishing: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 63. | Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 64. | Ni YH, Huo LJ, Li TT. Antioxidant axis Nrf2-keap1-ARE in inhibition of alcoholic liver fibrosis by IL-22. World J Gastroenterol. 2017;23:2002-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Pan H, Hong F, Radaeva S, Gao B. Hydrodynamic gene delivery of interleukin-22 protects the mouse liver from concanavalin A-, carbon tetrachloride-, and Fas ligand-induced injury via activation of STAT3. Cell Mol Immunol. 2004;1:43-49. [PubMed] [Cited in This Article: ] |
| 66. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 67. | Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 465] [Cited by in F6Publishing: 471] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 68. | Wu LY, Liu S, Liu Y, Guo C, Li H, Li W, Jin X, Zhang K, Zhao P, Wei L, Zhao J. Up-regulation of interleukin-22 mediates liver fibrosis via activating hepatic stellate cells in patients with hepatitis C. Clin Immunol. 2015;158:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 69. | Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y, Jin L, Zhou C, Fu J, Gao B, Fu Y, Wang FS. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 70. | Chen E, Cen Y, Lu D, Luo W, Jiang H. IL-22 inactivates hepatic stellate cells via downregulation of the TGF-β1/Notch signaling pathway. Mol Med Rep. 2018;17:5449-5453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Hassoba H, Leheta O, Sayed A, Fahmy H, Fathy A, Abbas F, Attia F, Serwah A. IL-10 and IL-12p40 in Egyptian patients with HCV-related chronic liver disease. Egypt J Immunol. 2003;10:1-8. [PubMed] [Cited in This Article: ] |
| 72. | Khanam A, Trehanpati N, Sarin SK. Increased interleukin-23 receptor (IL-23R) expression is associated with disease severity in acute-on-chronic liver failure. Liver Int. 2019;39:1062-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Bao S, Zheng J, Li N, Huang C, Chen M, Cheng Q, Li Q, Lu Q, Zhu M, Ling Q, Yu K, Chen S, Shi G. Role of interleukin-23 in monocyte-derived dendritic cells of HBV-related acute-on-chronic liver failure and its correlation with the severity of liver damage. Clin Res Hepatol Gastroenterol. 2017;41:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Meng Z, Wang J, Yuan Y, Cao G, Fan S, Gao C, Wang L, Li Z, Wu X, Wu Z, Zhao L, Yin Z. γδ T cells are indispensable for interleukin-23-mediated protection against Concanavalin A-induced hepatitis in hepatitis B virus transgenic mice. Immunology. 2017;151:43-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, Merad M, Luedde T, Trautwein C, Tacke F. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 551] [Cited by in F6Publishing: 564] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 76. | Li J, Wang Y, Ma M, Jiang S, Zhang X, Zhang Y, Yang X, Xu C, Tian G, Li Q, Wang Y, Zhu L, Nie H, Feng M, Xia Q, Gu J, Xu Q, Zhang Z. Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine. 2019;40:43-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 77. | Sui G, Cheng G, Yuan J, Hou X, Kong X, Niu H. Interleukin (IL)-13, Prostaglandin E2 (PGE2), and Prostacyclin 2 (PGI2) Activate Hepatic Stellate Cells via Protein kinase C (PKC) Pathway in Hepatic Fibrosis. Med Sci Monit. 2018;24:2134-2141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Pan G, Zhao Z, Tang C, Ding L, Li Z, Zheng D, Zong L, Wu Z. Soluble fibrinogen-like protein 2 ameliorates acute rejection of liver transplantation in rat via inducing Kupffer cells M2 polarization. Cancer Med. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Niu L, Cui X, Qi Y, Xie D, Wu Q, Chen X, Ge J, Liu Z. Involvement of TGF-β1/Smad3 Signaling in Carbon Tetrachloride-Induced Acute Liver Injury in Mice. PLoS One. 2016;11:e0156090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 80. | Shi J, Zhao J, Zhang X, Cheng Y, Hu J, Li Y, Zhao X, Shang Q, Sun Y, Tu B, Shi L, Gao B, Wang FS, Zhang Z. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-β-dependent emperipolesis in HBV cirrhotic patients. Sci Rep. 2017;7:44544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 81. | Hu Z, Qin F, Gao S, Zhen Y, Huang D, Dong L. Paeoniflorin exerts protective effect on radiation-induced hepatic fibrosis in rats via TGF-β1/Smads signaling pathway. Am J Transl Res. 2018;10:1012-1021. [PubMed] [Cited in This Article: ] |
| 82. | Lee JH, Jang EJ, Seo HL, Ku SK, Lee JR, Shin SS, Park SD, Kim SC, Kim YW. Sauchinone attenuates liver fibrosis and hepatic stellate cell activation through TGF-β/Smad signaling pathway. Chem Biol Interact. 2014;224:58-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Yang JH, Kim SC, Kim KM, Jang CH, Cho SS, Kim SJ, Ku SK, Cho IJ, Ki SH. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-β/Smad signaling and relieving oxidative stress. Eur J Pharmacol. 2016;783:92-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 84. | Kim JY, An HJ, Kim WH, Gwon MG, Gu H, Park YY, Park KK. Anti-fibrotic Effects of Synthetic Oligodeoxynucleotide for TGF-β1 and Smad in an Animal Model of Liver Cirrhosis. Mol Ther Nucleic Acids. 2017;8:250-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Ghafoory S, Varshney R, Robison T, Kouzbari K, Woolington S, Murphy B, Xia L, Ahamed J. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018;2:470-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 86. | Li Y, Lua I, French SW, Asahina K. Role of TGF-β signaling in differentiation of mesothelial cells to vitamin A-poor hepatic stellate cells in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2016;310:G262-G272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Lei Y, Wang QL, Shen L, Tao YY, Liu CH. MicroRNA-101 suppresses liver fibrosis by downregulating PI3K/Akt/mTOR signaling pathway. Clin Res Hepatol Gastroenterol. 2019;pii:S2210-7401(19)30041-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, Lan HY, Sung JJ, Yu J. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget. 2015;6:7325-7338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 89. | Liang C, Bu S, Fan X. Suppressive effect of microRNA-29b on hepatic stellate cell activation and its crosstalk with TGF-β1/Smad3. Cell Biochem Funct. 2016;34:326-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Feili X, Wu S, Ye W, Tu J, Lou L. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-β1/Smad3 pathway in hepatic stellate cells. Cell Biol Int. 2018;42:1370-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Wang Y, Du J, Niu X, Fu N, Wang R, Zhang Y, Zhao S, Sun D, Nan Y. MiR-130a-3p attenuates activation and induces apoptosis of hepatic stellate cells in nonalcoholic fibrosing steatohepatitis by directly targeting TGFBR1 and TGFBR2. Cell Death Dis. 2017;8:e2792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 92. | Ning ZW, Luo XY, Wang GZ, Li Y, Pan MX, Yang RQ, Ling XG, Huang S, Ma XX, Jin SY, Wang D, Li X. MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1β Axis via Targeting Smad7 and Spry1. Antioxid Redox Signal. 2017;27:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 93. | Yu F, Lu Z, Huang K, Wang X, Xu Z, Chen B, Dong P, Zheng J. MicroRNA-17-5p-activated Wnt/β-catenin pathway contributes to the progression of liver fibrosis. Oncotarget. 2016;7:81-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Yu F, Guo Y, Chen B, Dong P, Zheng J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab Invest. 2015;95:781-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 95. | Yang X, Dan X, Men R, Ma L, Wen M, Peng Y, Yang L. MiR-142-3p blocks TGF-β-induced activation of hepatic stellate cells through targeting TGFβRI. Life Sci. 2017;187:22-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Besheer T, Elalfy H, Abd El-Maksoud M, Abd El-Razek A, Taman S, Zalata K, Elkashef W, Zaghloul H, Elshahawy H, Raafat D, Elemshaty W, Elsayed E, El-Gilany AH, El-Bendary M. Diffusion-weighted magnetic resonance imaging and micro-RNA in the diagnosis of hepatic fibrosis in chronic hepatitis C virus. World J Gastroenterol. 2019;25:1366-1377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Ma T, Cai X, Wang Z, Huang L, Wang C, Jiang S, Hua Y, Liu Q. miR-200c Accelerates Hepatic Stellate Cell-Induced Liver Fibrosis via Targeting the FOG2/PI3K Pathway. Biomed Res Int. 2017;2017:2670658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Saikia P, Bellos D, McMullen MR, Pollard KA, de la Motte C, Nagy LE. MicroRNA 181b-3p and its target importin α5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology. 2017;66:602-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 99. | Ju B, Nie Y, Yang X, Wang X, Li F, Wang M, Wang C, Zhang H. miR-193a/b-3p relieves hepatic fibrosis and restrains proliferation and activation of hepatic stellate cells. J Cell Mol Med. 2019;23:3824-3832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Ma L, Ma J, Ou HL. MicroRNA‑219 overexpression serves a protective role during liver fibrosis by targeting tumor growth factor β receptor 2. Mol Med Rep. 2019;19:1543-1550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Yang L, Dong C, Yang J, Yang L, Chang N, Qi C, Li L. MicroRNA-26b-5p Inhibits Mouse Liver Fibrogenesis and Angiogenesis by Targeting PDGF Receptor-Beta. Mol Ther Nucleic Acids. 2019;16:206-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 102. | Men R, Wen M, Zhao M, Dan X, Yang Z, Wu W, Wang MH, Liu X, Yang L. MircoRNA-145 promotes activation of hepatic stellate cells via targeting krüppel-like factor 4. Sci Rep. 2017;7:40468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 103. | Mandrekar P. Epigenetic regulation in alcoholic liver disease. World J Gastroenterol. 2011;17:2456-2464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 104. | Fakhir FZ, Lkhider M, Badre W, Alaoui R, Meurs EF, Pineau P, Ezzikouri S, Benjelloun S. Genetic variations in toll-like receptors 7 and 8 modulate natural hepatitis C outcomes and liver disease progression. Liver Int. 2018;38:432-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | El-Bendary M, Neamatallah M, Elalfy H, Besheer T, Elkholi A, El-Diasty M, Elsareef M, Zahran M, El-Aarag B, Gomaa A, Elhammady D, El-Setouhy M, Hegazy A, Esmat G. The association of single nucleotide polymorphisms of Toll-like receptor 3, Toll-like receptor 7 and Toll-like receptor 8 genes with the susceptibility to HCV infection. Br J Biomed Sci. 2018;75:175-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 106. | Liu J, Yu Q, Wu W, Huang X, Broering R, Werner M, Roggendorf M, Yang D, Lu M. TLR2 Stimulation Strengthens Intrahepatic Myeloid-Derived Cell-Mediated T Cell Tolerance through Inducing Kupffer Cell Expansion and IL-10 Production. J Immunol. 2018;200:2341-2351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 107. | Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, Lian Z, Wei H, Tian Z. Kupffer Cells Support Hepatitis B Virus-Mediated CD8+ T Cell Exhaustion via Hepatitis B Core Antigen-TLR2 Interactions in Mice. J Immunol. 2015;195:3100-3109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Bagheri V, Askari A, Arababadi MK, Kennedy D. Can Toll-Like Receptor (TLR) 2 be considered as a new target for immunotherapy against hepatitis B infection? Hum Immunol. 2014;75:549-554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Lee YS, Kim DY, Kim TJ, Kim SY, Jeong JM, Jeong WI, Jung JK, Choi JK, Yi HS, Byun JS. Loss of toll-like receptor 3 aggravates hepatic inflammation but ameliorates steatosis in mice. Biochem Biophys Res Commun. 2018;497:957-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Gong XW, Xu YJ, Yang QH, Liang YJ, Zhang YP, Wang GL, Li YY. Effect of Soothing Gan (Liver) and Invigorating Pi (Spleen) Recipes on TLR4-p38 MAPK Pathway in Kupffer Cells of Non-alcoholic Steatohepatitis Rats. Chin J Integr Med. 2019;25:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 111. | Seo W, Eun HS, Kim SY, Yi HS, Lee YS, Park SH, Jang MJ, Jo E, Kim SC, Han YM, Park KG, Jeong WI. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 2016;64:616-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 112. | Zhang M, Hu X, Li S, Lu C, Li J, Zong Y, Qi W, Yang H. Hepatoprotective effects of ethyl pyruvate against CCl4-induced hepatic fibrosis via inhibition of TLR4/NF-κB signaling and up-regulation of MMPs/TIMPs ratio. Clin Res Hepatol Gastroenterol. 2018;42:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Etienne-Mesmin L, Vijay-Kumar M, Gewirtz AT, Chassaing B. Hepatocyte Toll-Like Receptor 5 Promotes Bacterial Clearance and Protects Mice Against High-Fat Diet-Induced Liver Disease. Cell Mol Gastroenterol Hepatol. 2016;2:584-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 114. | Kwon EY, Choi MS. Luteolin Targets the Toll-Like Receptor Signaling Pathway in Prevention of Hepatic and Adipocyte Fibrosis and Insulin Resistance in Diet-Induced Obese Mice. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 115. | Roh YS, Zhang B, Loomba R, Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am J Physiol Gastrointest Liver Physiol. 2015;309:G30-G41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 116. | Roh YS, Park S, Kim JW, Lim CW, Seki E, Kim B. Toll-like receptor 7-mediated type I interferon signaling prevents cholestasis- and hepatotoxin-induced liver fibrosis. Hepatology. 2014;60:237-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | He YJ, Kuchta K, Deng YM, Cameron S, Lin Y, Liu XY, Ye GR, Lv X, Kobayashi Y, Shu JC. Curcumin Promotes Apoptosis of Activated Hepatic Stellate Cells by Inhibiting Protein Expression of the MyD88 Pathway. Planta Med. 2017;83:1392-1396. [PubMed] [DOI] [Cited in This Article: ] |
| 118. | Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, Österreicher CH, Stickel F, Ley K, Brenner DA, Kisseleva T. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-776.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 483] [Article Influence: 40.3] [Reference Citation Analysis (1)] |
| 119. | Miller AM, Wang H, Bertola A, Park O, Horiguchi N, Ki SH, Yin S, Lafdil F, Gao B. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54:846-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 120. | Campana L, Starkey Lewis PJ, Pellicoro A, Aucott RL, Man J, O'Duibhir E, Mok SE, Ferreira-Gonzalez S, Livingstone E, Greenhalgh SN, Hull KL, Kendall TJ, Vernimmen D, Henderson NC, Boulter L, Gregory CD, Feng Y, Anderton SM, Forbes SJ, Iredale JP. The STAT3-IL-10-IL-6 Pathway Is a Novel Regulator of Macrophage Efferocytosis and Phenotypic Conversion in Sterile Liver Injury. J Immunol. 2018;200:1169-1187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 121. | Katsounas A, Trippler M, Wang B, Polis M, Lempicki RA, Kottilil S, Gerken G, Schlaak JF. CCL5 mRNA is a marker for early fibrosis in chronic hepatitis C and is regulated by interferon-α therapy and toll-like receptor 3 signalling. J Viral Hepat. 2012;19:128-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 122. | Svinka J, Pflügler S, Mair M, Marschall HU, Hengstler JG, Stiedl P, Poli V, Casanova E, Timelthaler G, Sibilia M, Eferl R. Epidermal growth factor signaling protects from cholestatic liver injury and fibrosis. J Mol Med (Berl). 2017;95:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Kagan P, Sultan M, Tachlytski I, Safran M, Ben-Ari Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS One. 2017;12:e0176173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 124. | Choi S, Jung HJ, Kim MW, Kang JH, Shin D, Jang YS, Yoon YS, Oh SH. A novel STAT3 inhibitor, STX-0119, attenuates liver fibrosis by inactivating hepatic stellate cells in mice. Biochem Biophys Res Commun. 2019;513:49-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | Lee KJ, Jang YO, Cha SK, Kim MY, Park KS, Eom YW, Baik SK. Expression of Fibroblast Growth Factor 21 and β-Klotho Regulates Hepatic Fibrosis through the Nuclear Factor-κB and c-Jun N-Terminal Kinase Pathways. Gut Liver. 2018;12:449-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 126. | Ben-Shoshan SO, Kagan P, Sultan M, Barabash Z, Dor C, Jacob-Hirsch J, Harmelin A, Pappo O, Marcu-Malina V, Ben-Ari Z, Amariglio N, Rechavi G, Goldstein I, Safran M. ADAR1 deletion induces NFκB and interferon signaling dependent liver inflammation and fibrosis. RNA Biol. 2017;14:587-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10:98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 128. | Shen YH, Jiang HX, Qin SY, Wei LP, Meng YC, Luo W. [Activation of hepatocyte growth factor promotes apoptosis of hepatic stellate cells via the Rho pathway]. Zhonghua Gan Zang Bing Za Zhi. 2014;22:136-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 129. | Oh Y, Park O, Swierczewska M, Hamilton JP, Park JS, Kim TH, Lim SM, Eom H, Jo DG, Lee CE, Kechrid R, Mastorakos P, Zhang C, Hahn SK, Jeon OC, Byun Y, Kim K, Hanes J, Lee KC, Pomper MG, Gao B, Lee S. Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology. 2016;64:209-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 130. | Henderson WR, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309-14314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 131. | Sombetzki M, Fuchs CD, Fickert P, Österreicher CH, Mueller M, Claudel T, Loebermann M, Engelmann R, Langner C, Sahin E, Schwinge D, Guenther ND, Schramm C, Mueller-Hilke B, Reisinger EC, Trauner M. 24-nor-ursodeoxycholic acid ameliorates inflammatory response and liver fibrosis in a murine model of hepatic schistosomiasis. J Hepatol. 2015;62:871-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 132. | Lefebvre E, Gottwald M, Lasseter K, Chang W, Willett M, Smith PF, Somasunderam A, Utay NS. Pharmacokinetics, Safety, and CCR2/CCR5 Antagonist Activity of Cenicriviroc in Participants With Mild or Moderate Hepatic Impairment. Clin Transl Sci. 2016;9:139-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 133. | Qu K, Huang Z, Lin T, Liu S, Chang H, Yan Z, Zhang H, Liu C. New Insight into the Anti-liver Fibrosis Effect of Multitargeted Tyrosine Kinase Inhibitors: From Molecular Target to Clinical Trials. Front Pharmacol. 2016;6:300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 134. | Zhao Z, Pan G, Tang C, Li Z, Zheng D, Wei X, Wu Z. IL-34 Inhibits Acute Rejection of Rat Liver Transplantation by Inducing Kupffer Cell M2 Polarization. Transplantation. 2018;102:e265-e274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |