Published online Feb 21, 2018. doi: 10.3748/wjg.v24.i7.852
Peer-review started: October 25, 2017
First decision: November 22, 2017
Revised: December 31, 2017
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: February 21, 2018
To define predictors of functional benefit of direct-acting antivirals (DAAs) in patients with chronic hepatitis C virus (HCV) infection and liver cirrhosis.
We analysed a cohort of 199 patients with chronic HCV genotype 1, 2, 3 and 4 infection involving previously treated and untreated patients with compensated (76%) and decompensated (24%) liver cirrhosis at two tertiary centres in Germany. Patients were included with treatment initiation between February 2014 and August 2016. All patients received a combination regimen of one or more DAAs for either 12 or 24 wk. Predictors of functional benefit were assessed in a univariable as well as multivariable model by binary logistic regression analysis.
Viral clearance was achieved in 88% (175/199) of patients. Sustained virological response (SVR) 12 rates were as follows: among 156 patients with genotype 1 infection the SVR 12 rate was 90% (n = 141); among 7 patients with genotype 2 infection the SVR 12 rate was 57% (n = 4); among 30 patients with genotype 3 infection the SVR 12 rate was 87% (n = 26); and among 6 patients with genotype 4 infection the SVR 12 rate was 67% (n = 4). Follow-up MELD scores were available for 179 patients. A MELD score improvement was observed in 37% (65/179) of patients, no change of MELD score in 41% (74/179) of patients, and an aggravation was observed in 22% (40/179) of patients. We analysed predictors of functional benefit from antiviral therapy in our patients beyond viral eradication. We identified the Child-Pugh score, the MELD score, the number of platelets and the levels of albumin and bilirubin as significant factors for functional benefit.
Our data may contribute to the discussion of potential risks and benefits of antiviral therapy with individual patients infected with HCV and with advanced liver disease.
Core tip: Therapeutic regimens for patients with chronic hepatitis C virus (HCV) infection have substantially improved over the last few years. However real-life data in patients with cirrhosis are still limited, and predictors of functional benefit of direct-acting antivirals are not well defined. We analysed data from patients with HCV infection and liver cirrhosis to evaluate predictors of functional benefit for identifying patients profiting most from antiviral therapy beyond HCV eradication.
- Citation: Steinebrunner N, Stein K, Sandig C, Bruckner T, Stremmel W, Pathil A. Predictors of functional benefit of hepatitis C therapy in a ‘real-life’ cohort. World J Gastroenterol 2018; 24(7): 852-861
- URL: https://www.wjgnet.com/1007-9327/full/v24/i7/852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i7.852
The introduction of all oral direct-acting antivirals (DAAs) based hepatitis C virus (HCV) therapy has dramatically increased the number of patients who are eligible for anti-viral therapy[1-4] and high response rates can be achieved with various combinational treatment regimens[5-7]. However, patients infected with HCV and suffering from liver cirrhosis remain a challenging subgroup with lower sustained virological response (SVR) rates compared with those patients with mild or moderate hepatic impairment[7,8]. An estimated 25% of patients with HCV infection in the United States have cirrhosis, and this number is expected to rise to 37% by 2020[9-11]. These patients are at risk of disease-related complications, including hepatocellular carcinoma, liver decompensation, end-stage liver disease and finally the need for liver transplantation[7,12,13]. Liver decompensation and hepatocellular carcinoma associated with infection of HCV are the most common causes for liver transplantation in Europe and North America. Several trials have shown an early and long-term improvement of liver function associated with successful anti-viral treatment in the majority of cases[14-21]. However, a certain number of patients with compensated and decompensated liver cirrhosis show a deterioration of liver function in spite of successful viral clearance[17,22,23]. In this subset of patients with advanced liver disease, the prediction of functional benefit of anti-viral therapy in an individual patient is not well established. Therefore, in our real-life observational study, we analysed patients with HCV infection and liver cirrhosis to evaluate predictors for identifying those patients profiting most from antiviral therapy beyond HCV eradication with significant clinical improvement of hepatic function.
We analysed clinical and laboratory data sets of all consecutive patients aged 18 years or over with chronic HCV genotype 1, 2, 3 or 4 infection and liver cirrhosis receiving DAA-based antiviral therapy. Patients were included with treatment initiation between February 2014 and August 2016 in a retrospective, longitudinal study at two investigative sites in Germany. One patient with HCV genotype 1b infection on a 12-wk regimen of paritaprevir/ritonavir (PTV/r) + ombitasvir (OMV) + dasabuvir (DSV) ± ribavirin (RBV) prematurely terminated treatment due to deterioration of liver function and subsequently received a liver transplant. After liver transplantation, the viral load remained negative on follow-up. Another patient with HCV genotype 1b infection on a 12-wk regimen of sofosbuvir (SOF) + daclatasvir (DCV) prematurely terminated treatment due to myalgia. At the point of treatment discontinuation, the patient had no detectable viral load and it remained negative on follow-up. In two patients with HCV genotype 1b infection on a 12-wk regimen of SOF + simeprevir (SMV) + RBV the dose of RBV was discontinued after four weeks and after six weeks respectively, due to anaemia. Both patients showed SVR on follow-up. One patient was non-adherent to the antiviral treatment plan and showed no SVR. Three patients were started on antiviral therapy and failed to attend their follow-ups. These were, respectively, two patients with a 12-wk regimen of SOF + ledipasvir (LDV) + RBV (genotype 1a and genotype 1b) and one patient with a 12-wk regimen of SOF + DCV + RBV (genotype 3). These patients were classified as treatment failures. All patients were included in the intention-to-treat (ITT) analysis.
Patients received a combination treatment of one or more DAAs with or without RBV for either 12 or 24 wk, depending on genotype, pre-treatment history or contraindications according to local guidelines[1]. SOF was administered at 400 mg once daily or in combination with LDV 90 mg as a single tablet co-formulation. DCV was applied once daily at a dosing of 60 mg, with dose adjustment to 30 mg or 90 mg per day as recommended for patients with relevant potential drug-drug interactions. SMV was applied at a dosing of 150 mg once daily. Another treatment combination was PTV/r 150 mg/100 mg plus OMV 25 mg (once daily) and DSV 250 mg (twice daily) with or without RBV. RBV was administered twice daily, with the dose determined according to body weight (1000 mg per day for patients with a body weight of < 75 kg and 1200 mg per day in patients with a body weight ≥ 75 kg), according to the individual treatment protocol.
Patients were reviewed at treatment weeks 4, 12 and 24 and at additional time points, if deemed necessary, as well as 12 wk after the end of treatment. Serum HCV-RNA and standard laboratory tests were regularly assessed at baseline and at the following clinical visits. The lower limit of quantification (LLOQ) was 12 IU/mL [Abbott Real Time (ART) HCV assay (Abbott Molecular, Des Plaines, IL, United States)]. Liver cirrhosis was confirmed by liver histology or conducted by evaluation of data sets from non-invasive tests, comprising transient elastography (TE) (FibroScan, Echosens, Paris, France), with a cut-off value for cirrhosis of ≥ 12.5 kPa, ultrasound examination, imaging by computed tomography or magnetic resonance, presence of oesophageal varices and laboratory values. Classification was according to the Child-Pugh score. Liver stiffness measurements were performed according to current EASL guidelines[5,24] at baseline and 12 wk after the end of DAA treatment. The study was conducted in accordance with the Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Continuous data were expressed by mean values and standard deviation. Categorical variables were expressed as absolute and relative numbers. Continuous data were analysed with t-test and categorical data with the chi-square test. Predictors of functional benefit were assessed in a non-stepwise method for multivariable binary logistic regression analysis. To maintain the validity of the logistic regression analysis relative to the number of outcome events, a maximum of 7 variables was selected[25]. Variables were chosen due to clinical relevance. These variables were selected based on the clinical experience of the authors, as well as based on the data of other publications[23] and were used for univariable as well as for multivariable analyses. MELD score and Child-Pugh score were assessed for interaction and showed none. We performed the Hosmer-Lemeshow test, which showed significance (P = 0.02) and therefore demonstrated a low goodness of fit. The removal of creatinine from the multivariable assessments improved the goodness of fit considerably (P = 0.43) and therefore was excluded from further analyses. A P-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (IBM SPSS Statistics 24, Armonk, NY, United States) with additional analysis performed using SAS 9.4 (SAS Institute, Cary, NC, United States).
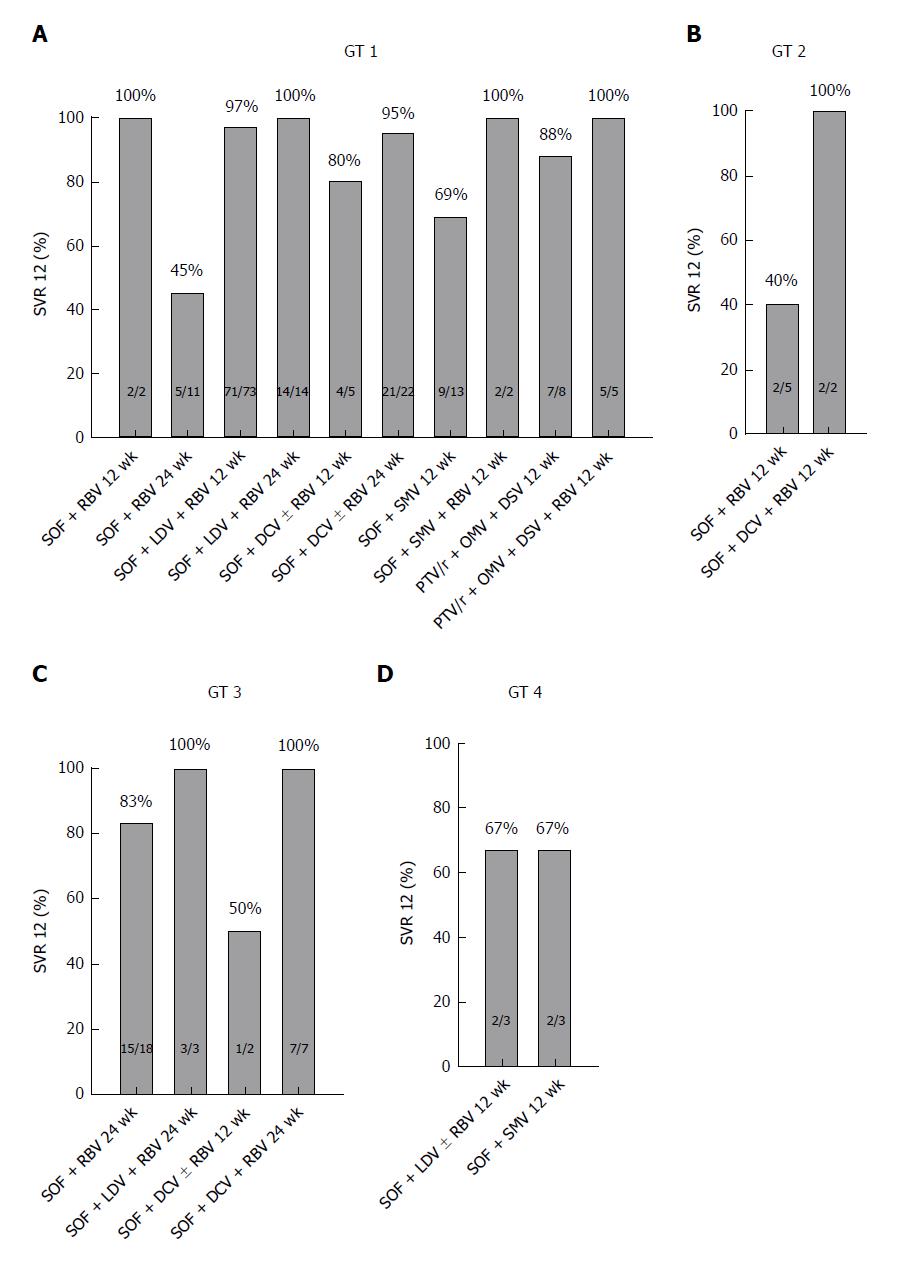
We enrolled 199 patients with chronic HCV infection and liver cirrhosis at two tertiary sites in Germany. HCV genotype 1 was present in 78% (n = 156) of patients, followed by genotype 3 in 15% (n = 30), genotype 2 in 4% (n = 7) and genotype 4 in 3% (n = 6) of patients. Our patient sample reflects existing data for the distribution of genotypes in Central Europe: Approximately 70% for genotype 1, followed by 21% for genotype 3, 3% for genotype 2 and 5% for genotype 4[26,27]. Of all patients, 56% (n = 112) were treatment experienced, and 4% (n = 8) had received a protease inhibitor in a previous therapy. At treatment initiation, 152 (76%) patients had compensated cirrhosis and 47 (24%) had decompensated cirrhosis. Baseline characteristics of the study cohort are shown in Table 1. A combination treatment of SOF+RBV was administered to 18% (n = 36) of patients, and 47% (n = 93) of patients received a therapy regime of SOF + LDV ± RBV. A therapy regime of SOF + DCV ± RBV was administered to 19% (n = 38) of patients. 18 patients (9%) were treated with a regimen of SOF + SMV ± RBV. A therapy regime of PTV/r + OMV + DSV ± RBV was applied in 7% (n = 14) of patients. Treatment duration was either 12 or 24 wk, depending on the individual treatment protocol. Details on the treatment protocols with respect to the different genotypes are shown in Table 2.
| Demographics | Value |
| Age (yr) | 59 ± 10 (27-83) |
| Male gender | 133 (67) |
| HCV genotype | |
| 1a | 47 (23) |
| 1b | 100 (50) |
| 1 (no confirmed subtype) | 9 (5) |
| 2 | 7 (4) |
| 3 | 30 (15) |
| 4 | 6 (3) |
| Viral load 106 (IU/mL) | 2.04 ± 3.46 (0.01-34.50) |
| Treatment history | |
| Treatment naive | 86 (43) |
| Treatment experienced | 113 (57) |
| Protease inhibitor experienced | 8 (4) |
| Liver/renal status | |
| Platelets (103/μL) | 116 ± 61 (26-341) |
| Total bilirubin (mg/dL) | 1.4 ± 0.9 (0.2-5.7) |
| INR | 1.34 ± 0.78 (0.40-5.70) |
| Creatinine (mg/dL) | 0.80 ± 0.22 (0.43-1.96) |
| TE score (kPa)1 | 24.5 ± 12.5 (5.5-75) |
| Average MELD score | 9 ± 3 (6-23) |
| MELD score | |
| < 10 | 130 (65) |
| 10-15 | 59 (30) |
| > 15 | 10 (5) |
| Child-Pugh score | |
| A | 152 (76) |
| B | 40 (20) |
| C | 7 (4) |
| Therapy regime | Treatment duration (wk) | GT 1 | GT 2 | GT 3 | GT 4 |
| SOF + RBV | 12 | 2 (1) | 5 (3) | ||
| SOF + RBV | 24 | 11 (5) | 18 (9) | ||
| SOF + LDV | 12 | 1 (1) | |||
| SOF + LDV + RBV | 12 | 73 (36) | 2 (1) | ||
| SOF + LDV + RBV | 24 | 14 (6) | 3 (2) | ||
| SOF + DCV | 12 | 2 (1) | 1 (1) | ||
| SOF + DCV | 24 | 19 (9) | |||
| SOF + DCV + RBV | 12 | 3 (2) | 2 (1) | 1 (1) | |
| SOF + DCV + RBV | 24 | 3 (2) | 7 (3) | ||
| SOF + SMV | 12 | 13 (6) | 3 (2) | ||
| SOF + SMV + RBV | 12 | 2 (1) | |||
| PTV/r + OMV + DSV | 12 | 8 (4) | |||
| PTV/r + OMV + DSV + RBV | 12 | 6 (3) |
Viral clearance was achieved in 88% (175/199) of patients. The SVR 12 rates according to the HCV genotype were as follows: 90% of patients with genotype 1 infection (141/156) and 57% of patients with genotype 2 infection (4/7) reached SVR 12. Among 30 patients with genotype 3 infection, the SVR 12 rate was 87% (26/30) and among 6 patients with genotype 4 infection, the SVR 12 rate was 67% (4/6) (Figure 1). Anti-viral treatment in our patient group was well tolerated. However, one patient treated with PTV/r + OMV + DSV ± RBV presented with acute liver failure within 10 d of treatment initiation. Therefore, antiviral therapy was terminated at this time. Deterioration of liver function was associated with a worsening of the MELD score from 12 to 29. This patient received a liver transplant within a short period of time with subsequently undetectable HCV RNA on follow-ups.
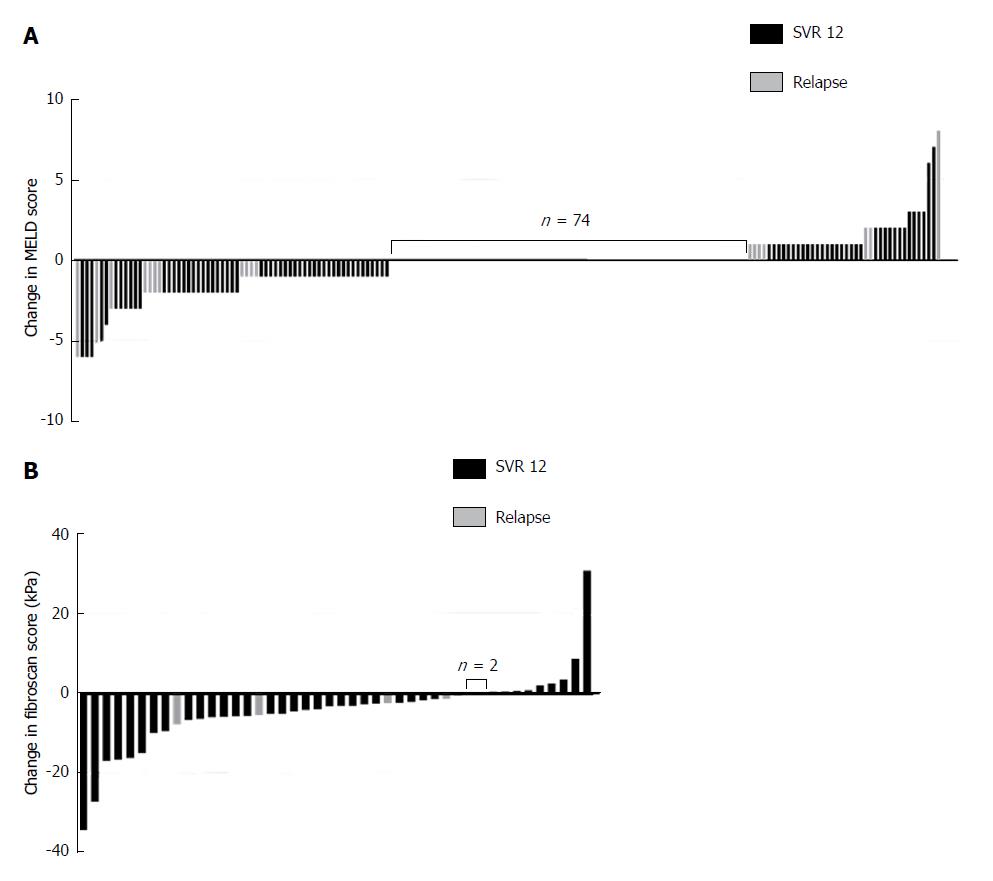
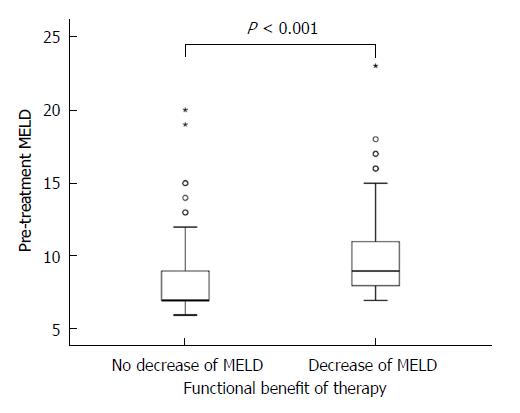
The MELD score consists of international normalized ratio (INR), serum bilirubin and creatinine levels[28]. At treatment initiation, the mean MELD score in our cohort was 9 ± 3 and the respective variables were as follows: INR was 1.34 ± 0.78; bilirubin was 1.4 ± 0.9 mgdl-1 and creatinine 0.80 ± 0.22 mgdl-1. Of all patients, 130 (65%) had a MELD score <10; 59 (30%) had a MELD score in the range of 10-15; and 10 (5%) had a MELD score >15. At 12 wk post-treatment, laboratory data were available for 179 patients. The average MELD score in the total number of our studied patients remained unchanged with 9 ± 3 at 12 wk post-treatment and the respective variables were as follows: INR was 1.13 ± 0.23; bilirubin was 1.4 ± 1.0 mgdl-1 and creatinine 0.80 ± 0.21 mgdl-1. A MELD score decrease was observed in 37% (65/179) of patients, no change of MELD score in 41% (74/179), and an increase was observed in 22% (40/179). In the subgroup of patients with a MELD decrease, 16% (11/65) experienced a relapse, compared to 25% (10/40) in the subgroup with a MELD increase. The difference between these groups for this parameter could not be deemed to be significant (P = 0.315). The highest increase of the MELD score was +8 in one patient, the largest reduction of the MELD score was -6 in four patients (Figure 2A). In one patient, the increase of the MELD score was 17 (from 12 to 29) within 10 d of treatment initiation. In this patient, antiviral treatment was terminated prematurely. Of the baseline factors examined (age, Child-Pugh score, MELD score, creatinine, platelets, albumin and bilirubin), the Child-Pugh score, the MELD score, the number of platelets and the levels of albumin and bilirubin were significant factors for functional benefit in the univariable analyses. Multivariable analyses showed a trend for MELD (P = 0.082) and for albumin (P = 0.057), however significance at the level of 5% was not reached (Table 3 and Figure 3).
| Baseline parameters | MELD decrease | P value (univariable) | P value (multivariable3) | OR (95%CI) | |
| Yes (n = 78, 44%) | No (n = 101, 56%) | ||||
| Age (yr) | 59 ± 9 | 59 ± 9 | 0.9781 | 0.815 | 0.996 (0.961-1.032) |
| Child-Pugh A | 53 (38) | 86 (62) | 0.0062 | 0.592 | 1.327 (0.472-3.725) |
| Child-Pugh B/C | 25 (62) | 15 (38) | |||
| MELD | 10 ± 3 | 8 ± 3 | < 0.0011 | 0.082 | 1.177 (0.980-1.414) |
| Creatinine (mg/dL) | 0.8 ± 0.3 | 0.9 ± 0.8 | 0.3441 | - | - |
| Platelets (/nL) | 104 ± 56 | 126 ± 64 | 0.0141 | 0.617 | 0.998 (0.992-1.005) |
| Albumin (g/L) | 36.7 ± 5.2 | 39.9 ± 4.9 | < 0.0011 | 0.057 | 0.928 (0.860-1.002) |
| Bilirubin (mg/dL) | 1.6 ± 1.0 | 1.1 ± 0.8 | < 0.0011 | 0.739 | 1.096 (0.638-1.883) |
Mean TE scores before treatment initiation were 24.5 ± 12.5 kPa. After antiviral therapy, TE scores decreased in 75% of patients (33/44) and increased in 20% of patients (9/44) 12 wk post-treatment. Two patients (5%) had no change in TE scores. Four patients had a relapse of HCV infection as well as a decrease in liver stiffness measured at that time (Figure 2B).
Interferon-free combinations of DAAs have profoundly improved the efficacy and safety of HCV treatment in patients with cirrhosis, which is the group of patients most difficult to cure. Overall response rates to DAA-based therapies in our patient group were high at 88% (175/199). This is consistent with previously published data of DAA treatment regimens in patients with an advanced stage of liver disease. However, there is restricted comparability of SVR rates of our patients with previously published trials, due to differences in study populations, including clinical characteristics of patients, pre-treatment history and the specific treatment plans and treatment duration. For patients with genotype 1 infection treated with SOF + LDV for 12 wk, in the ION-1 trial SVR was achieved in 97% of previously untreated patients and in the ION-2 trial in 93% of treatment-experienced patients. However, the ION-1 and ION-2 trial included only 15%-20% of patients with cirrhosis[29,30]. In the TURQUOISE-II trial, 191 (92%) of 208 treatment-naive or treatment-experienced patients with compensated cirrhosis treated for 12 wk with ritonavir-boosted PTV + OMV + DSV achieved SVR[31]. In the SOLAR-1 and SOLAR-2 trials, including patients with cirrhosis with HCV genotype 1 or 4 treated with SOF/LDV + RBV for 12 or 24 wk, SVR rates were 86% to 89%[22,32]. In the SOLAR trials, viral eradication was associated with an improvement of MELD scores, but it is unclear whether the benefits were related to viral eradication alone or due to a more stringent care of patients at specialized centres in the setting of a clinical trial[22,32].
In general, randomized controlled trials involve a more homogenous cohort of patients with typically extensive inclusion and exclusion criteria, while real-life cohorts represent a more diverse spectrum of patients with fewer restrictions. Thus, these cohorts help to better understand the risk of virological failure in a real-life clinical setting.
It has to be emphasised that current DAA treatments are expensive. Therefore, stratification and prioritisation of patients by identifying those with the most urgent need for therapy and the most likely to benefit from therapy are important until treatment costs decrease as several effective drugs offering a cure are introduced[7].
SVR has been shown to reduce both all-cause and liver-related mortality from HCV infection among patients with cirrhosis[21,33] and also an improvement of the MELD score has been reported[22]. In the SOLAR-2 study including patients with advanced cirrhosis with Child-Pugh class B and C, a majority of patients showed an improvement of the MELD score under successful DAA therapy[32]. Nevertheless, some patients showed no change or even a deterioration of the MELD score despite viral clearance.
Considering that not all patients with cirrhosis benefit from HCV therapy despite SVR, the key question is which patients profit. The Child-Pugh score is a prognostic model for liver cirrhosis, which has been a useful clinical tool in day-to-day clinical practice for over 50 years[34]. However, the Child-Pugh score includes subjective criteria (ascites and encephalopathy) besides the laboratory values of INR, bilirubin and albumin. Furthermore, the Child-Pugh score is not as accurate in predicting mortality of patients with liver cirrhosis as is the MELD score. In contrast to the Child-Pugh score, the MELD score has been derived from prospectively collected data rather than empirically constructed data. Also, the MELD score increases as the three variables (INR, bilirubin and creatinine) deteriorate, whereas the constituent parameters in the Child-Pugh score remain fixed once a defined threshold has been reached[35]. Therefore, the MELD score has been established as a classification system to determine the urgency for liver transplantation. Consequently, we suggest that the MELD score also might be a valuable tool in assessing the risk and benefit of DAA treatment and might be more reliable than the Child-Pugh score. This is in line with the results of the study by Foster et al[23], including patients with HCV and decompensated cirrhosis treated with SOF + LDV or SOF + DCV either with or without RBV for a total of 12 wk. This study analysed patient characteristics to identify patients most likely to benefit from therapy. Regression analysis of baseline characteristics and association with MELD change yielded baseline MELD score as the only significant independent factor[23].
However, it should be mentioned that benefits, including mortality, even in non-advanced liver disease such as low-grade fibrosis have been found in long-term studies following antiviral treatment[36,37].
In a study of the Irish Early Access Programme, 101 patients with advanced cirrhosis with HCV genotype 1, 3 or 4 were treated with SOF + LDV ± RBV for 12 wk. The overall SVR 12 rate for this cohort was 74.3%. This data depicts high SVR rates in this difficult to treat patient group. However, the authors also reported 8 deaths, representing an on-treatment mortality in patients with Child-Pugh B score at a baseline of 6% (4/67), and in patients with Child-Pugh C score at a baseline of 21% (4/19). The causes of death for the eight patients were: liver failure (n = 2), intracerebral haemorrhage (n = 2), sepsis (n = 2), complications from a pre-existing hepatocellular carcinoma (n = 1) and cardiac arrest (n = 1)[38]. However, with efficacy and safety of DAA therapy established in large patient cohorts, it appears that in these cases of adverse events were caused by the underlying disease process[39]. The data of our study support the general statement that DAA treatment is safe, even in patients with advanced liver disease.
As there was no significant difference in the rate of relapse in the group of patients with a MELD score increase compared to the group with no change in MELD score or with a decrease of the MELD score, the risk of a relapse seems to be independent of functional hepatic changes during therapy.
Transient elastography measurements are established for fibrosis and cirrhosis assessment in patients with HCV infection[5,6,40,41]. However, experience in the change of transient elastography following antiviral therapy is still limited. We observed a decrease of transient elastography measures in 75% of patients (33/44) following antiviral therapy. A recent study showed a significant reduction of liver stiffness assessed by transient elastography only for patients who achieve SVR[42]. However, in our patient cohort, four patients with a relapse presented with a decrease of transient elastography measures. It remains to be further examined whether the changes in liver stiffness indicate a regression of fibrosis or an attenuation of chronic liver inflammation caused by HCV replication. These results have to be validated in a larger cohort of patients, and it has to be determined whether these early changes persist with a longer follow-up time interval.
In conclusion, novel treatment algorithms with DAAs for chronic HCV infection yield excellent results even in a patient population with cirrhosis. However, it may be useful to pinpoint parameters to identify those patients who will actually benefit clinically from antiviral therapy beyond viral eradication. Our results suggest that the Child-Pugh score, the MELD score, the number of platelets and the levels of albumin and bilirubin may be predictors of functional benefit from DAA-based therapy. This association may serve as another tool for health care providers to discuss treatment options and assess the risks and benefits of antiviral therapy with patients on an individual basis.
To improve patient care by assisting health care professionals in the decision-making process for treatment of patients infected with hepatitis C virus (HCV).
Direct-acting antivirals (DAAs) have recently opened up promising new therapeutic options for patients with chronic HCV infection. Nevertheless, in order to study the efficacy of these regimens, especially in difficult-to-treat patient populations, such as patients with liver cirrhosis, real-life data is needed.
We investigated patients in our real-life cohort from two tertiary referral centres in Germany. We analysed data from patients with HCV infection and liver cirrhosis to evaluate predictors of functional benefit for identifying patients profiting most of antiviral therapy beyond HCV eradication.
Predictors of functional benefit were determined in a univariable as well as multivariable model assessed by binary logistic regression analysis. For reasons of validity of the logistic regression analysis (relative to the number of outcome events), a maximum of 7 clinically relevant variables was selected.
Our results indicate that the Child-Pugh score, the MELD score, the number of platelets and the levels of albumin and bilirubin may be predictors of functional benefit from DAA-based therapy, so that these variables may serve as another tool to guide antiviral therapy in affected patients.
With the introduction of DAAs, the indication for therapy for patients with HCV infection and with liver cirrhosis has dramatically expanded with vast improvements of SVR. However, it remains unclear, which patients profit most from antiviral therapy. Therefore, a simple and feasible clinical index may be beneficial to evaluate the presumed effect of antiviral therapy before treatment initiation. Based on the parameters studied, we suggest the MELD score as part of such an evaluation system.
The ultimate goal of antiviral therapy in patients with HCV infection is to avoid hepatic complications such as the development of hepatocellular carcinoma or hepatic decompensation. Further research with large patient groups and longer observation periods is warranted to define and confirm predictive markers to identify patients, which profit clinically most from antiviral therapy beyond viral eradication
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Inoue K, Marcos M S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Lin H, Zeng J, Xie R, Schulz MJ, Tedesco R, Qu J, Erhard KF, Mack JF, Raha K, Rendina AR. Discovery of a Novel 2,6-Disubstituted Glucosamine Series of Potent and Selective Hexokinase 2 Inhibitors. ACS Med Chem Lett. 2015;7:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | European Association for Study of Liver. EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 877] [Cited by in F6Publishing: 890] [Article Influence: 98.9] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J Hepatol. 2017;66:153-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 776] [Cited by in F6Publishing: 840] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 5. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1177] [Cited by in F6Publishing: 1178] [Article Influence: 130.9] [Reference Citation Analysis (0)] |
| 6. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1046] [Cited by in F6Publishing: 1011] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 7. | Staton-Tindall M, Webster JM, Oser CB, Havens JR, Leukefeld CG. Drug use, hepatitis C, and service availability: perspectives of incarcerated rural women. Soc Work Public Health. 2015;30:385-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, Freilich BF, Younes ZH, Harlan W, Ghalib R. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61:1127-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 500] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 9. | Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-521, 521.e1-521.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 10. | Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 704] [Cited by in F6Publishing: 707] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 11. | Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, Kumar R. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-2170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 12. | Steinebrunner N, Sandig C, Sommerer C, Hinz U, Giese T, Stremmel W, Zahn A. Reduced residual gene expression of nuclear factor of activated T cells-regulated genes correlates with the risk of cytomegalovirus infection after liver transplantation. Transpl Infect Dis. 2014;16:379-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Steinebrunner N, Sandig C, Sommerer C, Hinz U, Giese T, Stremmel W, Zahn A. Pharmacodynamic monitoring of nuclear factor of activated T cell-regulated gene expression in liver allograft recipients on immunosuppressive therapy with calcineurin inhibitors in the course of time and correlation with acute rejection episodes--a prospective study. Ann Transplant. 2014;19:32-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509-516.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, Almasio PL, Maisonneuve P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069-2076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009;50:387-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, Reddy KR, Lawitz E, Flamm SL, Schiano T. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med. 2015;373:2618-2628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 599] [Cited by in F6Publishing: 574] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 18. | George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 286] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 19. | Mira JA, Rivero-Juárez A, López-Cortés LF, Girón-González JA, Téllez F, de los Santos-Gil I, Macías J, Merino D, Márquez M, Ríos-Villegas MJ. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56:1646-1653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Poordad F, Schiff ER, Vierling JM, Landis C, Fontana RJ, Yang R, McPhee F, Hughes EA, Noviello S, Swenson ES. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology. 2016;63:1493-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 348] [Cited by in F6Publishing: 341] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 21. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1165] [Cited by in F6Publishing: 1128] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 22. | Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr, Fried MW, Terrault NA, O’Leary JG, Vargas HE, Kuo A, Schiff E, Sulkowski MS, Gilroy R, Watt KD, Brown K, Kwo P, Pungpapong S, Korenblat KM, Muir AJ, Teperman L, Fontana RJ, Denning J, Arterburn S, Dvory-Sobol H, Brandt-Sarif T, Pang PS, McHutchison JG, Reddy KR, Afdhal N; SOLAR-1 Investigators. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 637] [Cited by in F6Publishing: 656] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 23. | Foster GR, Irving WL, Cheung MC, Walker AJ, Hudson BE, Verma S, McLauchlan J, Mutimer DJ, Brown A, Gelson WT. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;64:1224-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 372] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 24. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 974] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 25. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [PubMed] [Cited in This Article: ] |
| 26. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1077] [Cited by in F6Publishing: 1066] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 27. | Petruzziello A, Marigliano S, Loquercio G, Cacciapuoti C. Hepatitis C virus (HCV) genotypes distribution: an epidemiological up-date in Europe. Infect Agent Cancer. 2016;11:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3434] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 29. | Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1065] [Cited by in F6Publishing: 1042] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 30. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1329] [Article Influence: 132.9] [Reference Citation Analysis (0)] |
| 31. | Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, Shiffman ML, Wedemeyer H, Berg T, Yoshida EM. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 698] [Cited by in F6Publishing: 731] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 32. | Manns M, Samuel D, Gane EJ, Mutimer D, McCaughan G, Buti M, Prieto M, Calleja JL, Peck-Radosavljevic M, Müllhaupt B. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicentre, open-label, randomised, phase 2 trial. Lancet Infect Dis. 2016;16:685-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 33. | Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL; Italian Association of the Study of the Liver Disease (AISF). Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 34. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] [Cited in This Article: ] |
| 35. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1967] [Cited by in F6Publishing: 1911] [Article Influence: 79.6] [Reference Citation Analysis (0)] |
| 36. | Zahnd C, Salazar-Vizcaya L, Dufour JF, Müllhaupt B, Wandeler G, Kouyos R, Estill J, Bertisch B, Rauch A, Keiser O; Swiss HIV; Swiss Hepatitis C Cohort Studies. Modelling the impact of deferring HCV treatment on liver-related complications in HIV coinfected men who have sex with men. J Hepatol. 2016;65:26-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | McCombs J, Matsuda T, Tonnu-Mihara I, Saab S, Hines P, L’italien G, Juday T, Yuan Y. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med. 2014;174:204-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Gray E, O’Leary A, Stewart S, Bergin C, Cannon M, Courtney G, Crosbie O, De Gascun CF, Fanning LJ, Feeney E, Houlihan DD, Kelleher B, Lambert JS, Lee J, Mallon P, McConkey S, McCormick A, McKiernan S, McNally C, Murray F, Sheehan G, Norris S; Irish Hepatitis C Outcomes and Research Network (ICORN). High mortality during direct acting antiviral therapy for hepatitis C patients with Child’s C cirrhosis: Results of the Irish Early Access Programme. J Hepatol. 2016;65:446-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Curry MP. Direct acting antivirals for decompensated cirrhosis. Efficacy and safety are now established. J Hepatol. 2016;64:1206-1207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 41. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P; FIBROSTIC study group. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 42. | Bachofner JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017;37:369-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |