Published online Nov 14, 2018. doi: 10.3748/wjg.v24.i42.4759
Peer-review started: August 8, 2018
First decision: October 5, 2018
Revised: October 19, 2018
Accepted: October 26, 2018
Article in press: October 26, 2018
Published online: November 14, 2018
To investigate whether Yiguanjian decoction (YGJ) has an anti-liver cirrhotic effect and whether it regulates hepatic stem cell differentiation.
A rat model of liver cirrhosis was established via subcutaneous injection of carbon tetrachloride (CCl4) for 8 wk. From the beginning of the ninth week, the rats received 2-acetylaminofluorene (2-AAF) by oral gavage and a DLK-1+ fetal liver stem/progenitor cell (FLSPC) transplant or an FLSPC transplant in combination with YGJ treatment for 4 wk. In vitro, lipopolysaccharide (LPS)-activated macrophages were co-cultured with WB-F344 cells, and the differentiation of WB-F344 cells was observed in the presence and absence of YGJ treatment.
FLSPC transplantation improved liver function and histopathology, and inhibited the activation of the non-canonical Wnt signaling pathway, while activating the canonical Wnt signaling pathway. YGJ enhanced the therapeutic effects of FLSPCs and also promoted the liver regeneration differentiation of FLSPCs into hepatocytes. In vitro, LPS-activated macrophages promoted the differentiation of WB-F344 cells into myofibroblasts, and the canonical Wnt signaling was inhibited while the non-canonical Wnt signaling was activated in WB-F344 cells. YGJ suppressed the activation of macrophages and then inhibited non-canonical Wnt signaling and promoted canonical Wnt signaling.
YGJ enhances FLSPC-mediated repair of liver cirrhosis through regulation of macrophage activation state, and YGJ in combination with stem cell transplantation may be a suitable treatment for end-stage liver cirrhosis.
Core tip: Stem cells play an important role in the treatment of end-stage liver cirrhosis, but details concerning their differentiation are still controversial. Our previous studies have indicated that Yiguanjian decoction (YGJ) has an anti-hepatic fibrosis effect. However, it remains unclear whether YGJ regulates stem cell differentiation. In this work, we found that YGJ may enhance fetal liver stem/progenitor cell-mediated repair of liver cirrhosis through regulation of macrophage activation state in cirrhosis, suggesting that YGJ in combination with stem cell transplantation may be a suitable treatment for end-stage liver cirrhosis.
- Citation: Xu Y, Fan WW, Xu W, Jiang SL, Chen GF, Liu C, Chen JM, Zhang H, Liu P, Mu YP. Yiguanjian decoction enhances fetal liver stem/progenitor cell-mediated repair of liver cirrhosis through regulation of macrophage activation state. World J Gastroenterol 2018; 24(42): 4759-4772
- URL: https://www.wjgnet.com/1007-9327/full/v24/i42/4759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i42.4759
Liver cirrhosis is the common endpoint of a wide variety of chronic liver disease processes. Liver transplantation is the most effective treatment for end-stage liver cirrhosis[1]. However, it is limited by the shortage of organs, transplant rejection, and high cost[2]. Thus, hepatic progenitor cells (HPCs) have been recommended as a potential therapeutic approach to end-stage liver cirrhosis, which can be isolated from developing and mature livers[3-6]. Wnt signaling is a key regulator for HPC differentiation[7,8]; canonical Wnt signaling promotes HPCs to differentiate into hepatocytes, while non-canonical Wnt signaling promotes HPCs to differentiate into myofibroblast and promotes the progression of liver fibrosis.
In addition, macrophages, also known as Kupffer cells (KCs) in the liver, regulate the development of fibrosis by modulating pro- and anti-inflammatory pathways[9,10]. Distinct macrophage subsets express different types of chemokines and surface markers and exhibit a diversity of functions[11-13]. M1 macrophages (classically activated) produce an abundance of pro-inflammatory cytokines after being induced by pro-inflammatory mediators, such as lipopolysaccharide (LPS). In contrast, M2 macrophages (alternatively activated) are believed to participate in the blockade of inflammatory responses and promotion of tissue repair[14,15]. A recent study demonstrated that scavenger receptor-AI positive M2 macrophages can protect against hepatitis C virus (HCV) infection-induced liver injury and fibrosis[16]. It has been reported that stem cell differentiation is also regulated by macrophage polarization, particularly by the Wnt ligands expressed by macrophages[17]. Therefore, it is important to elucidate the relationship between macrophages and Wnt signaling during HPC-mediated liver regeneration.
Yiguanjian decoction (YGJ), a classical recipe for treating liver injury,which has a long history in traditional Chinese medicine (TCM), consists of six medicinal herbs, i.e., Rehmanniae Radix (Rehmannia glutinosa Libosch., root, Shengdi), Glehniae Radix (Glehnia littoralis Fr. Schmidtex Miq., root, Beishashen), Ophiopogonis Radix [Ophiopogon japonicus (Linn. f.) Ker-Gawl., root, Maidong], Lycii Fructus (Lycium barbarum L., fruit, Gouqizi), Radix Angelicae Sinensis [Angelica sinensis (Oliv.) Diels., root, Danggui], and Toosendan Fructus (Melia toosendan Sieb. et Zucc., fruit, Chuanlianzi). Our previous studies indicated that YGJ exerts anti-fibrogenic effects on carbon tetrachloride (CCl4)-induced cirrhosis in rodent models by inhibiting hepatocyte apoptosis and hepatic stem cell (HSC) activation, regulating the function of KCs, repressing angiogenesis, and inhibiting the migration of bone marrow cells to the liver[18-20]. In the present study, we discovered that FLSPC transplantation promotes the repair of liver cirrhosis, and that YGJ may enhance this effect via mechanisms related to inhibition of pro-inflammatory macrophage activation and regulation of Wnt signaling.
YGJ consists of Rehmanniae Radix (Rehmannia glutinosa Libosch., root, Shengdi) 18 g, Glehniae Radix (Glehnia Littoralis Fr. Schmidtex Miq., root, Beishashen) 10 g, Ophiopogonis Radix [Ophiopogon japonicus (Linn. f.) Ker-Gawl., root, Maidong] 10 g, Lycii Fructus (Lycium barbarum L., fruit, Gouqizi) 12 g, Radix Angelicae Sinensis [Angelica sinensis (Oliv.) Diels., root, Danggui] 10 g, and Toosendan Fructus (Melia toosendan Sieb. et Zucc., fruit, Chuanlianzi) 4.5 g, which were provided by the Shanghai Huayu Herbs Co. Ltd. and prepared by Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, and were maintained at -20 °C.
Sorafinib (SORA) was purchased from Bayer (Leverkusen, Germany) and used as a positive control. Rabbit monoclonal antibody against hepatocyte nuclear factor 4 alpha (HNF4α, SC-8987) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Mouse monoclonal antibody against α-smooth muscle actin (α-SMA, Clone 1A4) was obtained from Sigma-Aldrich (St Louis, MO, United States). Mouse monoclonal antibody against hepatocyte specific antigen (Hep, GTX73779) was purchased from GeneTex (Alton Parkway Irvine, CA, United States). Horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-mouse and HRP-conjugated polyclonal swine anti-rabbit antibodies were obtained from Dako Denmark A/S (Glostrup, Denmark). Hybond-ECL nitrocellulose membranes and ECL detection reagents were obtained from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). Mouse monoclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Chemicon International (Temecula, CA, United States). All other reagents were purchased from Sigma Chemical or Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Male Wistar rats (aged 7-8 wk and weighing 160-180 g, n = 37) were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). Animals were kept in a constant temperature environment and supplied with laboratory chow and water ad libitum. The experimental protocol was approved by the Animal Research Committee of Shanghai University of TCM (No. 20130132).
Liver cirrhosis was induced as previously described[19]. Briefly, the rats received subcutaneous injections of 50% CCl4-olive oil solution (2 mL/kg) twice a week for 8 wk. At the beginning of the 9th wk, the dose of CCl4-olive oil solution was changed to 30% (2 mL/kg) and was administered for another 4 wk in order to maintain cirrhosis progression and reduce mortality, and all rats were treated with 2-AAF [10 mg/(kg∙d)] via intragastric administration once a day. The rats were randomly divided into a 2-AAF/CCl4 group (n = 8), an FLSPC group (n = 8), an FLSPC + YGJ group (n = 8), and an FLSPC + SORA group (n = 8). The FLSPC, FLSPC + YGJ, and FLSPC + SORA groups were treated with FLSCs via a single intra-splenic injection at the 9th wk. The FLSPC + YGJ and FLSPC + SORA groups were orally administrated at dosages of 3.56 g/kg and 1.0 mg/kg, respectively, once per day for 4 wk. Normal rats (N, n = 5) received an equal amount of subcutaneous olive oil and the same volume of oral physiological saline.
FLSPCs were isolated from ED14/15 fetal livers of pregnant Wistar rats as previously described[21]. The livers were cut into pieces and digested with 0.05% trypsin and 0.05% NB4 for 15 min. Next, a single-cell suspension was collected and stained with an anti-Dlk-1 antibody. Dlk-1 positive cells were sorted using a magnetic bead sorter instrument (Miltenyi Biotec). The purity of the Dlk-1 positive cells was analyzed by flow cytometry (BD Accuri C6, BD Biosciences) and was determined to be 60.58%. At the beginning of the 9th wk, the rats given FLSPC therapy were transplanted with Dio-stained Dlk-1+ FLSPCs (1 × 106 cells per rat) via intra-splenic injection.
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) were measured using standard laboratory methods.
Paraformaldehyde-fixed (4%) specimens were cut into 4 μm sections and stained with 0.1% (w/v) Sirius Red (Direct Red 80; Aldrich, Milwaukee, WI, United States), or hematoxylin and eosin (H&E).
Immunostaining was performed according to previously published methods[22]. Briefly, sections were de-paraffinized, washed, and pre-incubated in blocking solution, followed by incubation with anti-α-SMA (1:200), anti-HNF4α (1:200), and anti-Hep (1:200) antibodies. Next, the sections were incubated with HRP-conjugated secondary antibodies (1:1000), washed, stained with diaminobenzidine (DAB), and counterstained with hematoxylin. A Leica SCN 400 microscope was used to visualize the samples. For immunofluorescent staining, Alexa Fluor 488 and cyanine 3 secondary antibodies (Jackson ImmunoResearch, West Grove, PA, United States) were used with counterstaining. Images were obtained with a confocal laser scanning microscope (FV10i, Olympus, Japan).
WB-F344 cells (a rat oval cell line that is morphologically and functionally similar to freshly isolated HPCs[23]) and RAW264.7 cells (a murine macrophage cell line) were purchased from the Shanghai Cell Bank (Chinese Academy of Sciences, Shanghai, China). WB-F344 cells were cultured at 37 °C in an atmosphere containing 5% CO2 in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mmol/L glutamine, and penicillin/streptomycin (100 mg/mL). Activation of RAW264.7 cells was induced with LPS 100 ng/mL for 8 h at 37 °C in an atmosphere containing 5% CO2 in DMEM supplemented with 10% FCS[24], and then co-cultured with WB-F344 cells in Transwell chambers. A total of 2 × 104 WB-F344 cells were seeded into the upper compartment and 4 × 104 RAW264.7 cells were seeded into the lower compartment of the Transwell chamber. LPS was added to the culture medium, and the medium was replaced every 48 h for a total culture time of 7 d.
The mRNA expression of tumor necrosis factor-alpha (TNF-α), transforming growth factor beta 1 (TGF-β1), α-SMA, collagen type I [Col(1)], CD68, CD163, HNF4α, Hep, Wnt-1, -3A, -4, -5A, -5B, -8A, -8B, -10B, -11, β-catenin, frizzled (FZD)-1, -2, -3, -4, -5, -6, low-density lipoprotein receptor-related protein (LRP)-5, -6, and GAPDH was quantified using quantitative reverse transcription (RT)-PCR. Total RNA was extracted from the liver tissue using a total RNA purification kit (Lot. 250800) (TOYOBO, Osaka, Japan). RNA was reverse-transcribed to cDNA and gene expression was measured using SYBR Green Real-time PCR Master Mix (TaqMan) (Lot. 411900) (TOYOBO), and the ViiA 7 Real-Time PCR System (ABI, United States) was used. Primers and oligonucleotide probes were designed using Primer Express (Takara Chemical), and are presented in Table 1. Each PCR amplification was performed all samples in both the experimental and control groups. Individual gene expression was normalized to GAPDH expression levels. The conditions for the One-Step SYBR RT-PCR (Perfect Real Time) were as follows: an initial step at 42 °C for 15 min and 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 60 °C for 1 min.
| Gene | Primer sequence | Note | |
| α-SMA | Forward | AATGGCTCTGGGCTCTGTAA | SYBR |
| Reverse | TCTCTTGCTCTGGGCTTCAT | ||
| Col1 | Forward | TGACTGGAAGAGCGGAGAGT | SYBR |
| Reverse | GACGGCTGAGTAGGGAACAC | ||
| TGF-β | Forward | ATTCCTGGCGTTACCTTGG | SYBR |
| Reverse | AGCCCTGTATTCCGTCTCCT | ||
| TNF-α | Forward | GACGTGGAACTGGCAGAAGAG | SYBR |
| Reverse | TTGGTGGTTTGTGAGTGTGAG | ||
| CD68 | Forward | GGACCCACAACTGTCACTCAT | SYBR |
| Reverse | AAGCCCCACTTTAGCTTTACC | ||
| CD163 | Forward | TGGGATCGCCGTGACGCTTC | SYBR |
| Reverse | CAGCGACTGCCTCCACCGAC | ||
| HNF4α | Forward | CGGGCCACTGGCAAACAC | SYBR |
| Reverse | GTAATCCTCCAGGCTCAC | ||
| Hep | Forward | TAGCAGAGATGAGCCGTGTG | SYBR |
| Reverse | GCTTTGAGGCAGGCGTATT | ||
| β-catenin | Forward | GTCTGAGGACAAGCCACAGGACTAC | SYBR |
| Reverse | AATGTCCAGTCCGAGATCAGCA | ||
| Wnt 1 | Forward | GGGGAGCAACCAAAGTCG | SYBR |
| Reverse | TGGAGGAGGCTATGTTCACG | ||
| Wnt 3A | Forward | TCCGACTCTTGGCAGAACTT-3 | SYBR |
| Reverse | AATGGAATAGGTCCCGAACA | ||
| Wnt 4 | Forward | GGCACTCATGAACCTTCACAACA | SYBR |
| Reverse | CTTTACCTCACAGGAGCCTGACAC | ||
| Wnt 5A | Forward | GCGCTGCTGGAGTGGTAAAT | SYBR |
| Reverse | AGCCAGTCCCGAGGTAAGTC | ||
| Wnt 5B | Forward | CGAGCCCTCATGAACTTACAGAAC | SYBR |
| Reverse | GGAGACTCCGTGACATTTGCAG | ||
| Wnt 8A | Forward | CCTGGGAGCGGTGGAACT | SYBR |
| Reverse | CCTGGTGTGGGTTGAAAACTG | ||
| Wnt 8B | Forward | AAGGCTTACCTGGTCTACTC | SYBR |
| Reverse | CAGAGCTGATGGCGTGCACA | ||
| Wnt 10B | Forward | CCTCAAGCGCGGTTTCC | SYBR |
| Reverse | CAGCAGCCAGCATGGAGAA | ||
| Wnt 11 | Forward | TTGACCTGGAGAGAGGTACAC | SYBR |
| Reverse | GTCAGGGGAGCTCTGTAGATA | ||
| Frizzled 1 | Forward | GGGAATGCAGTCACCAGTACCA | SYBR |
| Reverse | CCAGACCCATAGCAGGTTCCA | ||
| Frizzled 2 | Forward | ACTGCAAGAGCCTAGCCATCC | SYBR |
| Reverse | ATCCAGAAGCCCGACGTGA | ||
| Frizzled 3 | Forward | ACACATGGCACCAGCATGAAC | SYBR |
| Reverse | CCATGCGAAGGCCAAGACTAA | ||
| Frizzled 4 | Forward | GACAACTTTCACGCCGCTCA | SYBR |
| Reverse | TTCAGGACTGGTTCACATCGTCTC | ||
| Frizzled 5 | Forward | CGAGAGCACAGCCACATTCACTA | SYBR |
| Reverse | GAGCTGGCCATGCCAAAGA | ||
| Frizzled 6 | Forward | CAGCAGCGTCCAACTCCAAG | SYBR |
| Reverse | TGCACTCCATCAGGCCAGTC | ||
| LRP5 | Forward | GACATTTACTGGCCCAATGG | SYBR |
| Reverse | CTGCCCTCCACCACCTTCT | ||
| LRP6 | Forward | TCTCCGGCGAATTGAAAG | SYBR |
| Reverse | GAGTCTTCTAGCACGATCCTGT | ||
| GAPDH | Forward | AAGGTCATCCATGACAACTTTGGC | SYBR |
| Reverse | ACAGTCTTCTGGGTGGCAGTGAT |
Protein expression was assessed by immunoblot analysis as previously described[22]. The liver tissue was lysed with RIPA buffer containing 50 mmol/L Tris-HCl (pH 7.2), 150 mmol/L NaCl, 1% NP-40, 0.1% SDS, 1 mmol/L ethylene diamine tetraacetic acid (EDTA), and 1 mmol/L phenylmethanesulfonyl fluoride (PMSF) and then homogenized in ice-cold water. After centrifugation for 10 min at 4 °C at 12000 r/min, the protein concentration of the supernatant was determined using the Bio-Rad Dc protein Assay Reagent (Bio-Rad, Hercules, CA, United States). Protein was electrophoretically resolved on a 10% or 12% sodium dodecyl sulfate (SDS) polyacrylamide gel and successively transferred to Hybond-ECL nitrocellulose membranes. The membranes were blocked with 5% non-fat dry milk solution in Tris-buffered saline (20 mmol/L Tris and 150 mmol/L NaCl, pH 7.4) with 0.1% Tween-20. Next, the membranes were incubated with the primary antibodies overnight at 4 °C and subsequently with secondary antibodies at room temperature for 1 h. The following dilutions of primary antibodies were used: α-SMA, 1:1000; HNF4α, 1:500; Hep, 1:500; GAPDH, 1:30000. Immune complexes were visualized using SuperSignal West Pico Chemiluminescent Substrate (ECL, Pierce, Rockford, IL, United States). Finally, band intensity was determined by scanning video densitometry.
All data are presented as the mean ± SD. Statistical analyses were performed using one-way analysis of variance with SPSS 17.0 software. P < 0.05 was considered statistically significant.
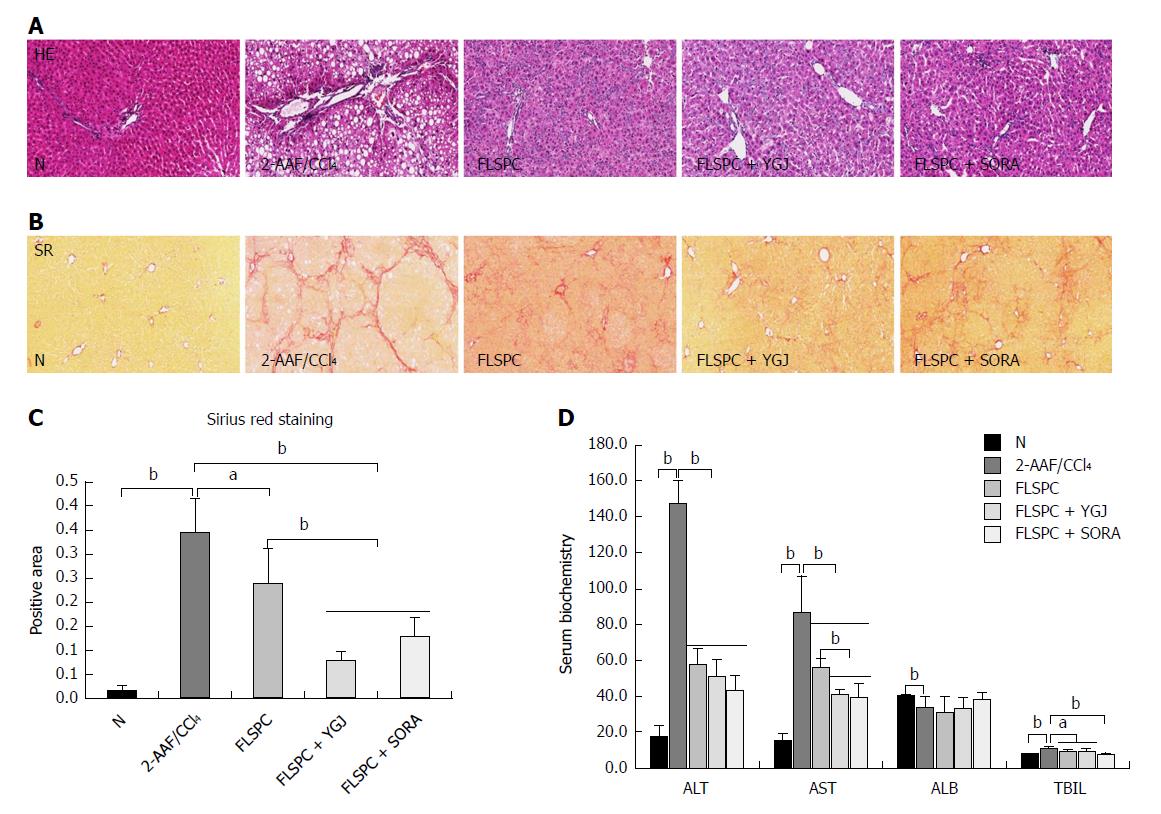
H&E staining showed an abnormal structure of the hepatic lobule, inflammatory infiltration in the portal area and fibrous septa, and more hepatic steatosis in the 2-AAF/CCl4 group, while hepatic steatosis and the inflammatory response were markedly attenuated in the FLSPC and FLSPC + YGJ groups compared to the 2-AAF/CCl4 group (Figure 1A).
Sirius red staining showed a large amount of collagen deposited in the portal area and some collagen fibers extended into the liver parenchyma and forming a pseudo-lobular structure in the 2-AAF/CCl4 group. In contrast, collagen deposition was markedly attenuated in the FLSPC and FLSPC + YGJ groups compared with the 2-AAF/CCl4 group (Figure 1B). The sirius red staining positive area was increased significantly in the 2-AAF/CCl4 group than in the N group (P < 0.01), while it was decreased significantly in the FLSPC and FLSPC + YGJ groups than in the 2-AAF/CCl4 group (P < 0.05 and P < 0.01, respectively), and in the FLSPC + YGJ group compared to the FLSPC group (P < 0.01) (Figure 1C).
Consistent with the pathological changes in the liver tissue, serum levels of ALT, AST, and TBIL were increased significantly in the 2-AAF/CCl4 group compared to the N group (P < 0.05 or P < 0.01), while they were decreased significantly in the FLSPC and FLSPC + YGJ groups compared to the 2-AAF/CCl4 group (P < 0.05 or P < 0.01). AST level was decreased significantly in the FLSPC + YGJ group compared to the FLSPC group (P < 0.01) (Figure 1D).
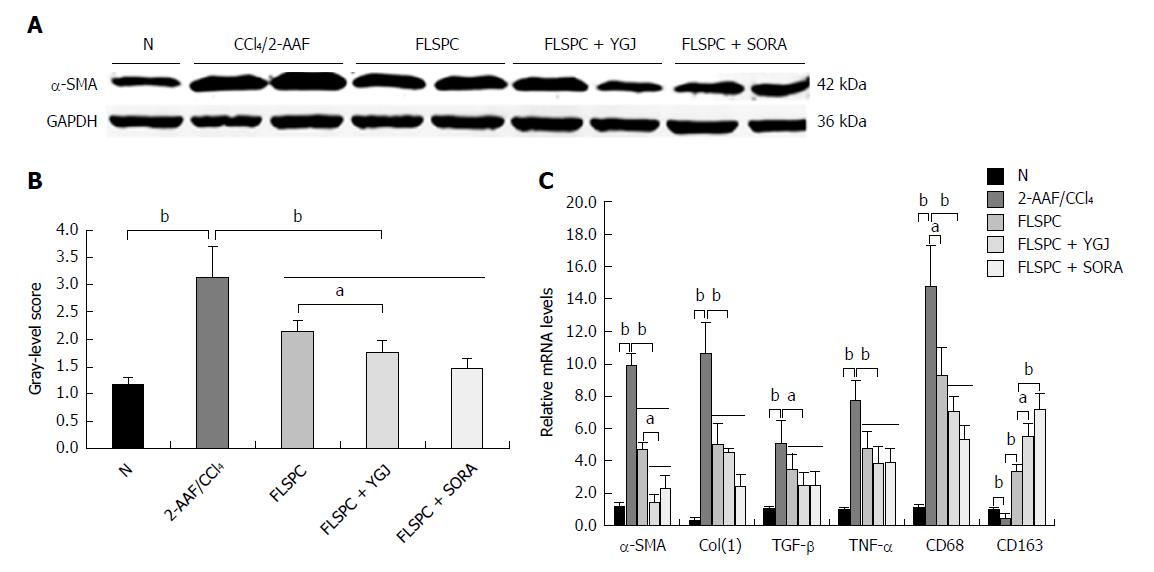
Immunoblotting analysis revealed that the expression of α-SMA was significantly higher in the 2-AAF/CCl4 group than in the N group (P < 0.01), but it was significantly lower in the FLSPC and FLSPC + YGJ groups than in the 2-AAF/CCl4 group (P < 0.01), and significantly lower in the FLSPC + YGJ group than in the FLSPC group (P < 0.05) (Figure 2A and B). Furthermore, the mRNA levels of α-SMA, Col(1), TGF-β, TNF-α, and CD68 were significantly higher in the 2-AAF/CCl4 group than in the N group (P < 0.01), while they were significantly lower in the FLSPC and FLSPC + YGJ groups compared to the 2-AAF/CCl4 group (P < 0.01). Consistent with the protein expression, the mRNA level of α-SMA was significantly lower in the FLSPC + YGJ group than in the FLSPC group (P < 0.05). In addition, the mRNA level of CD163 was significantly lower in the 2-AAF/CCl4 group than in the N group (P < 0.01), while it was significantly higher in the FLSPC and FLSPC + YGJ groups compared with the 2-AAF/CCl4 group (P < 0.01), and in the FLSPC + YGJ group compared to the FLSPC group (P < 0.05) (Figure 2C).
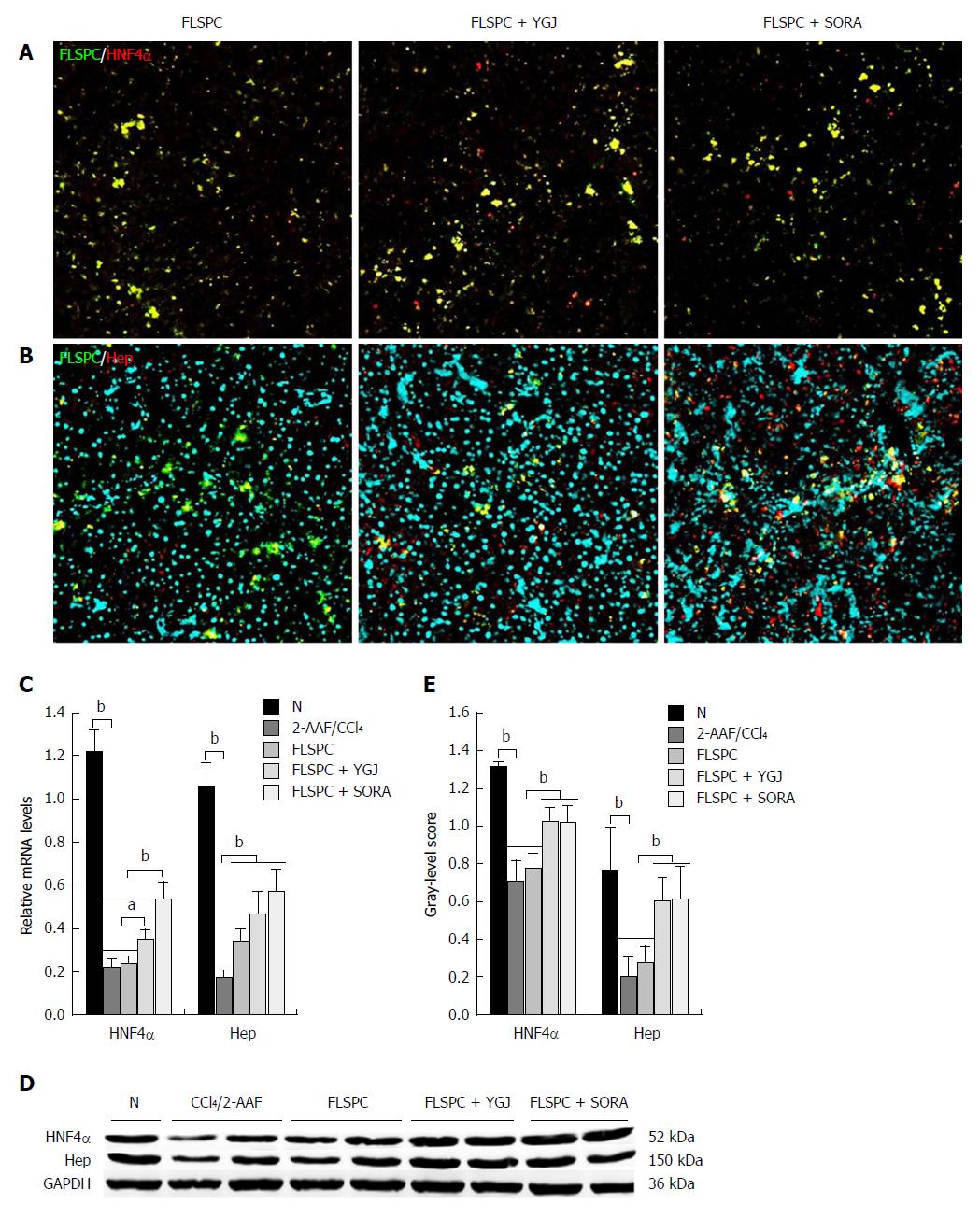
Immunofluorescence staining demonstrated that FLSPCs (red)/HNF4α (green), and FLSPCs (red)/Hep (green) were co-expressed in liver parenchyma (Figure 3A and B). The mRNA levels of the hepatocyte markers HNF4α and Hep were significantly lower in the 2-AAF/CCl4 group than in the N group (P < 0.01), while the mRNA level of HNF4α was significantly higher in the FLSPC + YGJ group than in the 2-AAF/CCl4 and FLSPC groups (P < 0.05), and the mRNA level of Hep was significantly higher in the FLSPC and FLSPC + YGJ groups compared to the 2-AAF/CCl4 group (P < 0.01) (Figure 3C).
Consistent with the mRNA expression patterns, the protein levels of HNF4α and Hep were also significantly lower in the 2-AAF/CCl4 group than in the N group (P < 0.01), while they were significantly higher in the FLSPC + YGJ group than in the FLSPC and 2-AAF/CCl4 groups (P < 0.01) (Figure 3D and E).
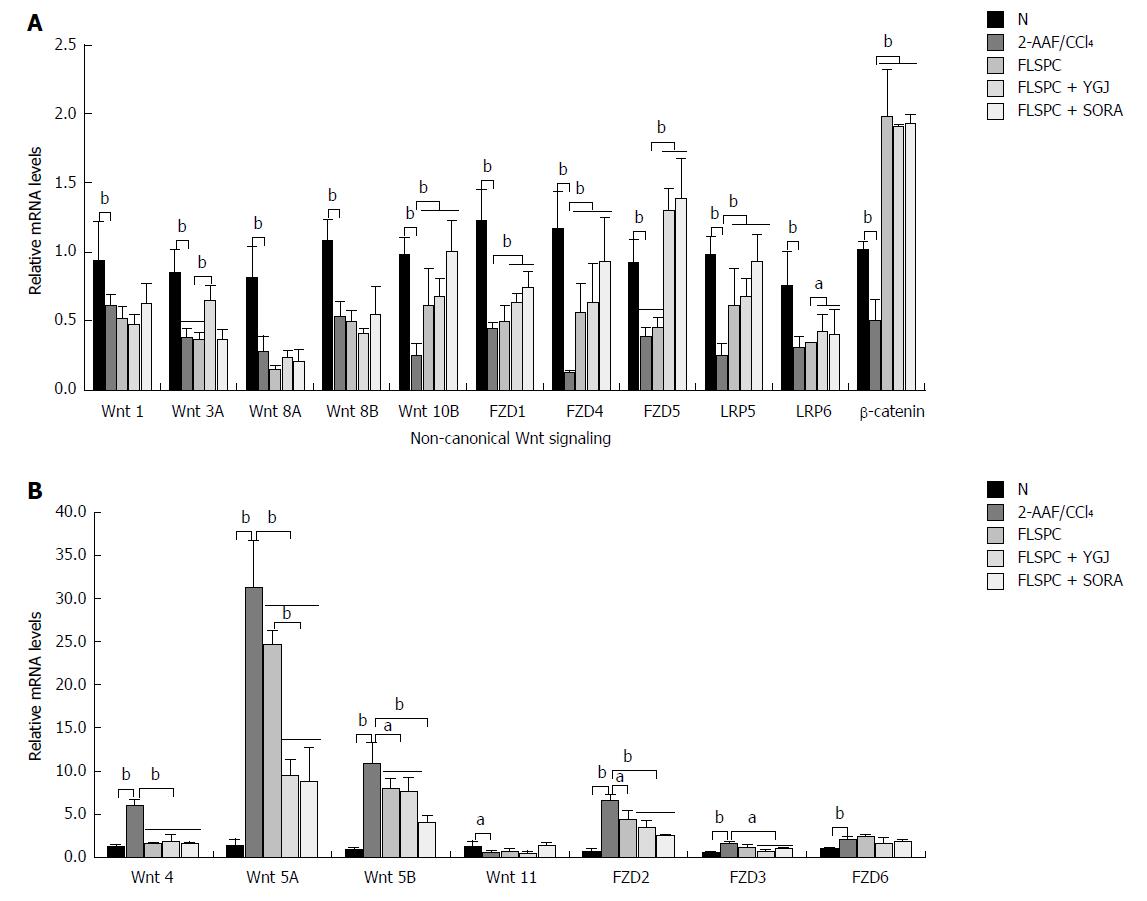
The mRNA levels of canonical Wnt pathway components including Wnt-1, -3A, -8A, -8B, -10B, FZD-1, -4, -5, LRP-5, -6, and β-catenin were decreased significantly in the 2-AAF/CCl4 group compared to the N group (P < 0.01), while the mRNA levels of Wnt 10B, FZD4, LRP5, and β-catenin were significantly higher in the FLSPC and the FLSPC + YGJ groups than in the 2-AAF/CCl4 group (P < 0.01). In addition, the mRNA levels of Wnt 3A, FZD-1, -5 and LRP6 were significantly higher in the FLSPC + YGJ group than in the 2-AAF/CCl4 group (P < 0.05 or P < 0.01), and the Wnt 3A, FZD1 and LRP6 were significantly higher than FLSPC group (P < 0.05 or P < 0.01) (Figure 4A).
The mRNA levels of non-canonical Wnt pathway components including Wnt-4, -5A, -5B, and FZD-2, -3, -6 were increased significantly in the 2-AAF/CCl4 group compared to the N group (P < 0.01), while the mRNA levels of Wnt-4, -5A, -5B, and FZD2 were significantly lower in the FLSPC and FLSPC + YGJ groups than in the 2-AAF/CCl4 group (P < 0.05 or P < 0.01), and FZD3 mRNA levels were significantly lower only in the FLSPC + YGJ group (P < 0.05). In addition, the expression of Wnt 5A was significantly lower in the FLSPC + YGJ group than in the FLSPC group (P < 0.01) (Figure 4B).
These results suggest that the Wnt signaling pathway was unbalanced in the development of liver cirrhosis induced with 2-AAF/CCl4. FLPSC transplantation promoted the activation of canonical Wnt signaling and suppressed the non-canonical Wnt signaling. Furthermore, these effects were augmented when FLSPC transplantation was combined with YGJ treatment.
As described above, we determined that the mRNA level of CD68 was lower and CD163 was higher after FLSPC transplantation, and these effects were more pronounced in the FLSPC + YGJ group. These results suggest that YGJ may regulate the activated state of KCs and then improve the repair function of FLSPCs. To confirm this hypothesis, we performed experiments with different concentrations of LPS (50 ng/mL, 100 ng/mL, and 200 ng/mL) for 48 h to induce RAW264.7 cells activation and assessed the mRNA levels of TGF-β, TNF-α, interleukin (IL)-1, and platelet-derived growth factor receptor (PDGF) as well as the protein level of TNF-α. These results indicate that the optimal LPS concentration to stimulate the RAW264.7 cells was 100 ng/mL (Supplementary Figure 1A and B). We also confirmed that treatment with 100 ng/mL LPS did not induce the differentiation of WB-F344 cells into myofibroblasts or macrophages by measuring the mRNA levels of α-SMA or CD68. In addition there was no apparent cytotoxicity to the WB-F344 cells in response to the LPS treatment, as determined by detecting the activation of LDH (Supplementary Figure 2A-C).
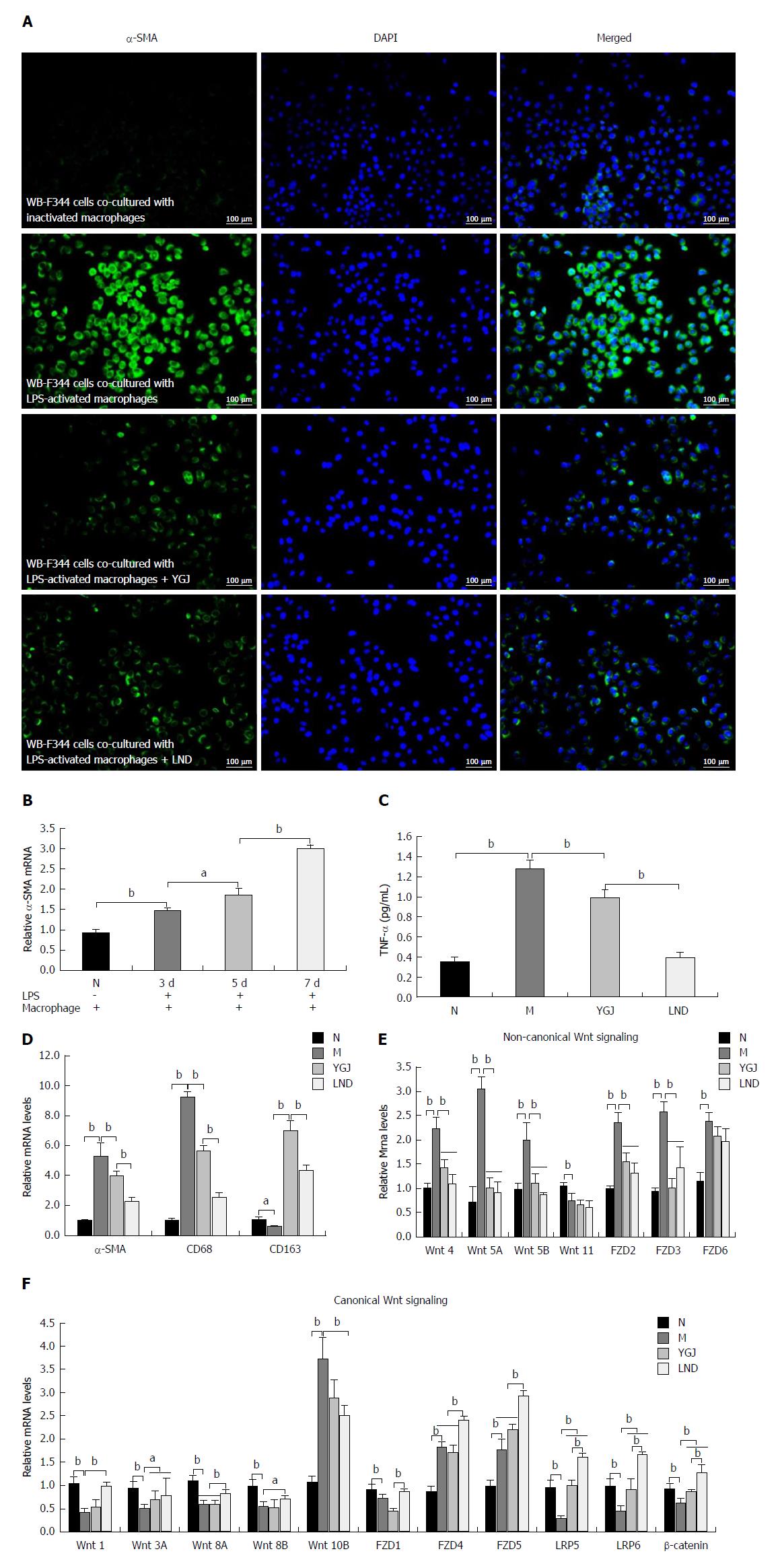
Thus, we co-cultured WB-F344 cells with RAW264.7 cells or LPS-activated (100 ng/mL) RAW264.7 cells in Transwell chambers. Immunofluorescence staining showed that the expression of α-SMA in WB-F344 cells was markedly increased after co-culture with the LPS-activated RAW264.7 cells (Figure 5A), and the mRNA expression of α-SMA in WB-F344 cells was steadily increased compared to the N group after 3, 5, and 7 d in culture (P < 0.05 or P < 0.01) (Figure 5B). These results indicate that activated KCs promote the differentiation of WB-F344 cells into myofibroblasts.
As shown in Figure 5C and D, when co-cultured WB-F344 cells with LPS-activated RAW264.7 cells were treated with YGJ or lenalidomide (LND, a TNF-alpha inhibitor, was used as a positive control drug) for 7 d, the mRNA levels of CD68 and α-SMA and the protein level of TNF-α were significantly higher (P < 0.01) and the mRNA level of CD163 was significantly lower (P < 0.01) in activated RAW264.7 cells than in the N group. The mRNA levels of CD68 and α-SMA and protein of TNF-α were significantly lower (P < 0.01) and the mRNA level of CD163 was significantly higher (P < 0.01) in the YGJ and LND groups than in the M group. These results suggest that YGJ regulates the activated state of macrophages (inhibition of pro-inflammatory macrophages and promotion of anti-inflammatory macrophages).
Furthermore, after the co-culture of WB-F344 cells with activated RAW264.7 cells, the mRNA levels of canonical Wnt signaling pathway components (Wnt-1, -3A, -8A, -8B, FZD1, LRP-5, -6, and β-catenin) in WB-F344 cells were significantly lower in the M group than in the N group (P < 0.01), and the mRNA levels of non-canonical Wnt signaling components (Wnt-4, -5A, -5B, and FZD-2, -3, -6) were significantly higher in the M group than in the N group (P < 0.01). The mRNA levels of Wnt 3A, LRP-5, -6, and β-catenin were markedly higher and the mRNA levels of Wnt-4, -5A, -5B, and FZD-2, -3 were significantly lower in the YGJ group and LND group than in the M group (P < 0.05 or P < 0.01) (Figure 5E and F). These results suggest that YGJ regulates the Wnt signaling pathway in FLSPCs through regulation of the KC activation state in vitro.
Liver transplantation is the best therapeutic option for patients with end-stage liver disease. However, donor organ scarcity is a major limitation and alternative strategies are urgently needed[25]. In recent years, stem cell transplantation has become a popular topic in the treatment of end-stage liver disease. Some studies have shown that bone marrow mesenchymal stromal cell (BM-MSC) transplantation can significantly reduce collagen deposition, inhibit the expression of TGF-β1 and α-SMA, reduce the degree of liver fibrosis, improve liver function, and reduce the mortality rate in rodent models of liver injury[26,27]. The latest report suggests that peripheral infusion of allogeneic BM-MSCs is safe and convenient for patients with hepatitis B virus (HBV)-related acute-on-chronic liver failure and significantly increases the 24-wk survival rate by improving liver function and decreasing the incidence of severe infections[28]. However, there are also reports that BM-MSCs transplanted into patients or mice with liver fibrosis differentiate into HSCs and myofibroblasts, which may increase the progression of liver fibrosis[29,30].
FLSPCs are considered more suitable for transplantation than BM-MSCs[31]. In the present study, we demonstrated that Dlk-1+ FLSPC transplantation inhibited inflammation and HSC activation, led to the improvement of liver function, and thereby prevented the development of cirrhosis. The combination of YGJ and FLSPC resulted in more effective repair of liver fibrosis. YGJ promoted the differentiation of Dlk-1+ FLSPCs into hepatocytes and accelerated wound healing after liver injury.
Macrophages play a crucial role in the regulation of HPC differentiation, in particular, dependent on the specific Wnt ligands expressed by macrophages[8]. A recent study demonstrated that cytokines including IL-6, IL-10, TNF-α, HGF, and TGF-β1 were significantly reduced in the liver when KCs were selectively depleted with liposome-encapsulated dichloromethylene-diphosphonate (Cl2MDP) after partial hepatectomy, which resulted in delayed liver regeneration[32]. Another study has shown that KC-derived Nogo-B promotes M1 polarization and exacerbates the progression of alcoholic liver disease (ALD), and Nogo-B depletion promotes M2 polarization and restores ALD[33]. The pro-inflammatory macrophages that are prevalent in pediatric NASH can be reprogrammed to an anti-inflammatory subset by docosahexaenoic acid (DHA) treatment. Wnt 3A was up-regulated in macrophages and β-catenin was activated in HPCs, resulting in the differentiation of HPCs into hepatocytes[17]. These studies demonstrate that macrophages are a critical regulator in maintaining liver homeostasis and the wound healing response. M1 macrophages that polarize to M2 result in the increased production of Wnt ligands that bind to receptors on the surface of HPCs, leading to the activation of Wnt/β-catenin signaling and differentiation to hepatocytes[34]. This suggests that the cross-talk between macrophages and HPCs regulates liver homeostasis of Yin and Yang in the liver.
In this study, we found that the levels of TNF-α and CD68 were increased, while the expression of CD163 was decreased in rat cirrhosis induced with 2-AAF/CCl4. However, the levels of expression of TNF-α and CD68 were significantly lower and CD163 expression higher after FLSPC transplantation. Moreover, this effect was further enhanced when FLSPCs were combined with YGJ administration, indicating that YGJ improved the therapeutic effect of FLSPCs, possibly through regulation of macrophage activation status.
In vitro, we found that when LPS-activated RAW264.7 cells were co-cultured with WB-F344 cells, the expression of α-SMA in WB-F344 cells was significantly higher, and it was significantly lower after administration of YGJ and LND. The expression of CD68 and TNF-α was significantly lower and that of CD163 was significantly higher in LPS-activated RAW264.7 cells after YGJ and LND treatment. These results demonstrate that LPS-activated macrophages promote HPC differentiation into myofibroblasts, which may be related to TNF-α secretion by activated macrophages. YGJ might inhibit the activation of pro-inflammatory macrophages and promote the activation of anti-inflammatory macrophages.
Wnt signaling consists of two major pathways, the β-catenin-dependent cascade, known as canonical Wnt signaling, and the β-catenin-independent cascade, known as non-canonical Wnt signaling. Recent studies have shown that canonical Wnt signaling regulates the differentiation of HPCs into hepatocytes. The activation of canonical Wnt signaling is associated with Wnt 3A, which is secreted by macrophages upon engulfing hepatocyte debris[35]. However, macrophage-derived TNF-α prompted HPCs to differentiate into myofibroblasts, mainly due to the activation of the Wnt5/FZD2 non-canonical cascade[8]. These results indicate that macrophages regulate the differentiation and proliferation of HPCs. The main mechanism underlying this process may be related to regulating the activation of the Wnt signaling pathway.
In the present study, in vivo, we found canonical Wnt signaling to be inhibited and the non-canonical Wnt pathway activated in rat cirrhosis induced with 2-AAF/CCl4. However, canonical Wnt signaling was restored and non-canonical signaling suppressed after FLSPC transplantation. This regulatory effect was further enhanced when FLSPC transplantation was combined with YGJ administration. FLSPC + YGJ showed significant enhancement of the Wnt 3A level and markedly decreased Wnt 5A level relative to the single FLSPC treatment group. This suggests that YGJ enhanced the therapeutic effect of FLSPCs, which may be associated with regulation of the Wnt signaling pathway.
Consistent with the results observed in vivo, canonical Wnt signaling in WB-F344 cells was significantly inhibited and non-canonical Wnt signaling was activated after co-culture with LPS-activated RAW264.7 cells in vitro. However, canonical Wnt signaling was activated and non-canonical Wnt signaling was suppressed after YGJ treatment, indicating that pro-inflammatory macrophages inhibited canonical Wnt signaling and activated the non-canonical cascade in HPCs. In this regard, YGJ was also shown to regulate Wnt signaling.
Overall, the proportion of pro-inflammatory and anti-inflammatory macrophage subsets determines the differentiation of FLSPCs. YGJ appears to enhance the therapeutic effect of FLSPCs in a rat model of liver cirrhosis, likely by regulating macrophage activation state and then regulating Wnt signaling. This results in controlling the differentiation of FLSPCs. These results suggest that the combination of YGJ with stem cell transplantation may be a more effective strategy for the treatment of end-stage liver cirrhosis.
Liver cirrhosis has emerged as a major contributor to the global health burden. Liver transplantation, a recognized treatment for end-stage liver cirrhosis, is limited by a shortage of organs, transplant rejection, high cost, and other problems. Stem cell transplantation is expected to replace liver transplantation, but finding a way to regulate its differentiation in vivo is a key scientific problem. Previous studies have confirmed that Yiguanjian decoction (YGJ) has a strong ability to prevent fibrosis and to promote hepatocyte regeneration, but whether YGJ can affect the differentiation of transplanted liver stem cells is still unclear.
To determine whether YGJ can affect the therapeutic effect of transplanted fetal liver stem/progenitor cells (FLSPCs) and identify possible mechanisms by which it may do so to further identify the active ingredients of YGJ and thus improve the treatment of liver cirrhosis.
To determine whether YGJ can improve the therapeutic efficacy of stem cells for liver cirrhosis, and so provide scientific evidence for YGJ combined with stem cell transplantation for liver cirrhosis.
Combination of stem cell transplantation with traditional Chinese medicine (TCM) may become a new method for the treatment of end-stage liver cirrhosis.
We here found that YGJ can enhance FLSPC-mediated repair of liver cirrhosis, and the key mechanism is related to regulation of macrophage activation state. This provides empirical evidence for the treatment of cirrhosis with YGJ. However, the molecular mechanism by which YGJ regulates macrophage activation is still unclear. This is the main question to be answered in the future.
YGJ enhances FLSPC-mediated repair of cirrhosis through regulation of macrophage activation state, and stem cell transplantation in combination with YGJ may be a suitable treatment for end-stage liver cirrhosis.
TCM has thousands of years of history and its practitioners have accumulated rich experience in the field of chronic liver disease. If TCM is combined with modern medicine, it may provide a more effective treatment for patients with chronic liver disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lv XP, Muriel P S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Huang Y
| 1. | Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Perera MT, Mirza DF, Elias E. Liver transplantation: Issues for the next 20 years. J Gastroenterol Hepatol. 2009;24 Suppl 3:S124-S131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA. Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am J Pathol. 2000;156:2017-2031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 142] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964-1978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973-1987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 447] [Cited by in F6Publishing: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159-2172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Lade AG, Monga SP. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow? Dev Dyn. 2011;240:486-500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Jiang F, Parsons CJ, Stefanovic B. Gene expression profile of quiescent and activated rat hepatic stellate cells implicates Wnt signaling pathway in activation. J Hepatol. 2006;45:401-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Chen Q, Xue Y, Sun J. Kupffer cell-mediated hepatic injury induced by silica nanoparticles in vitro and in vivo. Int J Nanomedicine. 2013;8:1129-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | You Q, Holt M, Yin H, Li G, Hu CJ, Ju C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem Pharmacol. 2013;86:836-843. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 11. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4403] [Cited by in F6Publishing: 4408] [Article Influence: 209.9] [Reference Citation Analysis (0)] |
| 12. | Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101-106. [PubMed] [Cited in This Article: ] |
| 14. | Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1361] [Cited by in F6Publishing: 1435] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 15. | Moestrup SK, Møller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347-354. [PubMed] [Cited in This Article: ] |
| 16. | Labonte AC, Sung SJ, Jennelle LT, Dandekar AP, Hahn YS. Expression of scavenger receptor-AI promotes alternative activation of murine macrophages to limit hepatic inflammation and fibrosis. Hepatology. 2017;65:32-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Carpino G, Nobili V, Renzi A, De Stefanis C, Stronati L, Franchitto A, Alisi A, Onori P, De Vito R, Alpini G. Macrophage Activation in Pediatric Nonalcoholic Fatty Liver Disease (NAFLD) Correlates with Hepatic Progenitor Cell Response via Wnt3a Pathway. PLoS One. 2016;11:e0157246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Mu Y, Liu P, Du G, Du J, Wang G, Long A, Wang L, Li F. Action mechanism of Yi Guan Jian Decoction on CCl4 induced cirrhosis in rats. J Ethnopharmacol. 2009;121:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Zhou YN, Mu YP, Fu WW, Ning BB, Du GL, Chen JM, Sun MY, Zhang H, Hu YY, Liu CH. Yiguanjian decoction and its ingredients inhibit angiogenesis in carbon tetrachloride-induced cirrhosis mice. BMC Complement Altern Med. 2015;15:342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wang XL, Jia DW, Liu HY, Yan XF, Ye TJ, Hu XD, Li BQ, Chen YL, Liu P. Effect of Yiguanjian decoction on cell differentiation and proliferation in CCl4-treated mice. World J Gastroenterol. 2012;18:3235-3249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 21. | Jensen CH, Jauho EI, Santoni-Rugiu E, Holmskov U, Teisner B, Tygstrup N, Bisgaard HC. Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am J Pathol. 2004;164:1347-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, Chen G, Liu C, Zern MA. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96:350-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984;154:38-52. [PubMed] [Cited in This Article: ] |
| 24. | Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 25. | Yovchev MI, Oertel M. Fetal Liver Stem/Progenitor Cell Transplantation: A Model to Study Tissue Mass Replacement and Cell-Based Therapies. Methods Mol Biol. 2017;1506:101-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Roderfeld M, Rath T, Voswinckel R, Dierkes C, Dietrich H, Zahner D, Graf J, Roeb E. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4-/- mice. Hepatology. 2010;51:267-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 28. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 29. | di Bonzo LV, Ferrero I, Cravanzola C, Mareschi K, Rustichell D, Novo E, Sanavio F, Cannito S, Zamara E, Bertero M. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut. 2008;57:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in F6Publishing: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Carvalho AB, Quintanilha LF, Dias JV, Paredes BD, Mannheimer EG, Carvalho FG, Asensi KD, Gutfilen B, Fonseca LM, Resende CM. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Oertel M, Menthena A, Chen YQ, Teisner B, Jensen CH, Shafritz DA. Purification of fetal liver stem/progenitor cells containing all the repopulation potential for normal adult rat liver. Gastroenterology. 2008;134:823-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambalgen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66-77. [PubMed] [Cited in This Article: ] |
| 33. | Park JK, Shao M, Kim MY, Baik SK, Cho MY, Utsumi T, Satoh A, Ouyang X, Chung C, Iwakiri Y. An endoplasmic reticulum protein, Nogo-B, facilitates alcoholic liver disease through regulation of kupffer cell polarization. Hepatology. 2017;65:1720-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253-2263. [PubMed] [Cited in This Article: ] |
| 35. | Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 577] [Cited by in F6Publishing: 559] [Article Influence: 46.6] [Reference Citation Analysis (0)] |