Published online Nov 14, 2018. doi: 10.3748/wjg.v24.i42.4750
Peer-review started: August 27, 2018
First decision: October 9, 2018
Revised: October 13, 2018
Accepted: October 21, 2018
Article in press: October 21, 2018
Published online: November 14, 2018
Gut microbiota are involved in the development or prevention of various diseases such as type 2 diabetes, fatty liver, and malignancy such as colorectal cancer, breast cancer and hepatocellular carcinoma. Alzheimer’s disease, osteoporosis, sarcopenia, atherosclerotic stroke and cardiovascular disease are major diseases associated with decreased activities of daily living (ADL), especially in elderly people. Recent analyses have revealed the importance of gut microbiota in the control of these diseases. The composition or diversity of these microbiota is different between patients with these conditions and healthy controls, and administration of probiotics or prebiotics has been shown effective in the treatment of these diseases. Gut microbiota may affect distant organs through mechanisms that include regulating the absorption of nutrients and/or the production of microbial metabolites, regulating and interacting with the systemic immune system, and translocating bacteria/bacterial products through disrupted mucosal barriers. Thus, the gut microbiota may be important regulators in the development of diseases that affect ADL. Although adequate exercise and proper diet are important for preventing these diseases, their combination with interventions that manipulate the composition and/or diversity of gut microbiota could be a promising strategy for maintaining health condition and preserving ADL. This review thus summarizes current understanding of the role of gut microbiota in the development or prevention of diseases closely associated with the maintenance of ADL.
Core tip: Gut microbiota are involved in the development or prevention of various diseases. The composition of gut microbiota is altered by conditions such as type 2 diabetes, fatty liver, and malignancy such as colorectal cancer, breast cancer and hepatocellular carcinoma. Moreover, gut microbiota have been associated with Alzheimer’s disease, osteoporosis, sarcopenia, atherosclerotic stroke and cardiovascular disease. All of these diseases are major causes of decreased activities of daily living (ADL). This review summarizes current understanding of the role of gut microbiota in the development or prevention of diseases closely associated with the maintenance of ADL.
- Citation: Shimizu Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J Gastroenterol 2018; 24(42): 4750-4758
- URL: https://www.wjgnet.com/1007-9327/full/v24/i42/4750.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i42.4750
More than > 1014 microorganisms live in the human gut, with most residing in the ileum and colon. Firmicutes and Bacteroidetes are the major phyla, followed by Proteobacteria, Actinobacteria, and Faecalibacteria[1]. The functions and role of these bacteria in maintaining homeostasis of energy, metabolism, and immunity have been extensively analyzed. Moreover, dysbiosis in the gut has been shown to be associated with various diseases, including obesity, type 2 diabetes, fatty liver, autism, inflammatory bowel diseases and malignancies such as colorectal cancer, breast cancer and hepatocellular carcinoma[1]. The repertoire of gut microbiota is thought to be formed within one to three years after birth, but may be altered by foods, stress, treatment with antibiotics and aging.
Several aging-related diseases are causes of decreased activities of daily living (ADL). These diseases, including Alzheimer’s disease (AD), osteoporosis, sarcopenia, atherosclerotic stroke and cardiovascular disease, are major causes of being bedridden in Japan[2]. Because the population of elderly people has been rapidly increasing, methods are urgently needed to prevent and treat these disorders.
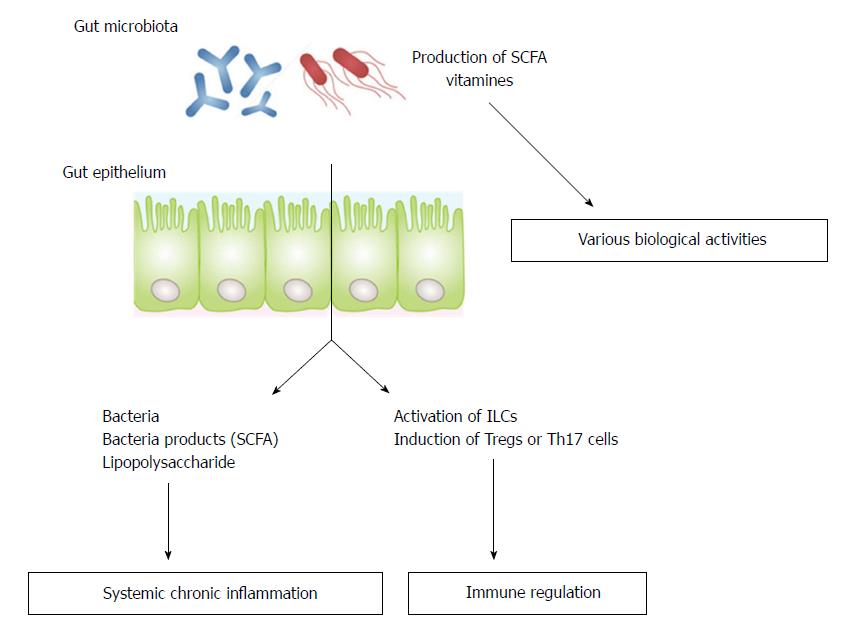
Gut microbiota have been shown to interact with the nervous[3-7], musculoskeletal[8-11] and cardiovascular[12] systems, suggesting that manipulation of gut microbiota may be a key strategy for the prevention or treatment of disorders associated with reduced ADL. There are three possible mechanisms by which gut microbiota can affect distant organs. The first is by regulating the absorption of nutrients from food or the production of microbial metabolites such as short-chain fatty acids (SCFA) or vitamins; the second is by regulating of the systemic immune system; and the third is by translocating bacteria/bacterial products through disrupted mucosal barriers (Figure 1)[8]. This review summarizes current knowledge of the role of gut microbiota in the regulation of the development of AD, osteoporosis, sarcopenia, and cerebro- and cardiovascular diseases.
AD is a neurodegenerative disease and the major cause of cognitive decline in elderly people. Pathologically, AD is thought to result from amyloid beta (Aβ) deposits in the brain. However, neuroinflammatory reactions caused by Aβ deposits, lipopolysaccharide (LPS), bacterial products, or neuroactive molecules may be more important for the development of AD than A deposits itself[13]. A microbiota-gut-brain axis has been identified. This axis allows gut microbiota to affect the physiology and pathology of the brain. Intestinal mucosal barriers are occasionally disrupted by pathogenetic bacteria and their products such as LPS, leading to an increase in the permeability of the blood-brain barrier. Thus, bacteria and/or bacterial products from the gut could cause chronic inflammatory responses in the brain, with these responses, in turn, inducing the formation of neurodegenerative lesions of the brain, such as those observed in AD[14]. Signaling pathways through the gut-brain axis may also participate in AD[15]. Moreover, the immune system may be an important regulator of gut-brain interactions. Gut microbes have been found to influence the maturation and function of microglia, the most abundant type of immune cells residing in the brain[16]. Gut microbes have also been shown to modulate activity of astrocytes, which, although they are not brain-resident immune cells, possess immune-related functions such antigen presentation or cytokine production[17]. Moreover, microbes modulate the activation and maturation of peripheral immune cells. All of these activities of gut microbiota may be associated with neuroinflammation, brain injury, and autoimmunity[16]. Conversely, the brain could regulate the gut through neurologic, immunologic, and hormonal messages[4].
Germ-free (GF) mice have provided important information on the role of the gut microbiota in the homeostasis of brain function. GF mice have been reported to show anxiety-like behavior, which may be due to upregulated expression of postsynaptic density protein 95[18]. GF mice were also shown to be deficit in spatial or working memory[19]. These data suggest that gut microbiota play a pivotal role in healthy functioning of the brain. However, gut microbiota have also been found to play a negative role. For example, experiments in a mouse model of AD found that Aβ deposition in the brain was lower in GF-Aβ precursor protein APP transgenic mice than in conventionally-raised transgenic APPPS1 mice. In addition, colonization of GF-APPPS1 mice with microbiota showed increased levels of Aβ deposit in the brain, indicating that gut microbiota are involved in Aβ deposits in the brain[20]. These seemingly inconsistent results suggest that the composition and/or balance of gut flora may determine the effect of gut microbiota on brain physiology and pathology.
Gut microbiota produce several substances, including SCFA, serotonin, and LPS, which act as important mediators not only of physiological functions but also for pathogenetic agents. Major SCFAs are butyrate, acetate and propionate, with each molecule having specific functions in the gut and/or brain. Butylate was shown to have protective properties in the brain[21], and administration of sodium butylate to a mouse model of AD improved learning and memory function[22]. Recently, four cultivable butylate-producing bacteria were isolated from fecal samples from Japanese AD patients, all of which are considered to be unique operational taxonomic units. The analysis for biochemical mechanism of butylate production from those bacteria may contribute the novel approach to stimulate the butylate production in the gut, possibly leading to the improvement of the memory function in patients with AD[23].
Gut microbiota have been shown to produce more than 90% of whole body serotonin, an important metabolite that regulates cognitive ability. Moreover, exogenous serotonin was shown effective in reducing Aβ deposits in the brain[7]. GF mice show decreased levels of serum serotonin, indicating that the gut microbiota is major sources of serum serotonin. To date, however, there has been no direct evidence showing that serotonin produced in the gut passes the blood-brain barrier in physiological conditions. Thus, the actual role of gut-derived serotonin in brain function remains unclear. Other gut microbiota metabolites associated with AD include mannitol, succinic acid and 3,4-dihydroxybenzeneacetic acid, all of which are related to AD cognitive decline or susceptibility to AD[5].
No specific gut microbe has been associated to date with AD development. Rather, a recent analysis revealed that reduced diversity of microbes is associated with AD[6]. A similar negative correlation between gut microbiota diversity and susceptibility to AD development was observed in a comparative analysis of gut microbes in subjects from developed and developing countries. Microbial diversity is lower, and AD prevalence higher, in developed than in developing countries[24].
The contribution of gut microbiota to cognitive function was indirectly suggested by the effect of probiotics on AD. Probiotics are microorganisms that maintain or restore beneficial bacteria and maintain a balance between “good” and “bad” bacteria in the gut. Probiotics containing Lactobacillus helvetics R0052 and Bifidobacterium longum R0175 were found to alleviate cognitive behavioral responses to external stimuli in healthy volunteers[25]. Recently, administration of Bifidobacterium breve strain A1 to AD model mice was shown to reverse the cognitive dysfunction[26]. These findings indicate that changes in the gut microbiota could sufficiently improve cognitive function, and suggest that probiotics may have possible therapeutic potential in patients with AD.
Osteoporosis is a bone disease characterized by reductions in bone density and quality, and is a major cause of vertebral compression and femoral bone fractures. These fractures often reduce patient ADL or quality of life and may result in patients being bed-ridden. The pathogenesis of the disease is complex, but common processes include elevated bone reabsorption, decreased bone formation, and increased inflammatory reactions in bone.
Links between gut microbiota and bone have been elucidated mostly from studies in mice. Compared with normal mice, GM mice showed increased trabecular bone volume/tissue volume, with these parameters becoming normalized after colonization[9]. The numbers of CD4+ T cells and osteoclast precursor cells (CD11b+/Gr1-), and the expression of mRNA encoding the osteolytic cytokine TNF-α are reduced in bone marrow of GM mice, with all of these factors leading to reduced osteoclastogenesis[9].
Gut microbiota were recently shown to increase serum concentrations of insulin-like growth factor-1 (IGF-1), which promotes bone formation and remodeling[27]. Depletion of microbiota by the administration of broad-spectrum antibiotics reduced serum IGF-1 levels, a reduction reversed by SCFA supplementation, suggesting that SCFA production by microbiota through the fermentation of dietary fiber may induce expression of IGF-1. These reports indicate that gut microbiota regulate bone metabolism, although their effects may differ according to genetic background, age, and/or sex. The role of gut microbiota in bone metabolism can also be analyzed by assessing the effects of probiotics or prebiotics on bone density. In mouse models, the effects of gut microbiota have been analyzed in male and ovariectomized female mice, the latter being a model of a postmenopausal, estrogen-deficient state. Probiotics such as Lactobacillus strains have been shown to increase bone mass along with changes in gut microbiota[28-32]. For example, Lactobacillus ruteri administered to ovariectomized mice was shown to protect these mice from bone loss, possibly by reducing expression of Trap 5 and receptor reactivator of NF-κB ligand, which are markers of osteoclast activation and bone resorption, leading to a reduction in osteoclastogenesis. Oral administration of Bifidobacterium longum for 16 wk to ovariextomized rats was found to upregulate bone formation, as shown by increases in serum osteocalcin concentrations and osteoblasts, and to downregulate bone absorption, as shown by decreased serum C-terminal telopeptide concentrations and osteoclasts, leading to an increase in bone mass density[33]. These effects were mediated by upregulation of the Bem-2 and Sparc genes, the former being a key gene for osteoblast differentiation and the latter being a gene involved in bone calcification.
In addition, several reports have described the effects of prebiotics on bone density. Prebiotics are non-digestible food ingredients that promote the growth of beneficial microorganisms in the gut. Major prebiotics include non-digestible oligosaccharides, such as fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), xylo-oligosaccharides, and inulin, with FOS and GOS shown to increase bifidobacteria in the intestine[34,35]. Administration of GOS or inulin to healthy male rats was shown to increase calcium absorption from the intestine, leading to an increase in bone mineral density[36,37]. FOS and inulin were also shown to enhance intestinal calcium absorption in gastrectomized male rats[38,39] and ovariectomized female rats[40]. FOS and GOS were shown to increase bone strength by enhancing calcium absorption[34,35,41]. The main mechanism by which prebiotics increase calcium absorption from the intestine may involve the production of SCFA by microbiota through fermentation of the prebiotics, with SCFA shown to directly stimulate calcium absorption by gut epithelium[35]. Collectively, supplementation with probiotics or prebiotics may be a potential therapeutic intervention for the prevention or treatment of osteoporosis in humans. However, there is limited evidence showing that prebiotics are effective in patients with postmenopausal or senile osteoporosis, although one study reported that supplementation with calcium and FOS had beneficial therapeutic effects on women with postmenopausal osteoporosis[42].
Sarcopenia is a condition in which muscle mass reduction is accompanied by reduced muscle performance. Causes of muscle weakness include aging, lack of physical exercise, malnutrition, and complications of malignancy. Mitochondrial dysfunction, accompanied by down-regulation of mitochondrial gene expression, has been observed in muscles of pre-frail elderly individuals[43], suggesting that activation of mitochondrial biogenesis may be important in preventing sarcopenia in frail elderly individuals.
Recent evidence has shown that gut microbiota influence skeletal muscle. Microbial metabolites from the intestines have been shown to act as nutrients or metabolic modulators in muscles. These metabolites include folate, vitamin B12, and tryptophan; bacteria involved in the production of each nutrient or metabolite have been identified[12]. Possible effects are thought to include biosynthesis of amino acids, DNA synthesis or methylation, prevention of oxidative stress or endothelial damage, and stimulation of anabolism or cell proliferation via IGF-1 synthesis[44-47]. Other metabolites include SCFAs, which target muscle mitochondria, leading to the promotion of mitochondrial biogenesis through binding to fatty acid receptors 2 and 3 in skeletal muscle cells[48,49]. Among the SCFAs, butylate has been shown to have the most prominent effect on skeletal muscles. Butylate may help prevent muscle loss and maintain muscle mass through anti-inflammatory effects and activation of regulatory pathways, resulting in increased ATP production and suppression of muscle protein catabolism and apoptosis[50,51]. Another microbial metabolite, urolithin A, was recently shown to preserve the biogenesis of skeletal muscle cell mitochondria and to improve exercise capacity in mice and rats[52]. Moreover, a randomized, double-blind, placebo-controlled trial demonstrated that oral intake of urolithin A modulated muscle and mitochondrial biomarkers[12].
The composition of gut microbiota characteristic of sarcopenic patients has not been determined. However, the effects of probiotics on sarcopenia have been analyzed. Supplementation with Lactobacillus species was found to prevent muscle mass reduction in mice with acute leukemia[53]. In humans, treatment of elderly patients with prebiotics containing FOS and inulin for 13 wk improved muscle function, as shown by decreases in exhaustion and improved handgrip strength[54]. Taken together, these findings indicate that muscle mass and functions are closely associated with the composition of gut microbiota.
Atherosclerosis is a cause of cardio- and cerebrovascular diseases, which are often lethal or linked to serious conditions leading to decreased quality of life. The composition of gut microbiota in patients with atherosclerotic diseases has been reported altered. For example, an analysis of gut microbiota in patients with symptomatic atherosclerosis and healthy controls showed that Collinsella was reduced in the patients, while Roseburia and Eubacterium were enriched in healthy persons[55]. In contrast, an analysis of gut microbiota in patients with atherosclerotic cardiovascular disease and healthy subjects found that Enterobacteriaceae and Streptococcus spp. were enriched in the patients, with the latter being oral bacteria shown to positively correlate with diastolic and systolic blood pressure[56]. Another report from Japan revealed that bacteria of the order Lactobacillales, such as Lactobacillus, Streptococcus, and Enterococcus, were significantly increased, while the phylum Bacteroidetes (Bacteroides and Prevotella) was significantly decreased, in patients with cardiovascular disease compared with healthy controls. Patients with atherosclerotic stroke or transient ischemic attack showed dysbiosis of gut microbiota, with an increase in opportunistic bacteria such as Enterobacter, Megasphaera, Oscillibacter and Desulfovibrio and a decrease in commensal bacteria such as Bacteroides, Prevotella and Fecalibacterium, with these changes correlating with disease severity[57]. Transplantation of gut microbiota in mice was found to result in the transmission of susceptibility to atherosclerosis, directly demonstrating the participation of gut microbiota in atherosclerosis[58].
Several mechanisms have been proposed by which gut microbiota regulate the development of atherosclerotic disease. For example, microbiota may enhance lipid metabolism. GM mice showed decreased lipolysis[59] and Bifidobacteria was shown to reduce cholesterol levels[60]. Another mechanism is associated with the ability of healthy gut microbiota to maintain gut permeability by reinforcing tight junctions of intestinal epithelium. Gut dysbiosis disrupts these tight junctions, increasing intestinal permeability and increasing the absorption of endotoxin, especially LPS, from the gut. These endotoxins can then enter the systemic circulation, causing chronic systemic inflammation, including inflammation of the cardiovascular system.
In addition to gut microbiota playing a protective role in the development of cardiovascular disease, gut microbiota may also play a promotional role in the development of this disease. Gut microbiota produce trimethylamine (TMA) from dietary choline and L-carnitine, with TMA subsequently oxidized in the liver, forming trimethylamine-N-oxides (TMAOs). TMAOs have been shown to induce macrophage foam cell formation and plaque formation in the aorta and coronary arteries[61], with high levels of TMAO in patients showing a positive correlation with the incidence of death from cardiovascular disease or myocardial infarction[62].
Hypertension is a major risk factor for atherosclerosis and has been associated with gut dysbiosis in animal models[12]. Administration of a combination of antibiotics has been shown to lower blood pressure in treatment-resistant patients with hypertension[63]. Moreover, hypertension, often observed in obese pregnant women, was shown to be associated with alterations in the composition of gut microbiota, with an abundance of the butyrate-producing genus Odoribacter associated with lower blood pressure[64]. These findings indicate that gut microbiota regulate blood pressure in humans, perhaps mediated by an interaction between SCFA and G-protein-coupled receptors, including Gpr41 and Olfr78[65].
A recent report using a mouse model of experimental transient cerebral ischemia induced by occlusion of middle cerebral artery showed that the composition of microbiota in young mice changed to that similar to uninjured aged mice after stroke, and transplantation of fecal gavage from young mice into those mice demonstrated beneficial effect on the outcome of stroke with less infarct size and less mortality. One possible mechanism of the effect could be due to increased SCFA production from youthful microbiota, which was accompanied by lower inflammatory cytokine levels in the plasma. The report clearly indicate that gut microbiota could modify the outcome of stroke, and intervention with youthful microbiota may have a therapeutic potential for the disease[66].
Probiotics have also been reported to affect blood pressure. Administration of Lactobacillus plantarum was shown to reduce the severity of myocardial infarction in a rat model[67], an effect thought to be associated with intestinal microbial metabolites[68]. In addition, administration of Lactobacillus rhamnosus GR-1 induced significant attenuation of left ventricular hypertrophy and improved systolic and diastolic left ventricular function in a rat experimental model of myocardial infarction[69]. Collectively, these data suggest a therapeutic strategy with modulation of gut microbiota can be used to prevent or treat cerebro- and cardiovascular diseases caused by atherosclerosis.
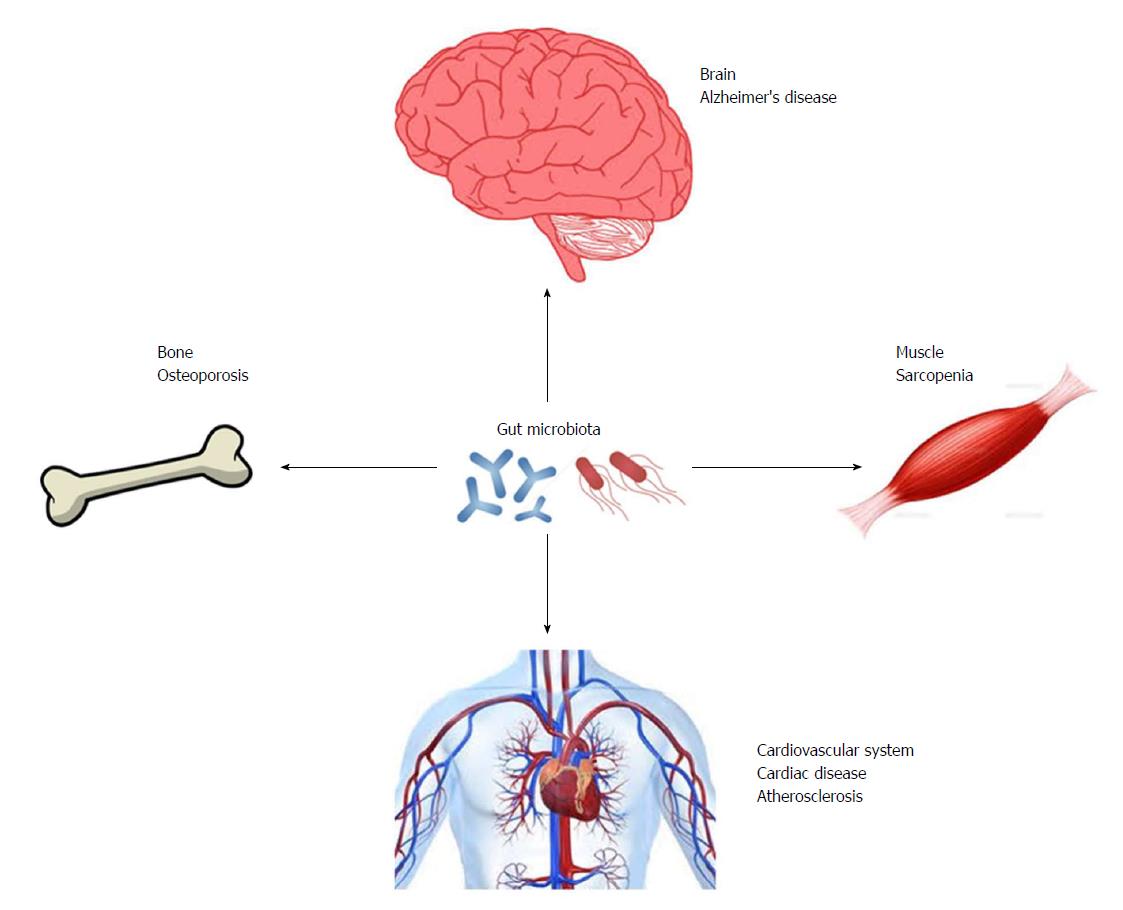
Gut microbiota may be important regulators in the development of diseases that affect ADL (Figure 2). Although, adequate exercise and a proper diet are important for the prevention of these diseases, the combination of these lifestyle interventions with methods that manipulate the composition and/or diversity of gut microbiota may be a promising strategy for maintenance of healthy conditions with preserved ADL.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Das U, Yu JS S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21:1691-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 110] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 2. | Yoshino H, Sakurai T, Hasegawa K, Yokono K. Causes of decreased activity of daily life in elderly patients who need daily living care. Geriatr Gerontol Int. 2011;11:297-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol Neurobiol. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 317] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 4. | Choi J, Hur TY, Hong Y. Influence of Altered Gut Microbiota Composition on Aging and Aging-Related Diseases. J Lifestyle Med. 2018;8:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Xu R, Wang Q. Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol. 2016;10 Suppl 3:63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 6. | Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7:13537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 801] [Cited by in F6Publishing: 1058] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 7. | Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016;59:1006-1023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 8. | Hernandez CJ, Guss JD, Luna M, Goldring SR. Links Between the Microbiome and Bone. J Bone Miner Res. 2016;31:1638-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 476] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 10. | Steves CJ, Bird S, Williams FM, Spector TD. The Microbiome and Musculoskeletal Conditions of Aging: A Review of Evidence for Impact and Potential Therapeutics. J Bone Miner Res. 2016;31:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Ticinesi A, Lauretani F, Milani C, Nouvenne A, Tana C, Del Rio D, Maggio M, Ventura M, Meschi T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut-Muscle Axis? Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 12. | Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res. 2017;120:1183-1196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 757] [Cited by in F6Publishing: 910] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 13. | Bronzuoli MR, Iacomino A, Steardo L, Scuderi C. Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res. 2016;9:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Feng Q, Chen WD, Wang YD. Gut Microbiota: An Integral Moderator in Health and Disease. Front Microbiol. 2018;9:151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 15. | Köhler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt KL, Carvalho AF. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr Pharm Des. 2016;22:6152-6166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 16. | Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 1062] [Article Influence: 151.7] [Reference Citation Analysis (0)] |
| 17. | Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 939] [Cited by in F6Publishing: 919] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 18. | Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1983] [Cited by in F6Publishing: 2060] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 19. | Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 611] [Cited by in F6Publishing: 599] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 20. | Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7:41802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 373] [Cited by in F6Publishing: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 21. | Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 22. | Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 2011;26:187-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 246] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Nguyen TTT, Fujimura Y, Mimura I, Fujii Y, Nguyen NL, Arakawa K, Morita H. Cultivable butyrate-producing bacteria of elderly Japanese diagnosed with Alzheimer’s disease. J Microbiol. 2018;56:760-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Fox M, Knapp LA, Andrews PW, Fincher CL. Hygiene and the world distribution of Alzheimer’s disease: Epidemiological evidence for a relationship between microbial environment and age-adjusted disease burden. Evol Med Public Health. 2013;2013:173-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Messaoudi M, Violle N, Bisson JF, Desor D, Javelot H, Rougeot C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes. 2011;2:256-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 363] [Cited by in F6Publishing: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 26. | Kobayashi Y, Sugahara H, Shimada K, Mitsuyama E, Kuhara T, Yasuoka A, Kondo T, Abe K, Xiao JZ. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep. 2017;7:13510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 27. | Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113:E7554-E7563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 377] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 28. | McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013;228:1793-1798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 29. | Chiang SS, Pan TM. Antiosteoporotic effects of Lactobacillus -fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011;59:7734-7742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Rodrigues FC, Castro AS, Rodrigues VC, Fernandes SA, Fontes EA, de Oliveira TT, Martino HS, de Luces Fortes Ferreira CL. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food. 2012;15:664-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, Parameswaran N, McCabe LR. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014;229:1822-1830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 32. | Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, Movérare-Skrtic S, Islander U, Sjögren K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014;9:e92368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 33. | Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, Ahmad Z, Ibrahim Z, Jamaluddin R. Probiotics (Bifidobacterium longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed Res Int. 2015;2015:897639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 34. | Weaver CM, Martin BR, Nakatsu CH, Armstrong AP, Clavijo A, McCabe LD, McCabe GP, Duignan S, Schoterman MH, van den Heuvel EG. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J Agric Food Chem. 2011;59:6501-6510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 35. | Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13:125-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Chonan O, Watanuki M. Effect of galactooligosaccharides on calcium absorption in rats. J Nutr Sci Vitaminol (Tokyo). 1995;41:95-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Roberfroid MB, Cumps J, Devogelaer JP. Dietary chicory inulin increases whole-body bone mineral density in growing male rats. J Nutr. 2002;132:3599-3602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Ohta A, Motohashi Y, Sakai K, Hirayama M, Adachi T, Sakuma K. Dietary fructooligosaccharides increase calcium absorption and levels of mucosal calbindin-D9k in the large intestine of gastrectomized rats. Scand J Gastroenterol. 1998;33:1062-1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 39. | Shiga K, Nishimukai M, Tomita F, Hara H. Ingestion of difructose anhydride III, a non-digestible disaccharide, improves postgastrectomy osteopenia in rats. Scand J Gastroenterol. 2006;41:1165-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Zafar TA, Weaver CM, Zhao Y, Martin BR, Wastney ME. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr. 2004;134:399-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Mathey J, Puel C, Kati-Coulibaly S, Bennetau-Pelissero C, Davicco MJ, Lebecque P, Horcajada MN, Coxam V. Fructooligosaccharides maximize bone-sparing effects of soy isoflavone-enriched diet in the ovariectomized rat. Calcif Tissue Int. 2004;75:169-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Slevin MM, Allsopp PJ, Magee PJ, Bonham MP, Naughton VR, Strain JJ, Duffy ME, Wallace JM, Mc Sorley EM. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J Nutr. 2014;144:297-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, Sgarioto N, Zovilé I, Barbat-Artigas S, Reynaud O, Alkaterji F, Lemieux FC, Grenon A. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle. 2017;8:213-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 790] [Cited by in F6Publishing: 823] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 45. | Kuo HK, Liao KC, Leveille SG, Bean JF, Yen CJ, Chen JH, Yu YH, Tai TY. Relationship of homocysteine levels to quadriceps strength, gait speed, and late-life disability in older adults. J Gerontol A Biol Sci Med Sci. 2007;62:434-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. 2017;49:2083-2090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 47. | Dukes A, Davis C, El Refaey M, Upadhyay S, Mork S, Arounleut P, Johnson MH, Hill WD, Isales CM, Hamrick MW. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition. 2015;31:1018-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol. 2013;305:G900-G910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 346] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 49. | den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325-2340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2408] [Cited by in F6Publishing: 2684] [Article Influence: 244.0] [Reference Citation Analysis (2)] |
| 50. | Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, Van Remmen H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14:957-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 51. | den Besten G, Gerding A, van Dijk TH, Ciapaite J, Bleeker A, van Eunen K, Havinga R, Groen AK, Reijngoud DJ, Bakker BM. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS One. 2015;10:e0136364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA, Williams EG, Jha P, Lo Sasso G, Huzard D. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879-888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 481] [Cited by in F6Publishing: 575] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 53. | Bindels LB, Beck R, Schakman O, Martin JC, De Backer F, Sohet FM, Dewulf EM, Pachikian BD, Neyrinck AM, Thissen JP. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS One. 2012;7:e37971. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 159] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, Verdejo Y, Mascarós MC, Peris C, Cauli O. Effect of a Prebiotic Formulation on Frailty Syndrome: A Randomized, Double-Blind Clinical Trial. Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 55. | Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Bäckhed F, Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 711] [Cited by in F6Publishing: 802] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 56. | Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 818] [Article Influence: 116.9] [Reference Citation Analysis (0)] |
| 57. | Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 58. | Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647-5660. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 59. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3967] [Cited by in F6Publishing: 4113] [Article Influence: 205.7] [Reference Citation Analysis (4)] |
| 60. | Tahri K, Crociani J, Ballongue J, Schneider F. Effects of three strains of bifidobacteria on cholesterol. Lett Appl Microbiol. 1995;21:149-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3327] [Cited by in F6Publishing: 3624] [Article Influence: 278.8] [Reference Citation Analysis (0)] |
| 62. | Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575-1584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2130] [Cited by in F6Publishing: 2163] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 63. | Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - A case report. Int J Cardiol. 2015;201:157-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 64. | Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M; SPRING Trial Group. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68:974-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 65. | Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep. 2017;19:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 66. | Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 67. | Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012;26:1727-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 68. | Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal Microbial Metabolites Are Linked to Severity of Myocardial Infarction in Rats. PLoS One. 2016;11:e0160840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 69. | Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |