Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.475
Peer-review started: October 25, 2017
First decision: November 14, 2017
Revised: November 23, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: January 28, 2018
Processing time: 95 Days and 13.3 Hours
To investigate expression of cell cycle-related and expression-elevated protein in tumor (CREPT) in colorectal cancer (CRC) and determine its prognostic value in response to 5-fluorouracil (5-FU).
The relative expression of CREPT in CRC tumor samples was determined using immunohistochemistry. The protein content in cell lines was analyzed by immunoblotting. Cell viability was measured with the CCK-8 assay. Cell cycle and apoptosis analyses were performed with flow cytometry.
CREPT was overexpressed in CRC tissues and correlated with histological grade. Clinicopathological analysis indicated that CREPT was positively related to tumor progression. Exogenous expression of CREPT stimulated cell proliferation and accelerated the cell cycle. More importantly, high expression of CREPT sensitized CRC cells to 5-FU treatment. Furthermore, we demonstrated that 5-FU elicited significant apoptosis in CREPT-positive cells.
Aberrant overexpression of CREPT contributes to tumorigenesis of CRC by promoting cell proliferation and accelerating the cell cycle, and confers sensitivity to 5-FU. CREPT is a potential prognostic biomarker for 5-FU in CRC.
Core tip: Cell cycle-related and expression-elevated protein in tumor (CREPT) is an oncogene that is preferentially expressed in diverse human tumors. Overexpression of CREPT promotes cell proliferation and tumorigenesis. However, the expression and mechanistic involvement of CREPT in colorectal cancer have not been fully investigated. Despite advances in clinical applications of 5-fluorouracil, drug resistance remains a significant limitation to its clinical use. A prognostic biomarker for administration of this drug is still urgently needed.
- Citation: Kuang YS, Wang Y, Ding LD, Yang L, Wang Y, Liu SH, Zhu BT, Wang XN, Liu HY, Li J, Chang ZJ, Wang YY, Jia BQ. Overexpression of CREPT confers colorectal cancer sensitivity to fluorouracil. World J Gastroenterol 2018; 24(4): 475-483
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/475.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.475
Colorectal cancer (CRC) is a malignant disease with symptoms such as blood in the stools, aberrant bowel movement, and weight loss[1]. Globally, CRC is the third most common malignancy, accounting for approximately10% of all cases. In China, there were 376 300 newly diagnosed cases of CRC and 191000 deaths in 2015[2]. With advances in early diagnosis and clinical therapeutics, the average five-year survival rate is approaching 70% in the United States. The diagnosis of CRC mainly relies on pathological examination of tissues collected via enteroscopy, and evaluation of cancer stage heavily depends on imaging technologies like computed tomography, positron emission tomography and magnetic resonance imaging[3].
Fluorouracil-based chemotherapy is still the mainstay for clinical management of CRC[4]. 5-Fluorouracil (5-FU) is an antimetabolite drug that inhibits the biosynthesis of DNA and thus induces tumor cell apoptosis[5]. The clinical application of 5-FU-based adjuvant chemotherapy in the treatment of late stage CRC improves overall and disease-free survival in 10%-15% of patients[6]. However, the provoked resistance in response to 5-FU seriously compromises its therapeutic efficiency. Therefore, identification and characterization of prognostic biomarkers for screening the potential sensitive population for this drug is crucial.
Cell cycle-related and expression-elevated protein in tumor (CREPT; also named RPR1B) was first identified as an oncoprotein that is highly expressed in most tumors[7]. Principally, CREPT functions as a transcriptional regulator in CCND1 expression in two distinct ways: promoting direct binding of RNA polymerase II on the promoter region to activate transcription, or on the termination region before the poly-A site to prevent release from the transcript and allow for recycling[7]. CREPT was later identified to function on the human RNA polymerase II C-terminal domain scaffold and participate in phosphorylation of the C-terminal heptapeptide repeat domain[8]. In addition, CREPT induces transcription of several other cell cycle-related genes including CDK2, CDK4, CDK6 and cyclin-E, which eventually accelerates the cell cycle and stimulates cell proliferation[9].
There is accumulating evidence for the crucial role of CREPT in tumor biology in a range of human cancers[10]. However, the expression pattern and mechanistic involvement of CREPT in CRC have not been fully investigated. In this study, we investigated the role of CREPT in tumorigenesis of CRC via inducing cell proliferation and stimulating the cell cycle. Overexpression of CREPT rendered cells sensitive to 5-FU, which reinforced the apoptotic response. We propose the prognostic biomarker function of CREPT for clinical application of 5-FU.
The expression plasmid for human CREPT was pCDH/HA-CREPT, which was constructed in our laboratory. The plasmid pBS/U6/CREPT-si was constructed according to a previous protocol. The siRNA target sequence (CREPT-si), GGACCTGAATTCACTAGAGA, was identical for humans and mice. Antibodies against PARP (5625S) were purchased from Cell Signaling Technology (Danvers, MA, United States), anti-actin (AC-15) antibody was obtained from Sigma-Aldrich (St. Louis, MO, United States), and anti-CREPT antibody (3E10) was raised in our laboratory.
Two hundred and three primary CRC and 13 colorectal adenoma patients who underwent surgical treatment were selected. Formalin-fixed, paraffin-embedded tissue blocks were cut into 4-µm paraffin sections, followed by immunohistochemical analysis. The slides were heated in a tissue-drying oven for 40 min at 65 °C, followed by deparaffinization in xylene and rehydration in a graded alcohol series. The slides were incubated in sodium citrate solution (pH 6.0) and heated in a boiling water bath for 20 min for antigen retrieval. After endogenous peroxidases were blocked by soaking the slides in 3% H2O2, the slides were incubated with anti-CREPT primary antibody (1:20) in a humidity chamber at 4 °C overnight. We washed the slides with phosphate-buffered saline (PBS) three times, and applied the EnVision Kit (Dako, Glostrup, Denmark) to the sections on the slides and incubated in a humidified chamber at room temperature for 30 min. Signal detection was performed using diaminobenzidine in the EnVision Kit (Dako). All the slides were examined under a microscope by two blinded pathologists. The proportion of positive cancer cell staining was classified as follows: grade 1 (-) = no positive cells; grade 2 (1+) < 25%; grade 3, 25%-75%; and grade 4, > 75%. All patients gave informed consent for participation in the study. The tissue collection procedure with informed consent was approved by the Ethics Committee of the Chinese PLA General Hospital, Beijing, China.
Human CREPT gene was subcloned into pCDH-vector with an HA-tag. Short hairpin RNA (shRNA) was designed to downregulate expression of CREPT. Nonoverlapped sequences were designed (shRNA, 5’-GCAAGAACGAAGUGUUAUTT-3’). The shRNA targeting CREPT was selectively subcloned into lentiviral vector pLVX-IRWS-ZsGreen1. pCDH-HA-CREPT was also subsequently cloned into pLVX-IRWS-ZsGreen1. Lentivirus was produced and the titration of purified virus was determined according to our previous study[10]. The virus was stored at -80 °C until use.
Human colorectal adenocarcinoma cell lines DLD1 and SW620 were purchased from American Type Culture Collection (Manassas, VA, United States). DLD1 cells were cultured in RPMI 1640 (Life Technologies, Carlsbad, CA, United States) and SW620 cells were cultured in L-15 supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel), penicillin (100 U/mL) and streptomycin (100 mg/mL). DLD1 cells were maintained at 37 °C in a 5% CO2-containing atmosphere and SW620 cells were kept at 37 °Cwith 100% air.
Cells were harvested and homogenized in RIPA buffer (Cell Signaling Technology), followed by determination of protein concentration using the BCA kit (Life Technologies). Proteins were resolved by 10% SDS-PAGE, and then transferred to 0.45-mm polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 5% skimmed milk in Tris-buffered saline with Tween 20 (TBST) at 37 °C for 1 h and incubated with primary antibody at 4 °C overnight. The PVDF membranes were rigorously washed with TBST and subjected to secondary antibody hybridization. The protein bands were visualized using enhanced chemiluminescence (Millipore, Temecula, CA, United States).
Cells were counted by hemocytometer and then seeded into 96-well plates at 104/well, and 5-FU (50 μm/mL) was added 12 h later. CCK-8 (Dojindo, Kumamoto, Japan) buffer was diluted as protocol indicated and added to the wells at indicated time. Absorbance at 450 nm was recorded with a reference filter of 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, United States)
Cells were seeded in six-well plates 12 h before 5-FU was added. After incubation for 48 h, cells were harvested and subjected to apoptosis detection by FITC-Annexin V Apoptosis Detection Kit with PI (BioLegend, San Diego, CA, United States). The cells were analyzed by BD FACSCalibur (San Jose, CA, United States).
Data were expressed as mean ± standard deviation and were analyzed with unpaired t test and analysis of variance followed by a post hoc t test. Differences between proportions were assessed by the χ2 test. Survival analysis was performed by Kaplan-Meier method. All the analyses were conducted using SPSS 17.0 software. Statistical significance was defined as P < 0.05.
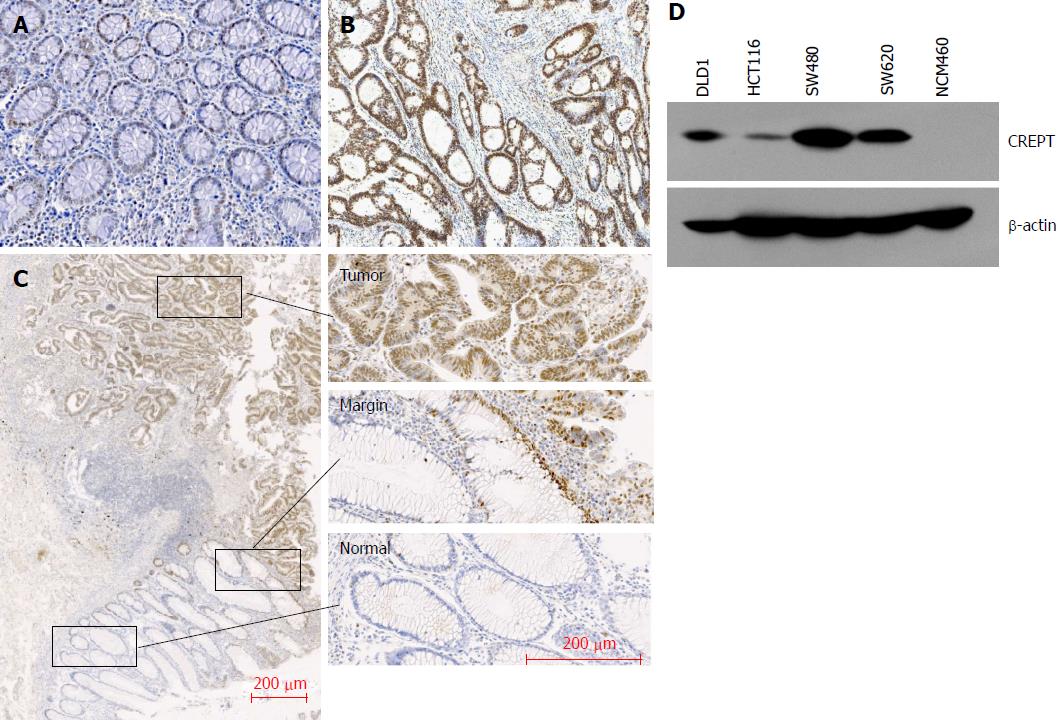
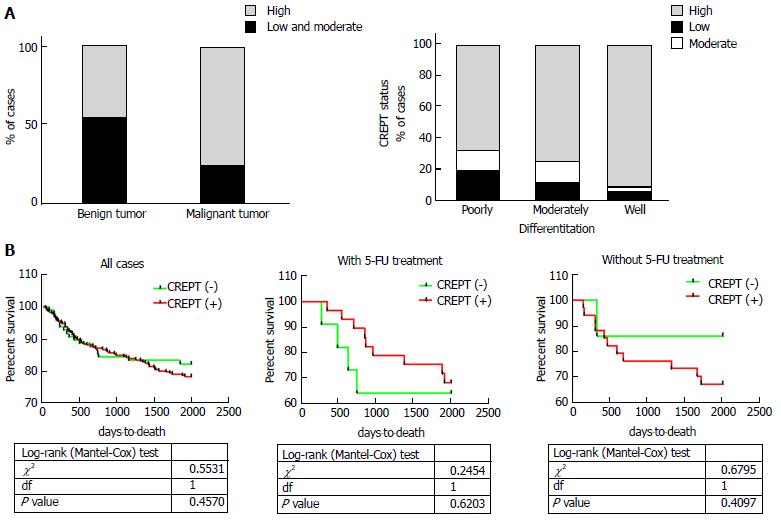
To determine the expression pattern of CREPT in CRC clinical samples, 203 CRC tissue slides and 13 benign colorectal adenoma tissues as controls were collected for immunohistochemistry. A significant increase in CREPT was detected in the CRC tissues in comparison with benign tissues (77% vs 46%; Figure 1A and B, Table 1). Abundant expression of CREPT was observed in well-differentiated tumors compared to moderately and poorly differentiated tumors (Figure 2A and Table 2). The intensive staining signal was enriched in the malignant region, in contrast to the margin (the benign stromal tissue at the tumor periphery) and normal counterparts in the same slide (Figure 1C).
| CREPT expression | |||||
| Low | Intermediate | High | P-value | ||
| Benign tumor | 1 | 6 | 6 | 0.035a | |
| Malignant tumor | 25 | 21 | 157 | ||
| CREPT expression | P value | |||
| Low | Intermediate | High | ||
| Tumor | ||||
| T1 | 2 | 0 | 4 | 0.770 |
| T2 | 3 | 2 | 25 | |
| T3 | 6 | 2 | 20 | |
| T4 | 14 | 17 | 108 | |
| Stage | ||||
| I | 4 | 2 | 23 | 0.700 |
| II | 11 | 12 | 64 | |
| III | 8 | 3 | 56 | |
| IV | 2 | 4 | 14 | |
| Histological grade | ||||
| Poor | 9 | 6 | 31 | 0.004b |
| Moderate | 12 | 13 | 71 | |
| Well | 4 | 2 | 55 | |
| Lymph node metastasis | ||||
| Negative | 15 | 15 | 92 | 0.487 |
| Positive | 10 | 6 | 65 | |
We analyzed expression of CREPT in CRC cell lines by western blotting (Figure 1D). NCM460, a normal human colon mucosal epithelial cell line, was used for comparative purpose. We did not detect CREPT protein in NCM460 cells. In contrast, CREPT levels were aberrantly upregulated in all four CRC cell lines examined: DLD1, HCT116, SW480 and SW620. Our in vitro expression analysis consolidated the observations from clinical samples.
Next, we attempted to analyze if CREPT had any correlation with clinicopathological features. Our data unambiguously demonstrated the positive association of high CREPT expression with pathological type (P < 0.05) and histological grade (P < 0.005) (Figure 2A and Tables 1 and 2). Our data suggested that CREPT expression was upregulated with CRC progression, which implicated a crucial role of CREPT in this disease.
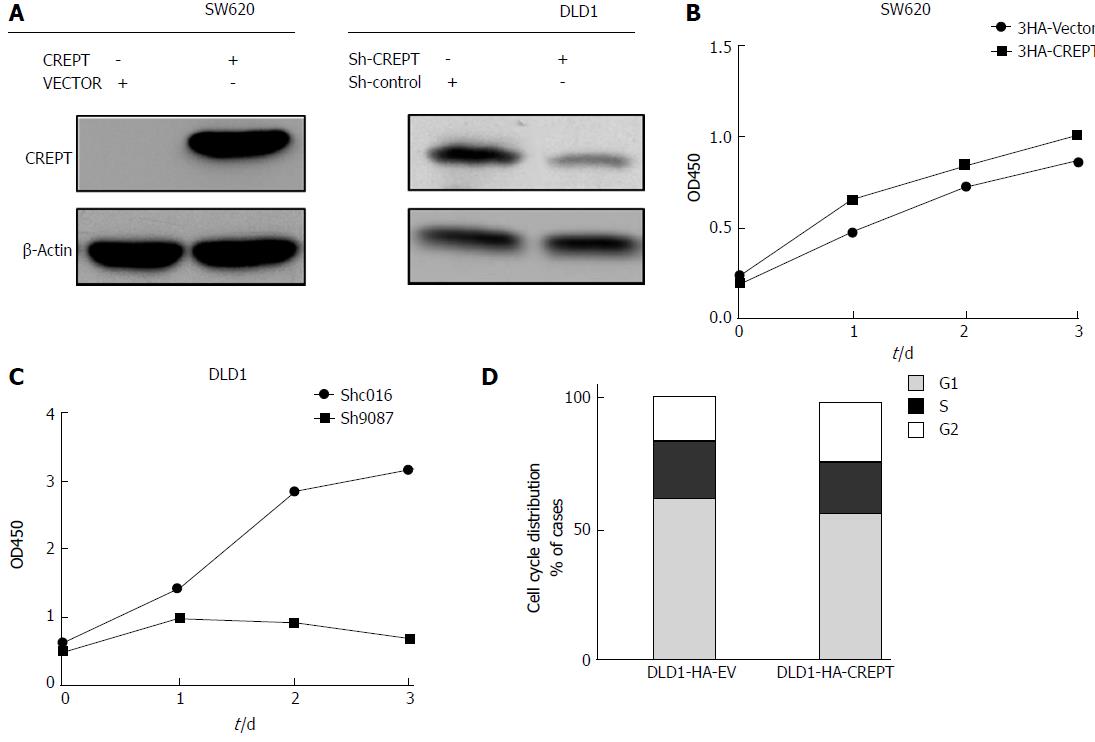
Our previous results characterized the aberrant high expression of CREPT in CRC both in vitro and in vivo. In view of the essential physiological role of CREPT in cell cycle modulation, we investigated whether CREPT was involved in cell proliferation and cell cycle regulation in CRC. To investigate whether CREPT had any influence on the viability of CRC cells, we constructed recombinant lentivirus lenti-HA-CREPT to generate stable overexpression cell lines, and the lentivirus lenti-sh-CREPT to knock down endogenous expression of CREPT. SW620 and DLD1 cells were infected with lentivirus. Both the ectopic expression of CREPT and knockdown efficiency were evaluated by immunoblotting (Figure 3A). Our results confirmed establishment of stable cell lines for further analysis.
Cell viability was determined using CCK-8 assay. Forced expression of CREPT in SW620 cells significantly promoted cell growth, while cell viability was markedly suppressed by CREPT depletion in DLD1 cells (Figure 3B and C). Previous studies have indicated that CREPT affects G1 to S phase transition[10]. In line with this notion, our cell cycle analysis by flow cytometry clearly demonstrated an increase of S and decrease of G phase cells upon exogenous expression of CREPT in DLD1 cells (Figure 3D). All the results suggested that CREPT overexpression played a critical role in stimulation of cell proliferation and the cell cycle in CRC cell lines, which might underlie its oncogenic potential in this disease.
The aforementioned data demonstrated the aberrant overexpression and oncogenic activity of CREPT via promoting cell proliferation and the cell cycle. We investigated whether high expression of CREPT was linked to chemotherapy resistance, especially for 5-FU. We retrieved the relevant data from the Cancer Genome Atlas (TCGA) database, and there was a trend that abundance of CREPT was a favorable indicator for CRC patients who received 5-FU-based chemotherapy (Figure 2B). Therefore, we set out to validate this observation in our in vitro system.
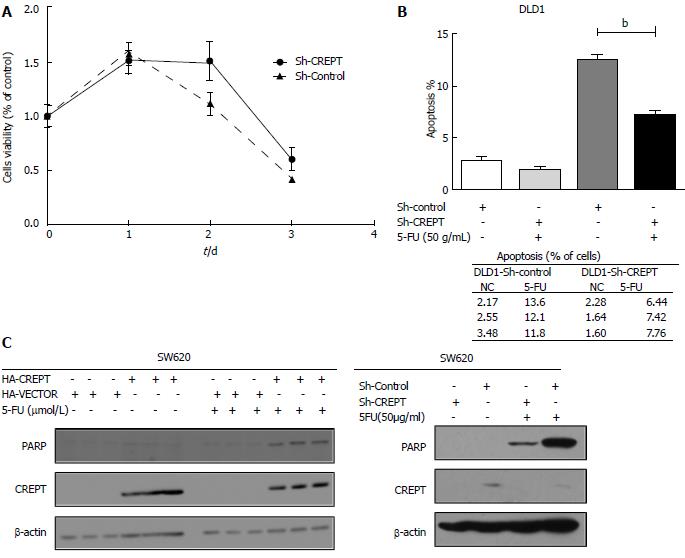
We measured the cytotoxic effect of 5-FU in CREPT-silenced DLD1 cells using the CCK-8 method. Knockdown of CREPT markedly suppressed cell proliferation. However, the cell viability of CREPT-silenced DLD1 cells was significantly increased in comparison with control cells in response to 5-FU (50 μg/mL) treatment, which suggested that drug resistance was induced by CREPT deficiency (Figure 4A). We showed that 5-FU elicited dramatic apoptosis in DLD1 cells, while this cytotoxic effect was significantly compromised upon CREPT knockdown (Figure 4B and D).
All these results implied a close link between CREPT expression and 5-FU sensitivity in CRC cells. This observation was consolidated through apoptotic pathway analysis in CREPT-manipulated SW620 and DLD cells. 5-FU treatment stimulated a significantly higher level of poly (ADP-ribose) polymerase (PARP) in CREPT-expressing SW620 cells than in the control ones. Consistently, the PARP level was markedly decreased in CREPT-silenced DLD cells upon 5-FU treatment (Figure 4C). All the results clearly demonstrated that CREPT conferred cells sensitivity to 5-FU in vitro.
CREPT protein is essentially a transcription regulator that acts via modulation of expression of multiple cell cycle-related factors[9]. For example, our previous study indicated that CREPT enhances expression of cyclin D1 by promoting RNA polymerase II recycling during transcription of this gene[7]. A later study showed that CREPT promotes transcriptional activity of the β-catenin/TCF4 (transcription factor 4) complex and in turn enhances the Wnt signaling pathway as well[8]. The Wnt pathway consequently regulates diverse biological processes, including cell proliferation, survival, migration and polarity[8].
Accumulating evidence indicates the oncogenic activity associated with aberrant overexpression of CREPT in various human malignances. For instance, Wang et al[9] demonstrated that CREPT promotes tumor growth by accelerating the cell cycle in endometrial cancer. Zhang et al[8] reported that CREPT is highly expressed in tumors and enhances the β-catenin/TCF4 transcriptional activity in response to Wnt signaling. She et al[11] suggested that CREPT expression correlates with poor prognosis in patients with retroperitoneal leiomyosarcoma. Similarly, high expression of CREPT in CRC promotes tumor growth and is correlated with poor prognosis[12]. Liu et al[13] demonstrated that inhibition of CREPT reduces proliferation and migration of non-small cell lung cancer cells by downregulating cell cycle-related proteins.
Consistent with all these reports, here we demonstrated that CREPT is highly expressed in both CRC tissues and cell lines, and is intimately linked to the pathological stage. Although there was no correlation between overall survival and CREPT expression in any of the CRC cases, stratification into non- and 5-FU treatment groups revealed a significant difference in respect to CREPT status. In line with its well-established role in cell cycle modulation, we further elucidated that CREPT promoted cell growth and accelerated the cell cycle in both CRC cell lines. Consistent with the results from TCGA database analysis, our in vitro experiments consolidated that CREPT level was positively associated with sensitivity to 5-FU.
5-FU is the mainstay chemotherapy drug for clinical treatment of CRC. However, only 5%-10% of all CRC patients manifested a favorable response to 5-FU-based regimens, whereas the majority had apparent drug resistance[5]. Several mechanisms underlying the refractory effect have been elucidated. For example, the elevated expression of DNA repair gene ERCC6 confers resistance to 5-FU and is associated with poor patient survival in CRC[14]. Liu et al[15] demonstrated that epigenetic silencing of ASPP1 confers 5-FU resistance in clear cell renal carcinoma by preventing p53 activation. In addition, overexpression of long noncoding RNA UCA1 is related to multidrug resistance including 5-FU and cisplatin[16]. The miRNA miR-1290 functions as a biomarker in DNA mismatch repair-deficient colon cancer and promotes resistance to 5-FU by directly targeting hMSH2[17].
Several strategies have been exploited to surmount the resistance developed in response to clinical use of 5-FU. The synthesized peptide of SPARC (secreted protein acidic and rich in cysteine) interferes with the interaction between caspase 8 and Bcl2 to resensitize chemo-resistant tumors and enhance their regression in vivo[18]. With respect to miRNAs, overexpression of miR-122 resensitizes 5-FU-resistant colon cancer cells through inhibition of PKM2 in vitro and in vivo[19]. Moreover, the chemotherapy response is associated with subsets of tumor-infiltrating lymphocytes in gastric cancer[20]. Together with all these results, here we provide novel evidence that overexpression of CREPT confers sensitivity to 5-FU on CRC cells, which suggests that CREPT has great potential as a prognostic biomarker for clinical application of 5-FU. Therefore, we proposed that relative expression of CREPT in CRC tissues should be determined during biopsy and could serve as prerequisite for the decision to use 5-FU.
Although 5-FU exerts its maximum therapeutic outcome in CRC, it is a broad-spectrum antitumor drug. In the first-line chemotherapy of breast and gastric cancer, 5-FU plays an irreplaceable role. According to our previous study, CREPT has a similar effect on CRC, breast and gastric cancer. This suggests that CREPT is a potential chemotherapy sensitivity indicator in these cancers and further research to verify this is needed.
Despite the well-acknowledged oncogenic role of CREPT in a range of human cancers, here we indicate that high expression of CREPT is favorable in respect to 5-FU administration. However, the molecular events underlying the drug sensitivity induced by CREPT are still elusive. In view of its nature as a transcription regulator of multiple cell cycle-related factors, we hypothesize that CREPT accelerates the cell cycle and exacerbates thymineless death in CRC cells in response to 5-FU challenge. Beyond this study, the issue still to be addressed is how CREPT is upregulated in CRC. Cui et al reported that miR-1188 at the imprinted Dlk1-Dio3 domain acts as a tumor suppressor in hepatoma cells and suppresses CREPT expression, which sheds light on the regulatory mechanism underlying the overexpression of CREPT in CRC.
Colorectal cancer (CRC) is the third leading cancer and the third most frequent cause of cancer-related death in the United States. Cell cycle-related and expression-elevated protein in tumor (CREPT) is preferentially expressed in many kinds of carcinomas. However, the correlation between CREPT and CRC clinicopathological patterns remains unclear. Study of the impact of CREPT expression on the anticancer drug 5-fluorouracil (5-FU) resistance in CRC has been limited.
We investigated the expression pattern of CREPT in CRC and explored if CREPT rendered CRC cells sensitive to 5-FU.
We investigated the expression pattern of CREPT in CRC. To the best of our knowledge, this is the first study to explore the correlation between CREPT and CRC cell sensitivity to 5-FU. Our results lead us to consider CREPT as a potential chemotherapy predictive biomarker. Moreover, further study on the impact of CREPT on chemotherapy outcome in other cancers and on other antitumor drugs is needed.
We analyzed tissue sections from 203 primary CRC patients and 13 benign colorectal adenoma patients using immunohistochemistry with anti-CREPT antibody. CREPT overexpressing/knockdown cell lines were established by lentivirus infection. Expression of CREPT in these cell lines was analyzed by western blotting and the cell viability was measured by CCK-8 assay. The cell lines were subjected to 5-FU treatment. The cytotoxic effect of 5-FU was measured by CCK-8 assay and poly (ADP-ribose) polymerase/flow cytometry analysis.
CREPT expression correlates with clinicopathological features in CRC. CREPT was abundantly expressed in CRC tissues compared with benign tissues. A significant increase in CREPT was detected in more highly differentiated tumors. The intensive staining signal was enriched in the malignant region in contrast to the margin and normal counterparts in the same slide. CREPT stimulated cell proliferation and the cell cycle in CRC cells. Cell growth was significantly enhanced when CREPT was overexpressed via exogenous transfection, while CREPT depletion markedly suppressed cell viability. Overexpression of CREPT sensitized CRC cells to 5-FU-induced apoptosis. Knock down of CREPT markedly suppressed cell proliferation. However, viability of CREPT-silenced DLD1 cells was significantly increased in comparison with control cells in response to 5-FU treatment, indicating that drug resistance was induced by CREPT deficiency. 5-FU elicited dramatic apoptosis in DLD1 cells.
The impact of CREPT on CRC cell response to 5-FU was identified for the first time. We hypothesize that this phenomenon is attributed to an accelerated cell cycle induced by high expression of CREPT. However, the mechanism of this finding requires further study. Clinically, biomarkers for chemotherapy efficacy prediction are urgently needed and this research provides a candidate.
There were a few limitations to this study. For example, compared to a public database, first-hand follow-up data of patients are more convincing. For the future, we are working on animal experiments to verify our findings in vivo. Then, we will investigate the mechanisms of action of CREPT, and the possibility of clinical application of CREPT as a prognostic indicator.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jeong KY, Kir G, Luchini C S- Editor: Chen K L- Editor: Filipodia E- Editor: Ma YJ
| 1. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2294] [Article Influence: 208.5] [Reference Citation Analysis (1)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Kekelidze M, D’Errico L, Pansini M, Tyndall A, Hohmann J. Colorectal cancer: current imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation. World J Gastroenterol. 2013;19:8502-8514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 115] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (4)] |
| 4. | Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 382] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 5. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3630] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 6. | Ma J, Ren Y, Zhang L, Kong X, Wang T, Shi Y, Bu R. Knocking-down of CREPT prohibits the progression of oral squamous cell carcinoma and suppresses cyclin D1 and c-Myc expression. PLoS One. 2017;12:e0174309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Liu C, Duan X, Ren F, Li S, Jin Z, Wang Y, Feng Y, Liu Z, Chang Z. CREPT/RPRD1B, a recently identified novel protein highly expressed in tumors, enhances the β-catenin·TCF4 transcriptional activity in response to Wnt signaling. J Biol Chem. 2014;289:22589-22599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Qiu H, Hu W, Li S, Yu J. RPRD1B promotes tumor growth by accelerating the cell cycle in endometrial cancer. Oncol Rep. 2014;31:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Lu D, Wu Y, Wang Y, Ren F, Wang D, Su F, Zhang Y, Yang X, Jin G, Hao X. CREPT accelerates tumorigenesis by regulating the transcription of cell-cycle-related genes. Cancer Cell. 2012;21:92-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | She Y, Liang J, Chen L, Qiu Y, Liu N, Zhao X, Huang X, Wang Y, Ren F, Chang Z. CREPT expression correlates with poor prognosis in patients with retroperitoneal leiomyosarcoma. Int J Clin Exp Pathol. 2014;7:6596-6605. [PubMed] |
| 11. | Zheng G, Li W, Zuo B, Guo Z, Xi W, Wei M, Chen P, Wen W, Yang AG. High expression of CREPT promotes tumor growth and is correlated with poor prognosis in colorectal cancer. Biochem Biophys Res Commun. 2016;480:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Liu T, Li WM, Wang WP, Sun Y, Ni YF, Xing H, Xia JH, Wang XJ, Zhang ZP, Li XF. Inhibiting CREPT reduces the proliferation and migration of non-small cell lung cancer cells by down-regulating cell cycle related protein. Am J Transl Res. 2016;8:2097-2113. [PubMed] |
| 13. | Zhao Z, Zhang G, Li W. Elevated Expression of ERCC6 Confers Resistance to 5-Fluorouracil and Is Associated with Poor Patient Survival in Colorectal Cancer. DNA Cell Biol. 2017;36:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Wang X, Cheng Y, Zhu Y, Li H, Ge W, Wu X, Zhao K, Yuan J, Li Z, Jiang S. Epigenetic silencing of ASPP1 confers 5-FU resistance in clear cell renal cell carcinoma by preventing p53 activation. Int J Cancer. 2017;141:1422-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wang H, Guan Z, He K, Qian J, Cao J, Teng L. LncRNA UCA1 in anti-cancer drug resistance. Oncotarget. 2017;8:64638-64650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Ye L, Jiang T, Shao H, Zhong L, Wang Z, Liu Y, Tang H, Qin B, Zhang X, Fan J. miR-1290 Is a Biomarker in DNA-Mismatch-Repair-Deficient Colon Cancer and Promotes Resistance to 5-Fluorouracil by Directly Targeting hMSH2. Mol Ther Nucleic Acids. 2017;7:453-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Rahman M, Chan AP, Tang M, Tai IT. A peptide of SPARC interferes with the interaction between caspase8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One. 2011;6:e26390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | He J, Xie G, Tong J, Peng Y, Huang H, Li J, Wang N, Liang H. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Lee JY, Son T, Cheong JH, Hyung WJ, Noh SH, Kim CB, Park CG, Kim HI. Association between Chemotherapy-Response Assays and Subsets of Tumor-Infiltrating Lymphocytes in Gastric Cancer: A Pilot Study. J Gastric Cancer. 2015;15:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Cui W, Huang Z, He H, Gu N, Qin G, Lv J, Zheng T, Sugimoto K, Wu Q. MiR-1188 at the imprinted Dlk1-Dio3 domain acts as a tumor suppressor in hepatoma cells. Mol Biol Cell. 2015;26:1416-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |