Published online Oct 21, 2018. doi: 10.3748/wjg.v24.i39.4482
Peer-review started: July 23, 2018
First decision: August 30, 2018
Revised: September 13, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 21, 2018
Processing time: 88 Days and 5.6 Hours
To evaluate the safety and efficacy of agitation thrombolysis (AT) combined with catheter-directed thrombolysis (CDT) for the treatment of non-cirrhotic acute portal vein thrombosis (PVT).
Nine patients with non-cirrhotic acute PVT who underwent AT combined with CDT were analyzed retrospectively. Portography was carried out via the transjugular intrahepatic portosystemic (commonly known as TIP) or percutaneous transhepatic (commonly known as PT) route, followed by AT combined with CDT. Complications of the procedure, and the changes in clinical symptoms, hemodynamics of the portal vein and liver function were recorded. Follow-up was scheduled at 1, 3 and 6 mo after treatment, and every 6 mo thereafter, or when the patients developed clinical symptoms related to PVT. Color Doppler ultrasound and contrast-enhanced computed tomography/magnetic resonance imaging were performed during the follow-up period to determine the condition of the portal vein.
AT combined with CDT was successfully performed. The portal vein was reached via the TIP route in 6 patients, and via the PT route in 3 patients. All clinical symptoms were relieved or disappeared, with the exception of 1 patient who died of intestinal necrosis 9 d after treatment. Significant differences in the changes in portal vein hemodynamics were observed, including the maximum lumen occupancy of PVT, portal vein pressure and flow velocity between pre- and post-treatment (P < 0.05). During the follow-up period, recurrence was observed in 1 patient at 19 mo after the procedure, and the portal vein was patent in the remaining patients.
AT combined with CDT is a safe and effective method for the treatment of non-cirrhotic acute PVT.
Core tip: Agitation is a common phenomenon in daily life, and can accelerate the rate of solute dissolution in a solvent. As the thrombus is porous in the acute stage, it can easily be broken into smaller fragments by agitation, which increases the contact area between the thrombus and thrombolytics, and increases the speed of thrombus dissolution. According to our research, agitation thrombolysis combined with catheter-directed thrombolysis is a safe and effective method for the treatment of non-cirrhotic acute portal vein thrombosis, with a good short- to middle-term efficacy. However, the long-term efficacy of agitation thrombolysis combined with catheter-directed thrombolysis in a large population requires further investigation.
- Citation: Wang CY, Wei LQ, Niu HZ, Gao WQ, Wang T, Chen SJ. Agitation thrombolysis combined with catheter-directed thrombolysis for the treatment of non-cirrhotic acute portal vein thrombosis. World J Gastroenterol 2018; 24(39): 4482-4488
- URL: https://www.wjgnet.com/1007-9327/full/v24/i39/4482.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i39.4482
Non-cirrhotic portal vein thrombosis (PVT) has a low incidence with a prevalence of approximately 1%, and partial obstruction of the portal vein is often clinically imperceptible[1]. However, complete obstruction due to acute thrombus can lead to a series of clinical symptoms, such as abdominal pain, ascites, and even intestinal necrosis. Common treatment options, including anticoagulation or indirect thrombolysis, are useful for relieving symptoms, but the outcome is unsatisfactory as most patients developed cavernous transformation of the portal vein due to incomplete recanalization[2,3]. Catheter-directed thrombolysis (CDT) via the transjugular intrahepatic portosystemic (TIP) or percutaneous transhepatic (PT) route is a minimally invasive method, which can dissolve the thrombus and relieve symptoms rapidly with a lower dose of thrombolytics[4]. In addition, agitation thrombolysis (AT) can break the thrombus into smaller fragments and accelerate the speed of thrombolysis[5]. The objective of this study was to evaluate the safety and efficacy of AT combined with CDT for the treatment of non-cirrhotic acute PVT.
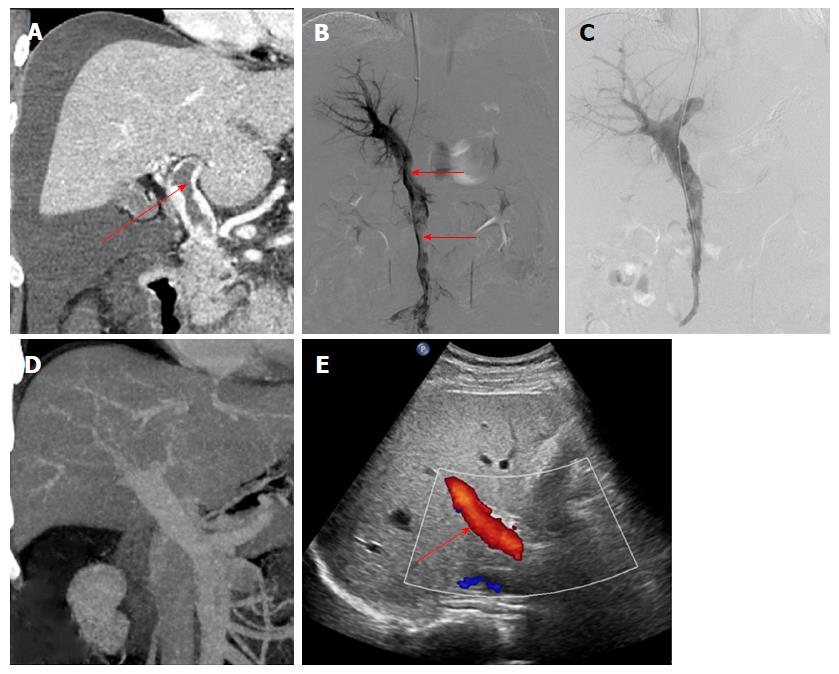
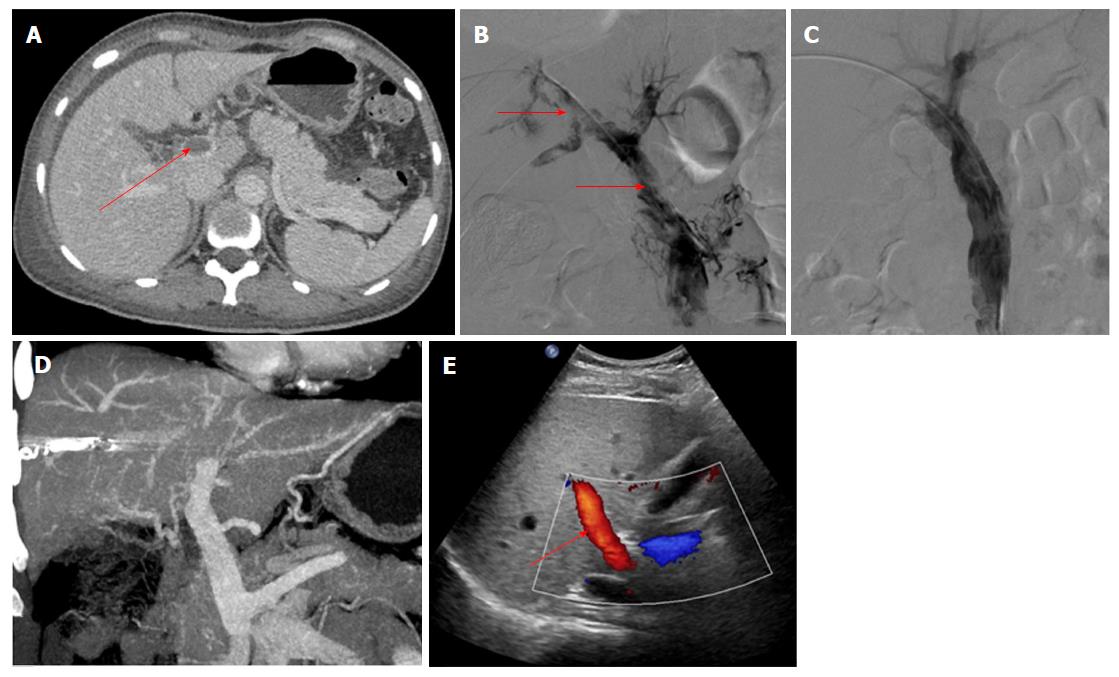
The data from 9 patients with non-cirrhotic acute PVT who underwent AT combined with CDT in our hospital between September 2015 and December 2017 were analyzed retrospectively. The patients consisted of 3 men and 6 women, with a mean age of 47.9 ± 10.6 years. All patients met the following eligibility criteria: (1) the PVT was diagnosed definitively by color Doppler ultrasound (CDUS) and enhanced-contrast computed tomography (CT) or magnetic resonance imaging (MRI) (Figures 1A and 2A); (2) absence of massive periportal collaterals and features of liver cirrhosis in the imaging findings; and (3) excluded malignant tumor. In addition, the following information was collected for each patient before treatment: clinical symptoms, days from onset to operation, routine blood examination, liver function, etiology, the maximum lumen occupancy of PVT and the flow velocity of the portal vein measured by CDUS (Table 1).
| Patient No. | Age (yr) | Sex | Etiology | Symptoms | Time from onset to treatment (d) |
| 1 | 29 | M | Myelodysplastic syndromes | Abdominal pain, abdominal distension | 4 |
| 2 | 39 | F | Protein S deficiency | Abdominal pain, abdominal distension, vomiting | 11 |
| 3 | 43 | M | Myelodysplastic syndromes | Abdominal pain, vomiting | 14 |
| 4 | 48 | F | Protein C deficiency | Abdominal pain, abdominal distension | 5 |
| 5 | 40 | F | Nephrotic syndrome | Abdominal pain, vomiting | 8 |
| 6 | 53 | M | Protein S deficiency | Abdominal pain, diarrhea | 2 |
| 7 | 61 | F | Splenectomy after trauma | Abdominal pain, abdominal distension, vomiting | 10 |
| 8 | 36 | F | Myelodysplastic syndromes | Abdominal pain, abdominal distension | 5 |
| 9 | 57 | F | Unknown | Abdominal pain | 16 |
The procedures were performed under digital subtraction angiographic guidance (Artis zeego; Siemens, Munich, Germany). The choice of TIP or PT route depended on if the patients had ascites.
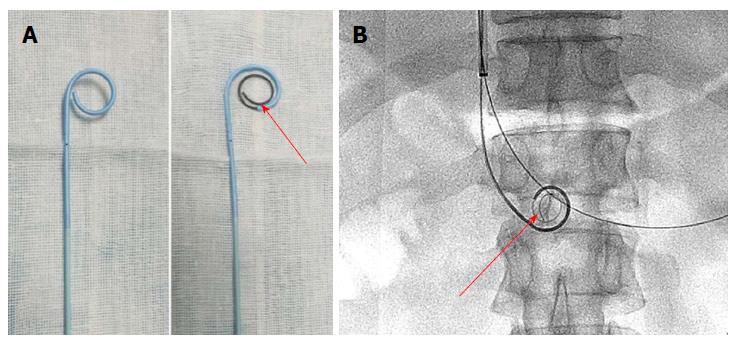
The portal vein was reached via the TIP route in 6 patients with ascites. A Rosch-Uchida set (Cook, Bloomington, IN, United States) was used to gain access to the portal vein branch from the hepatic vein under fluoroscopic guidance. When a 0.035-in hydrophilic guidewire (Terumo, Tokyo, Japan) reached the superior mesenteric vein or splenic vein in cooperation with a Cobra catheter (Cook), a pigtail catheter (Cook) was exchanged to perform portography (Figure 1B) and measure the portal pressure. The Rosch-Uchida sheath was then introduced into the portal vein following intrahepatic dilatation by a 6-mm balloon catheter (Boston Scientific, Natick, MA, United States). A pigtail catheter was inserted into the thrombus through the sheath, and a guidewire with a helical tip molded in vitro was advanced through the catheter (Figure 3). The guidewire and pigtail catheter were pushed and drawn together, and then rotated clockwise and anticlockwise alternatively, to agitate the thrombus into smaller fragments. In addition, 2 × 105 IU urokinase (Tianjin Biochemical Pharmaceutical Co., Ltd., Tianjin, China) was injected through the catheter by intermittent pulsatile delivery. The AT procedure was continued for approximately 10 min. The Rosch-Uchida sheath was then removed and the indwelling pigtail catheter was left in the PVT.
The portal vein was reached via the PT route in 3 patients without ascites. After successful puncture of a portal vein branch using a 22-gauge Chiba needle (Cook), a 6F coaxial dilator (Cook) was inserted into the portal vein. Then, a 5F sheath (Cook) and a pigtail catheter were exchanged. AT was performed after portography (Figure 2B), and the AT procedures were similar to those carried out via the TIP route. The indwelling pigtail catheter was left in the PVT.
Six hundred thousand IU of urokinase was infused continuously via the pigtail catheter each day, unless complications developed. In addition, 5000 IU of low molecular weight heparin was injected twice a day, and warfarin was administered after treatment at an initial dose of 5 mg. The low molecular weight heparin was discontinued when the international normalized ratio was maintained at 2.0-3.0, and warfarin was continued for at least 12 mo. Portography was repeated every 24 h, and termination of CDT was based on the patency of the portal vein assessed by imaging and an obvious improvement in clinical symptoms. The portal vein pressure was measured again before the pigtail catheter was removed.
Follow-up was scheduled at 1, 3 and 6 mo after treatment, and every 6 mo thereafter, or when the patients developed clinical symptoms related to PVT. Observations during follow-up mainly included clinical symptoms, routine blood examination, liver function and the condition of the portal vein evaluated by CDUS and contrast-enhanced CT/MRI. The end of follow-up was April 30, 2018.
Data are shown as mean ± standard deviation (SD). Paired Student’s t-test was used to determine statistically significant differences between pre- and post-treatment values. Significance was set at P < 0.05. Statistical analysis was performed using SPSS (version 19.0; IBM, Armonk, NY, United States) software.
AT combined with CDT was successful in all patients. Immediate portography after AT showed greater blood flow than pre-AT. One patient treated via the TIP route underwent intestinal resection as a result of congestive necrosis 5 d after the procedure; however, this patient died 9 d later. In the remaining patients, CDT was continued for 2.1 ± 1.1 d with a total dose of urokinase of 0.8-2.6 × 106 IU (Table 2). All clinical symptoms were relieved or disappeared, except in 1 patient who continued to experience abdominal distension after meals. There was a significant difference in the maximum lumen occupancy of PVT (Figures 1C and 2C), portal vein pressure and flow velocity between pre- and post-treatment (P < 0.05). No significant differences in the changes in liver function, such as alanine aminotransferase, albumin and bilirubin were observed (P > 0.05) (Table 3).
| Patient No. | Treatment route | Duration of CDT (d) | Dose of urokinase (× 106 IU) | Complications | Follow-up time (mo) and results |
| 1 | TIP | 2 | 1.0 | Hematuresis | 27/patent |
| 2 | PT | 2 | 1.4 | None | 23/patent |
| 3 | TIP | - | - | Death | Death |
| 4 | TIP | 1 | 0.8 | None | 31/patent |
| 5 | PT | 3 | 2.0 | Hemorrhage from the puncture tract | 5/patent |
| 6 | TIP | 4 | 2.6 | Subcapsular hematoma | 22/reappearance |
| 7 | TIP | 1 | 0.8 | None | 9/patent |
| 8 | TIP | 1 | 0.8 | None | 12/patent |
| 9 | PT | 3 | 2.0 | Hemorrhage from the puncture tract | 18/patent |
| Pre-treatment | Post-treatment | P value | |
| Albumin, g/L | 34.9 min | 36.7 min | 0.872 |
| Alanine aminotransferase, U/L | 38.2 inea | 33.6 inea | 0.934 |
| Total bilirubin, sferase | 17.9 lbi | 17.4 lbi | 0.991 |
| Flow velocity of the portal vein, cm/s | 4.2 tal | 16.3 alv | < 0.05 |
| Maximum lumen occupancy of PVT, % | 78.2 occ | 14.1 occ | < 0.01 |
| Portal pressure, mmHg | 31.0 alp | 14.1 alp | < 0.05 |
One patient developed subcapsular hemorrhage during puncture of the portal vein via the TIP route and experienced a rapid heartbeat. The patient’s vital signs were stable after erythrocyte transfusion, and CDT was started 3 d later. One patient via PT route had hematuresis during CDT. These symptoms disappeared when urokinase was discontinued (Table 2).
The follow-up period ranged from 5 mo to 31 mo. During this period, 1 patient who had been taking warfarin for 12 mo had an irregular poor appetite at 19 months after treatment, and contrast-enhanced CT showed that cavernous transformation of the portal vein had developed in the right portal vein. In the remaining patients, the portal vein was patent and none of the clinical symptoms related to PVT reappeared (Table 2). Liver function and routine blood examination were normal in all patients during follow-up (Figures 1D and E, 2D and E).
PVT is mostly seen in liver cirrhosis, with a prevalence ranging from 10% to 23%[6]. Non-cirrhotic PVT rarely occurs, with a prevalence of approximately 1%, and has an intimate relationship with inherited or acquired coagulation diseases, such as primary myeloproliferative disorders, Protein C/S deficiency, and abdominal surgery[7]. In addition to the etiology of PVT, it is particularly important to distinguish acute PVT from chronic PVT as the treatment is different. The American Association for the Study of Liver Diseases (commonly known as AASLD) describes acute PVT as the sudden formation of thrombus in the portal vein, and chronic PVT occurs when the obstructed portal vein is replaced by periportal collaterals bypassing the PVT[8]. However, the time boundary is not mentioned. In our study, acute PVT was diagnosed by clinical symptoms and the absence of massive periportal collaterals on imaging.
For non-cirrhotic acute PVT with obvious symptoms, the aim of treatment is recanalization of the obstructed portal vein and the prevention of complications. Common treatment methods including anticoagulation or indirect thrombolysis can be useful for relieving symptoms and recanalizing the portal vein. A meta-analysis involving 353 patients showed a significantly higher rate of recanalization following anticoagulation compared with the control group, which did not receive anticoagulation (71% vs 42%; P < 0.01)[2]. However, less than 20% of the patients achieved complete recanalization, and the others developed cavernous transformation of the portal vein due to incomplete recanalization, which can lead to chronic portal hypertension. Indirect thrombolysis via a peripheral vein or the superior mesenteric artery is less technically demanding, whereas the effect is limited as the thrombolytics are diverted from the patent branches or collaterals[3]. In addition, a high dose of thrombolytics may increase the risk of gastrointestinal hemorrhage.
CDT is an effective treatment for acute thrombus, which can enhance the efficacy of thrombolytics at a lower dose compared to indirect thrombolysis[4]. CDT via the PT route is relatively simple to perform, but it is not suitable for patients with massive ascites as it may cause hemorrhage through the PT tract during subsequent anticoagulation or thrombolysis. In addition, patients often felt pain when breathing as the indwelling catheter traversed the hepatic capsule. CDT via the TIP route can avoid these complications, but puncturing the portal vein from the hepatic vein is more difficult than using the PT route. Chen et al[9] reported that direct portography via the PT route, then a balloon catheter inflated with contrast medium to 80% of its volume positioned at the site of the bifurcation of the right and left portal veins can improve the success rate. Wang et al[10] reported the successful treatment of 12 patients with acute PVT treated with aspiration combined with CDT via the TIP route. The CDT time was 7.6 h. Eight patients achieved complete recanalization and four patients had partial recanalization, and AT combined with CDT was more effective than anticoagulation or indirect thrombolysis.
Agitation is a common phenomenon in daily life, and can accelerate the rate of solute dissolution in a solvent. AT was first reported by Ding et al[5] following the successful treatment of acute inferior vein thrombus in Budd-Chiari syndrome, which showed a good long-term efficacy[11], but has not yet been reported in the treatment of PVT. As the thrombus is porous in the acute stage, it can easily be broken into smaller fragments by agitation, which increases the contact area between the thrombus and thrombolytics, and increases the speed of thrombus dissolution. The shape of the catheter tip is important. In this study, the pigtail catheter was chosen as an agitator, as it did not injure the vessel wall and resulted in greater fragmentation of the thrombus during rotation. In addition, a guidewire with a helical tip can enhance the fracture resistance of the pigtail catheter, and increase the range of agitation. Compared with other methods, such as endovascular aspiration or mechanical thrombectomy[12], AT is simpler and safer, with less blood loss. In our study, all the patients had a smoother blood flow after treatment than before treatment, and the duration of CDT was shorter and the rate of recanalization was higher than those reported by Wang et al[10].
There are some limitations in this study. Firstly, the study was retrospective, with a limited number of cases; therefore, the data may have been affected by various potential biases. Secondly, puncture of the portal vein is difficult to perform under fluoroscopic guidance, and required extensive experience and a good understanding of imaging data including contrast-enhanced CT/MRI; thus, the treatment is restricted to University Hospitals.
In conclusion, AT combined with CDT is a safe and effective method for the treatment of non-cirrhotic acute PVT, with a good short- to middle-term efficacy. However, the long-term efficacy of AT combined with CDT in a large population requires further investigation.
Non-cirrhotic portal vein thrombosis (PVT) has a low incidence, with a prevalence of approximately 1%, and partial obstruction of the portal vein is often clinically imperceptible. However, complete obstruction due to acute thrombus can lead to a series of clinical symptoms, such as abdominal pain, ascites, and even intestinal necrosis. Common treatment opinions including anticoagulation or indirect thrombolysis are useful for relieving symptoms, but the outcome is unsatisfactory as most patients develop into cavernous transformation of the portal vein due to incomplete recanalization.
Catheter-directed thrombolysis (CDT) via the transjugular intrahepatic portosystemic (commonly known as TIP) or percutaneous transhepatic (PT) route is a minimally invasive method, which can dissolve the thrombus and relieve symptoms rapidly with a lower dose of thrombolytics. In addition, agitation thrombolysis (AT) can break the thrombus into smaller fragments and accelerate the speed of thrombolysis.
The objective of this study was to evaluate the safety and efficacy of AT combined with CDT for the treatment of non-cirrhotic acute PVT.
Nine patients with non-cirrhotic acute PVT who underwent AT combined with CDT via TIP or percutaneous transhepatic route were analyzed retrospectively. AT had not been reported for the treatment of PVT so far. The changes in clinical symptoms, hemodynamics of the portal vein and liver function were recorded and followed up to evaluate the safety and efficacy.
According to our research, AT combined with CDT is a safe and effective method for the treatment of non-cirrhotic acute PVT, with a good short- to middle-term efficacy. However, the long-term efficacy of AT combined with CDT in a large population requires further investigation.
Agitation is a common phenomenon in daily life, and can accelerate the rate of solute dissolution in a solvent. AT was first reported for the treatment of acute inferior vein thrombus in Budd-Chiari syndrome, which showed good long-term efficacy, but has not yet been reported in the treatment of PVT. As the thrombus is porous in the acute stage, it can easily be broken into smaller fragments by agitation, which increases the contact area between the thrombus and thrombolytics, and increases the speed of thrombus dissolution. Compared with other methods, such as endovascular aspiration or mechanical thrombectomy, AT is simpler and safer, with less blood loss.
The shape of the catheter tip is important. In this study, the pigtail catheter was chosen as an agitator, as it did not injure the vessel wall and resulted in greater fragmentation of the thrombus during rotation. In addition, a guidewire with a helical tip can enhance the fracture resistance of the pigtail catheter, and increase the range of agitation. The future research we will focus on involves the long-term efficacy of AT combined with CDT in a large population.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eleftheriadis NP S- Editor: Ma RY L- Editor: Filipodia E- Editor: Huang Y
| 1. | Basit SA, Stone CD, Gish R. Portal vein thrombosis. Clin Liver Dis. 2015;19:199-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Loffredo L, Pastori D, Farcomeni A, Violi F. Effects of Anticoagulants in Patients With Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;153:480-487.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 3. | Liu K, Li WD, Du XL, Li CL, Li XQ. Comparison of Systemic Thrombolysis Versus Indirect Thrombolysis via the Superior Mesenteric Artery in Patients with Acute Portal Vein Thrombosis. Ann Vasc Surg. 2017;39:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Avgerinos ED, Chaer RA. The ATTRACTiveness of catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2018;6:303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Ding PX, Li YD, Han XW, Wu G. Agitation thrombolysis for fresh iatrogenic IVC thrombosis in patients with Budd-Chiari syndrome. J Vasc Surg. 2010;52:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Harding DJ, Perera MT, Chen F, Olliff S, Tripathi D. Portal vein thrombosis in cirrhosis: Controversies and latest developments. World J Gastroenterol. 2015;21:6769-6784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Cruz-Ramón V, Chinchilla-López P, Ramírez-Pérez O, Aguilar-Olivos NE, Alva-López LF, Fajardo-Ordoñez E, Ponciano-Rodríguez G, Northup PG, Intagliata N, Caldwell SH. Thrombosis of the Portal Venous System in Cirrhotic vs. Non-Cirrhotic Patients. Ann Hepatol. 2018;17:476-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 652] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Ye P, Li Y, Ma S, Zhao J, Zeng Q. Percutaneous transhepatic balloon-assisted transjugular intrahepatic portosystemic shunt for chronic, totally occluded, portal vein thrombosis with symptomatic portal hypertension: procedure technique, safety, and clinical applications. Eur Radiol. 2015;25:3431-3437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Wang MQ, Liu FY, Duan F, Wang ZJ, Song P, Fan QS. Acute symptomatic mesenteric venous thrombosis: treatment by catheter-directed thrombolysis with transjugular intrahepatic route. Abdom Imaging. 2011;36:390-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Ding PX, He X, Han XW, Zhang Y, Wu Y, Liang XX, Liu C. An Individualised Strategy and Long-Term Outcomes of Endovascular Treatment of Budd-Chiari Syndrome Complicated by Inferior Vena Cava Thrombosis. Eur J Vasc Endovasc Surg. 2018;55:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Jun KW, Kim MH, Park KM, Chun HJ, Hong KC, Jeon YS, Cho SG, Kim JY. Mechanical thrombectomy-assisted thrombolysis for acute symptomatic portal and superior mesenteric venous thrombosis. Ann Surg Treat Res. 2014;86:334-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |