Published online Sep 14, 2018. doi: 10.3748/wjg.v24.i34.3958
Peer-review started: April 27, 2018
First decision: July 4, 2018
Revised: July 10, 2018
Accepted: July 22, 2018
Article in press: July 22, 2018
Published online: September 14, 2018
Castleman disease (CD) is a rare disorder of lymph nodes and related tissues. CD generally occurs in the mediastinum, as well as in cervical, retroperitoneal and axillary regions. The disease is classified into two major types: unicentric CD (UCD) and multicentric CD. The occurrence of UCD in the retroperitoneal peripancreatic region is quite rare. We encountered two cases of retroperitoneal peripancreatic UCD in our hospital during the past three years. Following a series of medical examinations, including magnetic resonance imaging, computed tomography, ultrasonography and postoperative histopathological examination, these two patients were diagnosed with UCD, which presented as a retroperitoneal peripancreatic mass. The mass in each patient was completely excised, and no postoperative radiochemotherapy was administered. Both patients recovered well without recurrence during a follow-up period of 30 mo and 8 mo.
Core tip: We report two typical cases of unicentric Castleman disease (UCD) presenting as a retroperitoneal peripancreatic mass. The clinical manifestations and imaging findings of these masses were nonspecific. UCD is difficult to diagnose prior to surgery, and the diagnosis is mainly by postoperative pathological examination. The occurrence of UCD in the abdominal cavity is very rare, especially in the retroperitoneal peripancreatic region. Importantly, both patients recovered well without postoperative radiochemotherapy. These case reports provide a valuable reference for the diagnosis and treatment of this disease.
- Citation: Cheng JL, Cui J, Wang Y, Xu ZZ, Liu F, Liang SB, Tian H. Unicentric Castleman disease presenting as a retroperitoneal peripancreatic mass: A report of two cases and review of literature. World J Gastroenterol 2018; 24(34): 3958-3964
- URL: https://www.wjgnet.com/1007-9327/full/v24/i34/3958.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i34.3958
Castleman disease (CD) is generally identified as a polyclonal lymphoid proliferation of unknown etiology, and is also known as angiofollicular lymph node hyperplasia. It was first described by Benjamin Castleman in 1954 as “localized mediastinal lymph node hyperplasia resembling thymoma”, and was named after him[1]. This disease can occur in any age group, but is mostly seen in patients aged 30-50 years, with no significant gender differences[2]. Clinically, the disease is divided into two major types: unicentric CD (UCD) and multicentric CD, based on its locations at a single lymph node and a series of lymph nodes, respectively[3]. CD, which develops in all lymph nodes, especially in the mediastinum, can also occur in the cervical, retroperitoneal, and axillary regions. However, invasion into the retroperitoneal peripancreatic region is quite rare[4]. We herein report two rare cases of retroperitoneal peripancreatic UCD over a three-year period from 2014 to 2017.
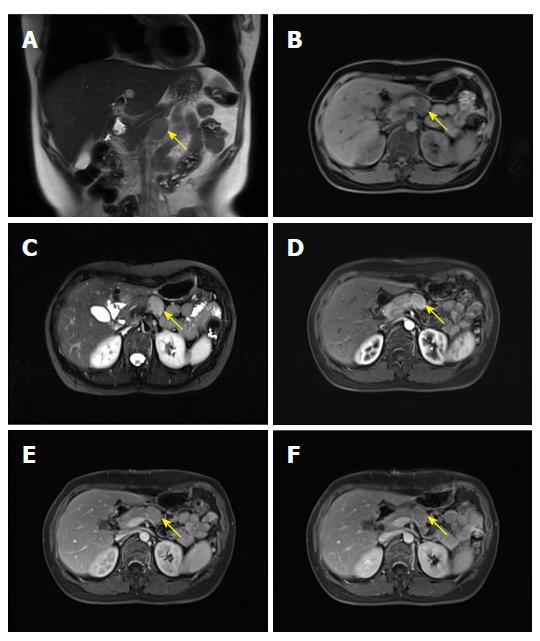
A 48 year old woman was referred to our department on April 28, 2014 for a detailed physical examination due to retroperitoneal peripancreatic lymph node enlargement over seven days. The patient initially had no apparent discomfort or noteworthy positive signs. Magnetic resonance imaging (MRI) of the epigastrium showed an elliptical hyperintense signal below the pancreas on the T1 weighted image (T1WI) (Figure 1B). The size of the lesion was 3.0 cm × 1.9 cm with a clear boundary, with slight hyperintensity on the fat-suppression T2 weighted image (T2WI) (Figure 1C). In addition, marked enhancement on the arterial phase (Figure 1D and E) and reduced enhancement on the delayed phase (Figure 1F) were observed, which were probably due to lymph node enlargement. Laboratory examinations revealed that the patient was negative for the human immunodeficiency virus (HIV) antibody. Tumor markers and other laboratory test results were also within the normal range.
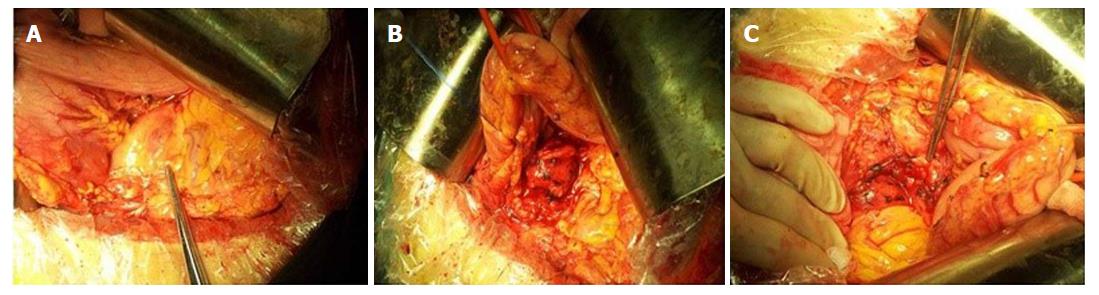
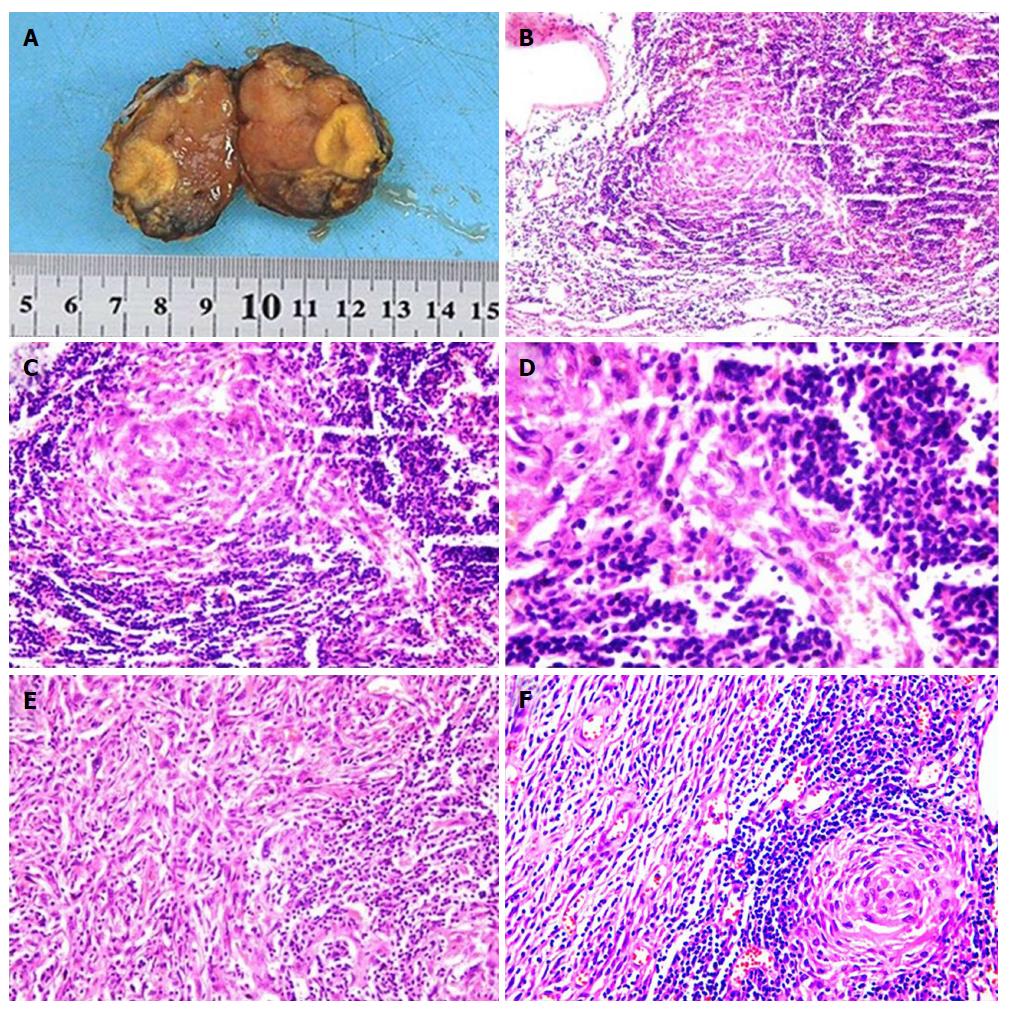
Based on the above results, a retroperitoneal peripancreatic mass was diagnosed preoperatively. Laparotomy was performed through an oblique incision along the left lower rib, and no ascites were found. When the gastrocolic ligament was incised, a tumor was discovered closely adhered to the lower edge of the pancreas (Figure 2A). The tumor volume was 3.0 cm × 2.0 cm × 2.0 cm, had a soft flexible texture and a clear boundary (Figure 2B). The tumor was completely removed with blunt and sharp separation for further testing (Figure 2C). Lymphoid tissue hyperplasia was identified in a frozen biopsy. Postoperative pathology indicated that the retroperitoneal peripancreatic single lymph node was compatible with CD (Figure 3B-D). Immunohistochemical staining revealed that cells were slightly positive for CD20, CD3, CD21, bcl-6, and bcl-2, a small number of cells were positive for CD138, and 20% of cells were positive for Ki-67. During the follow-up at 30 mo after surgery, the patient was recovering well with no signs of recurrence.
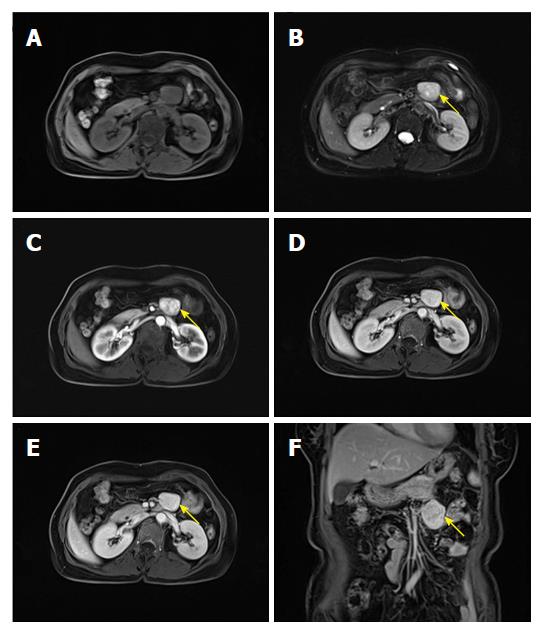
A 57 year old woman was admitted to our hospital on July 18, 2017 because of tiredness and fever. The patient had a body temperature of 39 degrees without other discomfort. Physical examination indicated no positive symptoms. MRI of the epigastrium showed that there was an elliptical hyperintense signal below the pancreas on the T1WI (Figure 4A) and slight hyperintensity on the fat-suppression T2WI (Figure 4B). The internal signal was heterogeneous and a clearly bounded tumor 3.4 cm × 2.8 cm in size was observed. Marked enhancement on the arterial phase was inhomogeneous (Figure 4C). In addition, the venous phase and delayed phase continued to intensify (Figure 4D-F). On account of the hypervascular mass located under the pancreas, it was highly possible that the mass was a nerve incretory tumor.
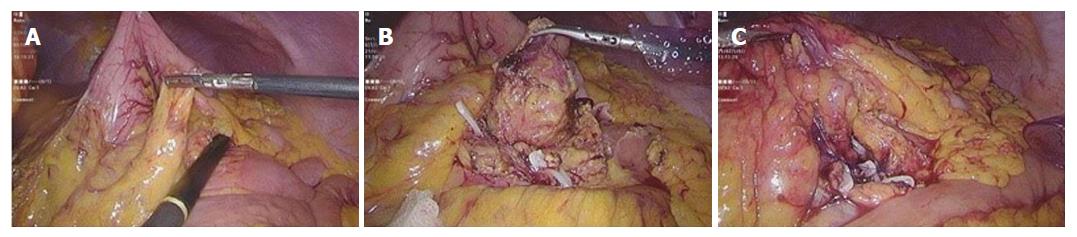
Laboratory examinations showed that the HIV antibody was negative and the white blood cell count was 14.6 × 109/L. Tumor markers and other laboratory test results were within the normal range. The preoperative diagnosis was an retroperitoneal neuroendocrine tumor. Laparoscopic surgery was performed to remove the retroperitoneal tumor. No ascitic fluid or obvious conglutination were found. When the gastrocolic ligament was incised, the lesion was located behind and below the transverse mesocolon. Intraoperative ultrasonography indicated that the tumor had an abundant blood supply, and its right side was close to the inferior mesenteric artery and vein (Figure 5A). The flexible tumor was 3.0 cm × 3.0 cm in size with a clear border (Figure 5B). The tumor was completely removed by blunt and sharp separation for further analysis (Figure 5C). Rapid histopathological examination revealed that the mass was possibly retroperitoneal CD together with a follicular dendritic cell tumor, which was confirmed by subsequent postoperative pathology (Figure 6B-F). Immunohistochemical staining showed that a portion of cells were positive for CD21 and negative for CK, a small number of cells were positive for S-100 and slightly positive for CD1a and CD23, and about five percent of cells were positive for Ki67. The patient recovered well after surgery without chemotherapy, and no relapse was found during the follow-up period.
CD is a rare lymphoproliferative disease with a favorable prognosis; however, the pathogenesis of this disease is unclear. Munshi et al[5] showed that the incidence of CD is approximately 21/million. This disease can occur in all lymph nodes, especially in the mediastinum, neck, retroperitoneum and axillary regions; however, its occurrence in the retroperitoneal peripancreatic region is more rare[4]. The etiology of CD is thought to be related to herpes simplex virus 8 and HIV[6-7]. Another report confirmed that the pathogenesis of CD was closely related to the proliferation of T cells and B cells stimulated by interleukin 6[8]. CD mainly manifests as a painless enlargement of deep or superficial lymph nodes, and may be accompanied by fever, fatigue, anemia, night sweats, and an accelerated erythrocyte sedimentation rate[9]. The two cases in this report showed some differences in clinical manifestation. The tumor in Case 1 was found by physical examination without apparent patient discomfort, and the other was diagnosed by the symptoms of fever and fatigue. In addition, there was no obvious abdominal discomfort in either patient.
Retroperitoneal peripancreatic UCD is usually concealed and difficult to diagnose. In addition, there is no specific manifestation to distinguish it from a neuroendocrine tumor, paraganglioma, or lymphoma[10]. Imaging manifestations of the disease are very difficult to distinguish from other diseases, and the preoperative imaging diagnosis does not usually agree with the postoperative histopathological diagnosis. These difficulties can lead to misdiagnosis. Computed tomography (CT), MRI, and ultrasound can provide reference values for the diagnosis of retroperitoneal UCD. On CT imaging, retroperitoneal UCD is generally a well-defined mass with different morphologies, such as elliptical, kidney-shaped, and dumbbell-shaped, which can easily be distinguished from a spherical paraganglioma. In these two cases, both masses were oval in shape. The plain CT scan images of this disease show low or equal density lesions. The majority of lesions exhibit inhomogeneous enhancement on enhanced CT images. In addition, some tumors have a rich vascular supply, accompanied by microcalcifications[11]. On the MRI, most retroperitoneal UCD lesions show an iso-intense or hypo-intense signal on T1WI, and a slight hyperintense or hyperintense signal on T2WI. Although the enhanced scan characteristics are similar in both CT and MRI, MRI is superior to CT in terms of identifying the relationship between the mass and neighboring vessels or tissues[12]. In addition, ultrasound also has some advantages in estimating the location of the mass and its adjacent feeding vessels. In Case 2 in our report, we quickly clarified the location of the mass and its relationship with surrounding blood vessels (such as the inferior mesenteric artery and vein) using intraoperative ultrasonography, effectively reducing the risk of bleeding during resection of the mass and significantly improving the safety of surgery.
Due to the unclear specificity in clinical manifestations and imaging findings, the diagnosis of retroperitoneal peripancreatic UCD is still dependent on histopathology. In addition, different pathological types also have a direct impact on the treatment and prognosis of the disease. In general, CD can be classified into three types based on pathological characteristics: hyaline vascular type (HV), plasma cell type (PC) and mixed type. The percentage of HV type and PC type is approximately 90% and 10%, respectively. Mixed type cases are rarely reported. Most HV type cases manifest as UCD, while PC type cases usually occur in the form of multicentric CD[13]. In microscopic images of HV type, the centers of the huge folliculus lymphaticus are atrophied, blood vessels degenerate with hyaline vascular changes during this period, and a portion have an onion-like skin appearance[2,14-15]. In microscopic images of PC type CD, the plasma cells are mainly spread throughout the interfollicular areas[16]. Pathologically, the two cases in this report were HV type UCD. In Case 1, mantle cell hyperplasia appeared as an onion-like skin surrounding a neurodegenerative vascularized germinal center. In Case 2, the single penetrated vessel appeared as a lollipop shape with hyaline degeneration.
At present, complete resection of the tumor is still the most effective treatment for retroperitoneal UCD with low recurrence and high cure rates[17]. However, recurrence has been reported in some cases, and both radiotherapy and chemotherapy were unsatisfactory for recurrent UCD[18]. Fortunately, no recurrence of retroperitoneal UCD was found in either of our two cases after surgery. During the resection of retroperitoneal UCD, special attention should be paid to the affected area between the tumor and surrounding vessels, tissues and organs, which is conducive to the complete resection of tumor and reducing blood loss during surgery. In Case 2, we performed laparoscopic resection of the tumor, which has the advantages of reduced surgical trauma, clear intraoperative vision, reduced intraoperative bleeding, high safety, and faster postoperative recovery. In Case 2, we found UCD together with follicular dendritic cell tumor (FDCT), which is a rare low-grade malignant tumor and may arise from CD[19]. However, it was difficult to determine whether the FDCT in Case 2 was secondary to CD. Surgical resection is the first choice in the treatment of FDCT, and recurrence has been observed after surgery. Adjuvant radiotherapy and chemotherapy can be followed after surgery, especially in patients with unresectable FDCT[20]. Case 2 did not undergo radiochemotherapy after surgery. We believe that the treatment of this patient was reasonable. In this report, we described two rare cases of retroperitoneal peripancreatic UCD, which will contribute to an improvement in the understanding of UCD and provide a significant reference for future cases.
Case 1 is a 48 year old female patient who presented with lymph node enlargement; and Case 2 is a 57 year old female patient who presented with tiredness and fever.
In Case 1, the patient initially had no apparent discomfort or noteworthy positive physical signs. In Case 2, the patient felt tired and had a fever with no other discomfort.
Neuroendocrine tumor, paraganglioma and lymphoma.
In Case 1, laboratory examinations revealed that the HIV antibody was negative. Carbohydrate antigen 19-9, carcinoembryonic antigen and other laboratory test results were also within the normal range. In Case 2, laboratory examinations showed that the HIV antibody was negative and the white blood cell count was 14.6 × 109/L. Carbohydrate antigen 19-9, carcinoembryonic antigen and other laboratory test results were also within the normal range.
In Case 1, MRI of the epigastrium showed that there was an elliptical hyperintense signal below the pancreas on the T1WI, and slight hyperintensity on the T2WI. In addition, marked enhancement on the arterial phase and reduced enhancement on the delayed phase were observed. In Case 2, MRI of the epigastrium showed that there was an elliptical hyperintense signal below the pancreas on the T1WI and slight hyperintensity on the T2WI. Marked enhancement on the arterial phase was inhomogeneous. In addition, the venous phase and delayed phase continued to intensify.
In Case 1, the retroperitoneal single lymph node was compatible with Castleman disease. In addition, immunohistochemical staining revealed that cells were slightly positive for CD20, CD3, CD21, bcl-6, and bcl-2, a small number of cells were positive for CD138, and 20% of cells were positive for Ki-67. In Case 2, the diagnosis was retroperitoneal Castleman disease together with a follicular dendritic cell tumor. Immunohistochemical staining showed that a portion of cells were positive for CD21 and cells were negative for CK, a small number of cells were positive for S-100 and slightly positive for CD 1a and CD23, and about five percent of cells were positive for Ki-67.
In Case 1, laparotomy was performed to remove the retroperitoneal peripancreatic tumor without subsequent radiochemotherapy. In Case 2, laparoscopic surgery was performed to remove the retroperitoneal peripancreatic tumor without subsequent radiochemotherapy.
Unicentric Castleman disease (UCD) is a rare disease of lymph nodes and related tissues, and occurrence in the retroperitoneal peripancreatic region is quite rare. Only a few cases of UCD together with follicular dendritic cell tumor have been identified. At present, complete resection of the tumor is still the most effective treatment for retroperitoneal peripancreatic UCD with low recurrence and high cure rates.
Follicular dendritic cell tumor is an uncommon neoplasm that has features of follicular dendritic cell differentiation, and typically presents as a slow-growing, painless mass without systemic symptoms. The tumor can occur on various parts of the body such as lymph nodes, tonsils, armpits and mediastinum, but is most common in the neck lymph nodes.
At present, complete resection of the tumor is still the most effective treatment for retroperitoneal peripancreatic UCD with low recurrence and high cure rates. The diagnosis of retroperitoneal peripancreatic UCD is still dependent on histopathology. The two rare cases of retroperitoneal peripancreatic UCD included in this report will improve our understanding of this disease and provide a significant reference for future cases.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
P- Reviewer: Govindarajan GK, Lee WJ S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9:822-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 2. | Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255:677-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Chan KL, Lade S, Prince HM, Harrison SJ. Update and new approaches in the treatment of Castleman disease. J Blood Med. 2016;7:145-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Charalabopoulos A, Misiakos EP, Foukas P, Tsapralis D, Charalampopoulos A, Liakakos T, Macheras A. Localized peripancreatic plasma cell Castleman disease. Am J Surg. 2010;199:e51-e53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Munshi N, Mehra M, van de Velde H, Desai A, Potluri R, Vermeulen J. Use of a claims database to characterize and estimate the incidence rate for Castleman disease. Leuk Lymphoma. 2015;56:1252-1260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Pria AD, Pinato D, Roe J, Naresh K, Nelson M, Bower M. Relapse of HHV8-positive multicentric Castleman disease following rituximab-based therapy in HIV-positive patients. Blood. 2017;129:2143-2147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Siegel MO, Ghafouri S, Ajmera R, Simon GL. Immune reconstitution inflammatory syndrome, human herpesvirus 8 viremia, and HIV-associated multicentric Castleman disease. Int J Infect Dis. 2016;48:49-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Musters A, Assaf A, Gerlag DM, Tak PP, Tas SW. Discovery of Innovative Therapies for Rare Immune-Mediated Inflammatory Diseases via Off-Label Prescription of Biologics: The Case of IL-6 Receptor Blockade in Castleman’s Disease. Front Immunol. 2015;6:625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Talarico F, Negri L, Iusco D, Corazza GG. Unicentric Castleman’s disease in peripancreatic tissue: case report and review of the literature. G Chir. 2008;29:141-144. [PubMed] [Cited in This Article: ] |
| 10. | Stevens EA, Strowd RE, Mott RT, Oaks TE, Wilson JA. Angiofollicular lymph node hyperplasia resembling a spinal nerve sheath tumor: a rare case of Castleman’s disease. Spine J. 2009;9:e18-e22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Shah D, Darji P, Lodha S, Bolla S. Unicentric Castleman’s disease of abdomen. J Radiol Case Rep. 2013;7:26-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Dong A, Dong H, Zuo C. Castleman disease of the porta hepatis mimicking exophytic hepatocellular carcinoma on CT, MRI, and FDG PET/CT. Clin Nucl Med. 2014;39:e69-e72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Ghosh A, Pradhan SV, Talwar OP. Castleman’s disease - hyaline vascular type - clinical, cytological and histological features with review of literature. Indian J Pathol Microbiol. 2010;53:244-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Hölzle F, Landers A, Platz-Baudin C, Basrei D, Wolff KD. [Castleman’s disease]. Mund Kiefer Gesichtschir. 2005;9:121-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Schaefer IM, Günnel H, Schweyer S, Korenkov M. Unicentric castleman’s disease located in the lower extremity: a case report. BMC Cancer. 2011;11:352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Semin Diagn Pathol. 2016;33:294-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Xu J, Zhou BO, Cao HL, Wang BO, Yan S, Zheng SS. Surgical management of isolated retroperitoneal Castleman’s disease: A case report. Oncol Lett. 2016;11:2123-2126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Baek HJ, Kook H, Han DK, Shin MG, Kim HS, Hwang TJ. Unicentric Castleman disease relapsed after rituximab-CHOP chemotherapy or radiation therapy in an adolescent. J Pediatr Hematol Oncol. 2012;34:e206-e208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Jain P, Prieto VG, Manning JT, Fowler N, Medeiros LJ, Kanagal-Shamanna R. Synchronous presentation of intra-nodal follicular dendritic cell sarcoma and Castleman disease. Am J Hematol. 2017;92:478-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. 2017;178:403-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |