Published online Aug 28, 2018. doi: 10.3748/wjg.v24.i32.3663
Peer-review started: May 31, 2018
First decision: July 4, 2018
Revised: July 6, 2018
Accepted: July 16, 2018
Article in press: July 16, 2018
Published online: August 28, 2018
To determine whether fasting C-peptide is an independent predictor for non-alcoholic fatty liver disease (NAFLD) in United States population.
Using the National Health and Nutrition Examination Survey (NHANES) 1988-1994, NAFLD participants aged 20 or greater without any other liver diseases were included in this study. Excessive alcohol intake is defined as > 2 drinks per day for males and > 1 drink per day for females. C-peptide and 27 other factors known to be associated with NAFLD (e.g., age, gender, body mass index, waist circumference, race/ethnicity, liver chemistries, and other diabetes tests) were tested in both univariate and multivariate level using logistic regression with a P-value 0.05.
Of 18825 participants aged ≥ 20, 3235 participants (n = 3235) met inclusion criteria. There were 23 factors associated with NAFLD by univariate analysis. 9 factors, ranked by the highest change in pseudo R2, were found to be significant predictors of NAFLD in multivariate model: waist circumference, fasting C-peptide, natural log of alanine aminotransferase (ALT), total protein, being Mexican American, natural log of glycated hemoglobin, triglyceride level, being non-Hispanic white, and ferritin level.
Together with waist circumference and ALT, fasting C-peptide is among three most important predictors of NAFLD in United States population in the NHANES data set. Further study is needed to validate the clinical utility of fasting C-peptide in diagnosis or monitoring insulin resistance in NAFLD patients.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a growing global epidemic and associated with many conditions and factors, including insulin resistance. However, C-peptide has not been used in practice to assess insulin resistance in NAFLD patients. Using a large national dataset, we demonstrated that three most important risk factors for NAFLD are waist circumference, fasting C-peptide, and alanine aminotransferase, respectively. Such results revealed that C-peptide superior to measurement of fasting insulin levels and can potentially be used for screening or monitoring the degree of insulin resistance in NAFLD.
- Citation: Atsawarungruangkit A, Chenbhanich J, Dickstein G. C-peptide as a key risk factor for non-alcoholic fatty liver disease in the United States population. World J Gastroenterol 2018; 24(32): 3663-3670
- URL: https://www.wjgnet.com/1007-9327/full/v24/i32/3663.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i32.3663
Non-alcoholic fatty liver disease (NAFLD) is a condition in which hepatic steatosis exists in the absence of excessive alcohol consumption. NAFLD is the most common cause of chronic liver disease in the United States with estimated prevalence around 30%-40%[1-3]. Given the epidemic of obesity, NAFLD is increasingly prevalent and challenging[4,5]. NAFLD can progress to more severe liver diseases, such as non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma.
Obesity and insulin resistance are among important risk factors for NAFLD[6,7]. Many studies found that indicators of obesity [i.e., body mass index (BMI) and waist circumference] and insulin resistance [i.e., glycated hemoglobin (HbA1c), insulin level, fasting glucose, and diabetes mellitus] are independently associated with NAFLD and/or severity of liver fibrosis in NAFLD[8-11]. C-peptide levels can be used to measure insulin secretion[12]. However, there is limited evidence of the association between NAFLD and C-peptide at the multivariate level[13,14].
Both C-peptide and insulin are produced and released in equimolar amounts. C-peptide can therefore be used to assess endogenous insulin secretion. However, the level of C-peptide and insulin level in blood are typically different deriving from the differences in clearance mechanisms and half-life[15]. In addition to diabetes and insulin resistance, C-peptide has been associated with many risk factors for NAFLD including cardiovascular diseases and metabolic syndrome[16-18].
Therefore, our primary objective was to determine if fasting C-peptide is independently associated with NAFLD using multivariate analysis in the United States general population.
The Third National Health and Nutrition Examination Survey (NHANES III) is a probability sample of 39695 persons aged 2 mo and older representing the United States population and conducted by the National Center for Health Statistics (NCHS) to evaluate health and nutritional status[19]. The survey collected multiple data sets, including demographic, interviews, physical examinations, and laboratory testing of biologic samples. NHANES III was conducted from 1988 to 1994. The NHANES protocol was approved by the NCHS Research Ethics Review Board.
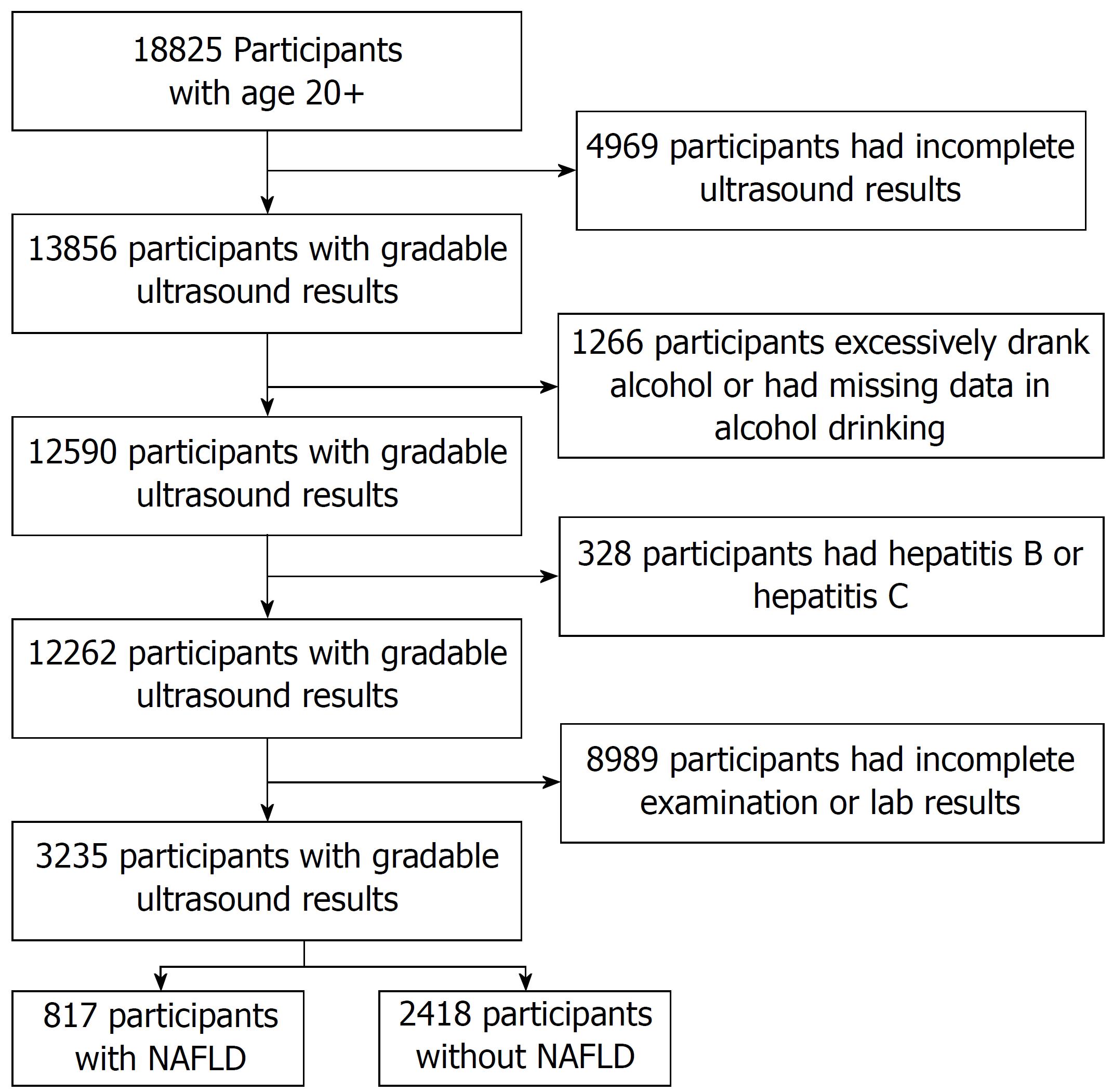
There were 18825 persons aged 20 years or older in NHANES III that met inclusion criteria for this study. The exclusion criteria included: (1) Ungradable or missing ultrasound results for hepatic steatosis, (2) excessive alcohol consumption, (3) hepatitis B or hepatitis C infection (4) fasting period outside of 8-24 h (5) incomplete or missing data on physical examination and laboratory testing. Participants were divided into two groups: NAFLD participants (study group) and non-NAFLD participants (control group).
As presented in Table 1, we included 28 factors associated with NAFLD as independent variables in this study: Demographic (i.e., age , gender, race/ethnicity), body measurement (i.e., BMI and waist circumference), general biochemistry tests [i.e., iron, total iron-binding capacity (TIBC), transferrin saturation, ferritin, cholesterol, triglyceride, HDL cholesterol, C-reactive protein, and uric acid], liver chemistry [aspartate aminotransferase (AST), Alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin, total protein, and albumin], and diabetes testing profile [i.e., HbA1c, fasting plasma glucose (FPG), fasting C-peptide, and fasting insulin]. Besides demographic variables, the above variables were selected as the risk factors based on the usage in clinical practice and the supporting evidence that demonstrated the association with NAFLD or its commonly accepted risk factors (i.e., obesity, insulin resistance, and liver fibrosis).
| NAFLD, n = 817 | Controls, n = 2418 | |
| Demographic | ||
| Age (yr) | 48.47 ± 15.75 | 44.36 ± 16.20 |
| Gender (male) | 368 (45.04) | 1004 (41.52) |
| Race/ethnicity | ||
| White (non-Hispanic) | 308 (37.70) | 1046 (43.13) |
| Black (non-Hispanic) | 193 (23.62) | 705 (29.16) |
| Mexican American | 280 (34.27) | 550 (22.75) |
| Others | 36 (4.41) | 120 (4.96) |
| Body measurement | ||
| Body mass index (kg/m2) | 30.38 ± 6.95 | 26.56 ± 5.42 |
| Waist circumference (cm) | 101.73 ± 16.32 | 90.84 ± 13.45 |
| Biochemistry tests | ||
| Iron (μg/dL) | 75.35 ± 29.71 | 77.71 ± 32.75 |
| TIBC (μg/dL) | 364.79 ± 58.05 | 359.86 ± 56.59 |
| Transferrin saturation (%) | 21.13 ± 8.73 | 22.09 ± 9.65 |
| Ferritin (ng/mL) | 161.75 ± 152.55 | 110.16 ± 114.71 |
| Cholesterol (mg/dL) | 212.23 ± 44.95 | 202.73 ± 42.19 |
| Triglyceride (mg/dL) | 202.91 ± 137.97 | 136.58 ± 95.79 |
| HDL cholesterol (mg/dL) | 46.72 ± 16.80 | 52.10 ± 15.61 |
| C-reactive protein (mg/dL)1 | 0.56 ± 0.80 | 0.45 ± 0.65 |
| Uric acid (mg/dL) | 5.62 ± 1.52 | 5.04 ± 1.42 |
| Liver chemistry | ||
| AST (U/L)1 | 24.76 ± 19.62 | 20.72 ± 14.71 |
| ALT(U/L)1 | 22.78 ± 17.86 | 15.96 ± 12.14 |
| GGT (U/L)1 | 42.87 ± 66.68 | 28.14 ± 41.69 |
| ALP (U/L)1 | 93.72 ± 33.61 | 86.04 ± 36.29 |
| Total bilirubin (mg/dL) | 0.55 ± 0.30 | 0.54 ± 0.28 |
| Total protein (g/dL) | 7.49 ± 0.46 | 7.37 ± 0.45 |
| Albumin (g/dL) | 4.11 ± 0.35 | 4.12 ± 0.36 |
| Diabetes testing profile | ||
| HbA1c (%)1 | 6.02 ± 1.62 | 5.50 ± 1.09 |
| FPG (mg/dL)1 | 114.50 ± 65.84 | 97.85 ± 35.68 |
| Fasting C-peptide (pmol/mL) | 1.11 ± 0.68 | 0.69 ± 0.53 |
| Fasting insulin (μU/mL)1 | 21.85 ± 27.82 | 12.76 ± 19.24 |
In this study, NAFLD is defined as: (1) Diagnosed with moderate to severe hepatic steatosis on ultrasound; (2) no history of excessive alcohol intake in the past 12 mo; (3) not infected with hepatitis B or hepatitis C.
To evaluate the presence and extent of hepatic steatosis, readers used five main criteria: (1) Parenchymal brightness, (2) liver to kidney contrast, (3) deep beam attenuation, (4) bright vessel walls, and (5) gallbladder wall definition. Based on the presence or absence of these five criteria, a main finding was categorized as normal, mild, moderate or severe[20]. It is worth nothing that participants aged above 74 were not eligible for ultrasound study in NHANES III For this reason, patients age above 74 were excluded from this study.
Excessive alcohol intake is defined as more than 2 drinks per day for men or 1 drink per day for women in the past 12 mo, in which one drink of alcoholic beverage is equivalent to a 12 oz beer, a 5 oz glass of wine, or 1.5 oz of liquor. The average number of drinks per day is calculated from number of drinking days × number of drinks on drinking day/365 d. To qualify as hepatitis viral infection, participants must have tested positive for serum hepatitis B surface antigen or serum hepatitis C antibody HCP (anti-HCV).
Statistical analyses were performed using STATA Release 14 (StataCorp LP, TX, United States). Numbers are presented in mean ± SD or number (%). All continuous factors were first tested for skewness; if the distributions were extremely skewed to the right (herein defined as skewness > 3), the factors were log transformed before using them as predictors in regression models. Since the response variable is dichotomous variable (NAFLD or non-NAFLD), logistic model is an appropriate model for determining if predictors are significantly associated with the response variable. As a result, logistic regression was used to determine if NAFLD is associated with any predictor in univariate level. Then, the significant factors from univariate analysis were included as predictors in step-wise logistic regression to determine the significant predictors in multivariate level. The significance level is 0.05.
Out of 18825 participants aged ≥ 20, there were 3235 participants (n = 3235) that passed the exclusion criteria as shown in Figure 1. Based on ultrasound findings, 817 (25.26%) participants were classified as NAFLD. Baseline characteristics of participants in study group and control group are summarized in Table 1.
For continuous variables, there were 8 factors having skewness greater than 3. Subsequently, the log transformation was applied to these factors, including C-reactive protein, AST, ALT, GGT, alkaline phosphatase, glycated hemoglobin, plasma glucose, and insulin. As shown in Table 2, there are 24 variables significantly associated with NAFLD in univariate level; the P-value of these significant factors mostly below 0.001.
| Beta | Standard error | Odds ratio | P value | |
| Demographic | ||||
| Age (yr) | 0.0157 | 0.0025 | 1.02 | < 0.001a |
| Gender (male) | 0.1435 | 0.0815 | 1.15 | 0.078 |
| Race/ethnicity | ||||
| White (non-Hispanic) | -0.226 | 0.0831 | 0.80 | 0.007a |
| Black (non-Hispanic) | -0.2857 | 0.0937 | 0.75 | 0.002a |
| Mexican American | 0.5715 | 0.0882 | 1.77 | < 0.001a |
| Others | -0.1248 | 0.1945 | 0.88 | 0.521 |
| Body measurement | ||||
| Body mass index (kg/m2) | 0.1004 | 0.0069 | 1.11 | < 0.001a |
| Waist circumference (cm) | 0.0506 | 0.003 | 1.05 | < 0.001a |
| Biochemistry tests | ||||
| Iron (μg/dL) | -0.0024 | 0.0013 | 1.00 | 0.069 |
| TIBC (μg/dL) | 0.0015 | 0.0007 | 1.00 | 0.032a |
| Transferrin saturation (%) | -0.0111 | 0.0044 | 0.99 | 0.012a |
| Ferritin (ng/mL) | 0.0029 | 0.0003 | 1.00 | < 0.001a |
| Cholesterol (mg/dL) | 0.005 | 0.0009 | 1.01 | < 0.001a |
| Triglyceride (mg/dL) | 0.0049 | 0.0004 | 1.00 | < 0.001a |
| HDL cholesterol (mg/dL) | -0.0261 | 0.0031 | 0.97 | < 0.001a |
| C-reactive protein (mg/dL)1 | 0.3398 | 0.0534 | 1.40 | < 0.001a |
| Uric acid (mg/dL) | 0.2649 | 0.0277 | 1.30 | < 0.001a |
| Liver chemistry | ||||
| AST (U/L)1 | 1.004 | 0.1153 | 1.02 | < 0.001a |
| ALT (U/L)1 | 1.0274 | 0.0777 | 1.04 | < 0.001a |
| GGT (U/L)1 | 0.7441 | 0.0612 | 1.01 | < 0.001a |
| ALP (U/L)1 | 0.9011 | 0.1303 | 1.01 | < 0.001a |
| Total bilirubin (mg/dL) | 0.0914 | 0.1395 | 1.09 | 0.512 |
| Total protein (g/dL) | 0.581 | 0.0896 | 1.79 | < 0.001a |
| Albumin (g/dL) | -0.0871 | 0.1141 | 0.92 | 0.445 |
| Diabetes testing profile | ||||
| HbA1c (%)1 | 2.2201 | 0.2184 | 1.34 | < 0.001a |
| FPG (mg/dL)1 | 1.3125 | 0.1428 | 1.01 | < 0.001a |
| Fasting C-peptide (pmol/mL) | 1.1976 | 0.0753 | 3.31 | < 0.001a |
| Fasting insulin (μU/mL)1 | 0.9646 | 0.059 | 1.02 | < 0.001a |
As presented in Table 3, the number of significant factors reduced from 24 to 9 in multivariate analysis. The top three factors ranked by the highest change in pseudo R2 (ΔR2) are waist circumference (OR = 1.03, ΔR2 = 2.13%, P < 0.001), C-peptide level (OR = 1.82, ΔR2 = 1.33%, P < 0.001), and loge of ALT (OR = 1.76, ΔR2 = 1.16%, P < 0.001). The pseudo R2 of the multivariate model is 16.68%.
| Beta | Standard error | Odds ratio | Change in pseudo R2 | P value | |
| Demographic | |||||
| Race/ethnicity | |||||
| White (non-Hispanic) | 0.2969 | 0.1162 | 1.35 | 0.18% | 0.011 |
| Mexican American | 0.5495 | 0.1207 | 1.73 | 0.57% | 0.020 |
| Body measurement | |||||
| Waist circumference (cm) | 0.0308 | 0.0035 | 1.03 | 2.13% | < 0.001 |
| Biochemistry tests | |||||
| Ferritin (ng/mL) | 0.0013 | 0.0004 | 1.00 | 0.15% | < 0.001 |
| Triglyceride (mg/dL) | 0.0008 | 0.0004 | 1.00 | 0.32% | < 0.001 |
| Liver chemistry | |||||
| ALT (U/L)1 | 0.5658 | 0.0875 | 1.76 | 1.16% | < 0.001 |
| Total protein (g/dL) | 0.5319 | 0.1045 | 1.70 | 0.72% | < 0.001 |
| Diabetes testing profile | |||||
| HbA1c (%)1 | 0.9266 | 0.2492 | 2.53 | 0.38% | < 0.001 |
| Fasting C-peptide (pmol/mL) | 0.6009 | 0.0877 | 1.82 | 1.33% | < 0.001 |
The most significant NAFLD risk factor in both univariate and multivariate levels is waist circumference. Since waist circumference and BMI are highly interrelated surrogate markers of obesity[21], it is not surprising to see one factor eliminated in multivariate level. Waist circumference-a measure of excess abdominal adiposity-has been identified as an independent risk factor for many obesity-related conditions, such as cardiovascular disease, type 2 diabetes, dyslipidemia, and hypertension, metabolic syndrome, polycystic ovary syndrome[22-27]. The results from this study support that waist circumference is also an independent and probably the most important risk factor for NAFLD in the United States population.
Insulin resistance is another well-known condition commonly found in NAFLD patients[28,29]. Indeed, all diabetes test profiles were positively correlated with NAFLD by univariate analysis. In fact, type 2 diabetes can be diagnosed directly from HbA1c level (≥ 6.5%) or FPG (≥ 126 mg/dL)[30], while C-peptide and insulin are not routinely used in clinical practice to diagnose type 2 diabetes. While there are situations where C-peptide or insulin levels are useful-the diagnosis of insulinoma[31], surreptitious use of insulin[32], the diagnosis of type 2 diabetes in the young[33], and the diagnosis of latent autoimmune diabetes in adults[34], direct measurement of insulin levels and not of C-peptide has been used to assess insulin resistance. Previous studies often found that C-peptide levels are raised in patients with NASH[35-37]. However, the application of C-peptide as a biomarker for interventions designed to improve insulin sensitivity remains to be determined. In case of NAFLD, there is only limited evidence; C-peptide was found to be associated with NAFLD in specific groups of population (i.e., obese adolescents and adults, latent autoimmune diabetes, and diabetes patients)[13,14,38]. Our results are the first to show that C-peptide has a role not only as an independent risk factor for NAFLD but can also be useful for screening or monitoring the degree of insulin resistance in NAFLD in the general population. Based on ∆R2, we conclude that insulin resistance, as indicated by fasting C-peptide, is the second most important condition leading to NAFLD, second only to obesity as diagnosed by waist circumference, and is superior to measurement of fasting insulin levels.
Liver chemistries are used as an indicator of liver inflammation or liver cell damage. Commonly used liver chemistries include AST, ALT, ALP, GGT, total bilirubin, total protein, and albumin. For example, predominance of AST and ALT indicates hepatocellular injury; predominance of ALP and total bilirubin indicates cholestatic injury; an elevated ALP of hepatic origin may be confirmed GGT[39-41]. As shown in Table 2, total protein and the natural log of AST, ALT, ALP, and GGT were positively associated with NAFLD in univariate analysis. However, only total protein and natural log of ALT were positively correlated with NAFLD in multivariate level. AST and ALT are the most widely used liver chemistries. The fact that ALT is included in multivariate model is not unexpected since ALT is generally higher than AST level in NAFLD[40]. On the other hand, total protein is a non-specific marker of health, nutrition and liver synthetic capacity. Due to the fact that total protein consists of albumin and multiple subtypes of globulin, further investigation into the association between NAFLD and each subtype of globulin may provide a clearer explanation of our findings.
Ferritin is a protein that mainly stores iron in the body and serum ferritin level is the most accurate blood test to diagnose iron deficiency anemia[42]. Recently, the role of ferritin as a biomarker in inflammatory diseases has been increasingly recognized[43-45]. As an acute phase response protein, ferritin concentrations increase during inflammation and may not reflect the size of total body iron stores[44]. Moreover, ferritin was found be associated with histologic severity and advanced fibrosis in patients with NAFLD[46-48].
Other factors significantly associated with NAFLD include race/ethnicity (non-Hispanic white and Mexican American), and triglyceride level. Race/ethnicity were often found associated with obesity-related diseases in United States based population[49,50] Triglyceride is an important biomarker of cardiovascular disease risk[51], another condition highly interrelated with NAFLD.
There are several limitations in this study. First, the diagnosis of NAFLD in this study is based on the hepatic ultrasound results although liver biopsy remains the gold standard for the diagnosis of NAFLD. Second, the statistical analysis used is logistic regression. Since the relationship among these factors are complex, interrelated, and non-linear, linearity assumptions embedded in logistic regression may not be able to address all aspects of NAFLD. Furthermore, given a pseudo R2 of 16.68%, only 16.68% of variation can be explained by multivariate model in Table 3.
In conclusion, NAFLD is associated with many conditions and factors. Three most important factors from multivariate model in this study are waist circumference, fasting C-peptide, and ALT. Further study is needed to validate the clinical utility of C-peptide in diagnosis or monitoring insulin resistance in NAFLD patients.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States. Additionally, NAFLD can progress to more severe liver diseases, such as non-alcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma. Many factors were found to be independently associated with NAFLD and/or severity of liver fibrosis in NAFLD. Nevertheless, there is limited evidence of the association between NAFLD and C-peptide.
Among many risk factors that are associated with NAFLD, obesity and insulin resistance are probably the most well-known ones. C-peptide levels can be used to measure insulin secretion and a surrogate marker of insulin resistance. However, C-peptide is not routinely used in clinical practice to diagnose type 2 diabetes or monitor insulin resistance status in NAFLD.
The objective of this study was to determine if fasting C-peptide is independently associated with NAFLD using multivariate analysis in the United States general population.
Using the National Health and Nutrition Examination Survey 1988-1994, NAFLD participants aged 20 or greater without any other liver diseases were included in this study. The participants with excessive alcohol intake (> 2 drinks per day for males and > 1 drink per day for female) were excluded from the study. C-peptide and 27 other factors known to be associated with NAFLD (e.g., age, gender, body mass index, waist circumference, race/ethnicity, liver chemistries, and other diabetes tests) were selected as predictors in regression model. Univariate logistic regression and multivariate step-wise logistic regression were used to determine if the significant predictors of NAFLD, respectively.
There were 3235 participants (n = 3235) that passed the exclusion criteria. Based on ultrasound findings, 817 (25.26%) participants were classified as NAFLD. Twenty-four variables were significantly associated with NAFLD in univariate level; the P-value of these significant factors mostly below 0.001. Using multivariate analysis, we found 9 out of 24 factors to be significantly associated with NAFLD. Ranked by ΔR2, the top three factors ranked are waist circumference (OR = 1.03, ΔR2 = 2.13%, P < 0.001), C-peptide level (OR = 1.82, ΔR2 = 1.33%, P < 0.001), and loge of ALT (OR = 1.76, ΔR2 = 1.16%, P < 0.001). The pseudo R2 of the multivariate model is 16.68%.
C-peptide is the second most important predictor of NAFLD in United States population after waist circumference.
Further prospective research is needed to validate the clinical utility of fasting C-peptide in diagnosis or monitoring insulin resistance in NAFLD patients. Moreover, C-peptide should be considered as a potential factor for calculative liver scores to evaluate the fibrosis level.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Abenavoli L, Gheita TA, Hegardt FG, Tziomalos K S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY
| 1. | Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814-1820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 247] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 3. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1522] [Cited by in F6Publishing: 1520] [Article Influence: 116.9] [Reference Citation Analysis (1)] |
| 4. | Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149:1471-1482.e5; quiz e17-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 5. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1508] [Cited by in F6Publishing: 1574] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 6. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1706] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 7. | Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP. Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Sci Rep. 2018;8:1466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 8. | Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2018] [Article Influence: 118.7] [Reference Citation Analysis (1)] |
| 10. | Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 545] [Cited by in F6Publishing: 553] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 11. | Ma H, Xu C, Xu L, Yu C, Miao M, Li Y. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterol. 2013;13:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41:368-377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 543] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Chen M, Li T, Zhang R, Li L, Lu J, Bao Y, Jia W. [Clinical features of non-alcoholic fatty liver disease and its relationship with serum C-peptide levels in patients with latent autoimmune diabetes in adults]. Zhonghua Yi Xue Za Zhi. 2015;95:3575-3578. [PubMed] [Cited in This Article: ] |
| 14. | Tricò D, Caprio S, Umano GR, Pierpont B, Nouws J, Galderisi A, Kim G, Mata MM, Santoro N. Metabolic Features of Nonalcoholic Fatty Liver (NAFL) in Obese Adolescents: Findings from a Multi-ethnic Cohort. Hepatology. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Leighton E, Sainsbury CA, Jones GC. A Practical Review of C-Peptide Testing in Diabetes. Diabetes Ther. 2017;8:475-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Wang L, Lin P, Ma A, Zheng H, Wang K, Li W, Wang C, Zhao R, Liang K, Liu F. C-Peptide Is Independently Associated with an Increased Risk of Coronary Artery Disease in T2DM Subjects: A Cross-Sectional Study. PLoS One. 2015;10:e0127112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Li Y, Li Y, Meng L, Zheng L. Association between serum C-peptide as a risk factor for cardiovascular disease and high-density lipoprotein cholesterol levels in nondiabetic individuals. PLoS One. 2015;10:e112281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Gonzalez-Mejia ME, Porchia LM, Torres-Rasgado E, Ruiz-Vivanco G, Pulido-Pérez P, Báez-Duarte BG, Pérez-Fuentes R. C-Peptide Is a Sensitive Indicator for the Diagnosis of Metabolic Syndrome in Subjects from Central Mexico. Metab Syndr Relat Disord. 2016;14:210-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | National Center for Health Statistics. Third National Health and Nutrition Examination Survey Data (NHANES III). Accessed May 29 2018 Available from: https://www.cdc.gov/nchs/nhanes/nhanes3.htm. [Cited in This Article: ] |
| 20. | National Center for Health Statistics. Third National Health and Nutrition Examination Survey: Hepatic Steatosis Ultrasound Images Assessment Procedures Manual. Accessed May 29, 2018 Available from: URL: https://www.cdc.gov/nchs/data/nhanes/nhanes3/hepatic_steatosis_ultrasound_procedures_manual.pdf. [Cited in This Article: ] |
| 21. | Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985-3023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1375] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 22. | Dimitriadis K, Tsioufis C, Mazaraki A, Liatakis I, Koutra E, Kordalis A, Kasiakogias A, Flessas D, Tentolouris N, Tousoulis D. Waist circumference compared with other obesity parameters as determinants of coronary artery disease in essential hypertension: a 6-year follow-up study. Hypertens Res. 2016;39:475-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7515] [Cited by in F6Publishing: 7813] [Article Influence: 411.2] [Reference Citation Analysis (0)] |
| 24. | Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1168] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 25. | Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1130] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 26. | Pazderska A, Kyaw Tun T, Phelan N, McGowan A, Sherlock M, Behan L, Boran G, Gibney J. In women with PCOS, waist circumference is a better surrogate of glucose and lipid metabolism than disease status per se. Clin Endocrinol (Oxf). 2018;88:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1333] [Cited by in F6Publishing: 1258] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 28. | Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 29. | Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218:R25-R36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 30. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2986] [Cited by in F6Publishing: 3132] [Article Influence: 313.2] [Reference Citation Analysis (15)] |
| 31. | Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K. Diagnosis and management of insulinoma. World J Gastroenterol. 2013;19:829-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 204] [Cited by in F6Publishing: 215] [Article Influence: 19.5] [Reference Citation Analysis (3)] |
| 32. | Waickus CM, de Bustros A, Shakil A. Recognizing factitious hypoglycemia in the family practice setting. J Am Board Fam Pract. 1999;12:133-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2006-2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabetes. 2006;7:352-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Stenström G, Gottsäter A, Bakhtadze E, Berger B, Sundkvist G. Latent autoimmune diabetes in adults: definition, prevalence, beta-cell function, and treatment. Diabetes. 2005;54 Suppl 2:S68-S72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Francque SM, Verrijken A, Mertens I, Hubens G, Van Marck E, Pelckmans P, Michielsen P, Van Gaal L. Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol. 2012;10:1162-1168; quiz e87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 671] [Cited by in F6Publishing: 668] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 37. | Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, Karim R, Lin R, Samarasinghe D, Liddle C. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 807] [Cited by in F6Publishing: 803] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 38. | Hua X, Li M, Pan F, Xiao Y, Cui W, Hu Y. Non-alcoholic fatty liver disease is an influencing factor for the association of SHBG with metabolic syndrome in diabetes patients. Sci Rep. 2017;7:14532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Kawachi I, Robinson GM, Stace NH. A combination of raised serum AST:ALT ratio and erythrocyte mean cell volume level detects excessive alcohol consumption. N Z Med J. 1990;103:145-148. [PubMed] [Cited in This Article: ] |
| 40. | Kwo PY, Cohen SM, Lim JK. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am J Gastroenterol. 2017;112:18-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 443] [Cited by in F6Publishing: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 41. | Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004;39:336-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 42. | Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87:98-104. [PubMed] [Cited in This Article: ] |
| 43. | Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6:748-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 361] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 44. | Abitbol V, Borderie D, Polin V, Maksimovic F, Sarfati G, Esch A, Tabouret T, Dhooge M, Dreanic J, Perkins G. Diagnosis of Iron Deficiency in Inflammatory Bowel Disease by Transferrin Receptor-Ferritin Index. Medicine (Baltimore). 2015;94:e1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Mecklenburg I, Reznik D, Fasler-Kan E, Drewe J, Beglinger C, Hruz P; Swiss IBD Cohort Study Group. Serum hepcidin concentrations correlate with ferritin in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:1392-1397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Sabrina N, Bai CH, Chang CC, Chien YW, Chen JR, Chang JS. Serum Iron:Ferritin Ratio Predicts Healthy Body Composition and Reduced Risk of Severe Fatty Liver in Young Adult Women. Nutrients. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Parikh P, Patel J, Ingle M, Sawant P. Serum ferritin levels predict histological severity in patients with nonalcoholic fatty liver disease in India. Indian J Gastroenterol. 2015;34:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, Sanyal AJ, Nelson JE; NASH Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 49. | Schmiegelow MD, Hedlin H, Mackey RH, Martin LW, Vitolins MZ, Stefanick ML, Perez MV, Allison M, Hlatky MA. Race and ethnicity, obesity, metabolic health, and risk of cardiovascular disease in postmenopausal women. J Am Heart Assoc. 2015;4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract. 2010;87:415-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 51. | Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626-635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 819] [Cited by in F6Publishing: 841] [Article Influence: 84.1] [Reference Citation Analysis (0)] |