Published online Jul 21, 2018. doi: 10.3748/wjg.v24.i27.2974
Peer-review started: April 13, 2018
First decision: May 9, 2018
Revised: May 26, 2018
Accepted: June 27, 2018
Article in press: June 27, 2018
Published online: July 21, 2018
Nonalcoholic fatty liver disease (NAFLD) has become the dominant form of chronic liver disease in children and adolescents with the increasing prevalence of obesity worldwide. NAFLD represents a wide spectrum of conditions, ranging from fatty liver - which generally follows a benign, non-progressive clinical course - to non-alcoholic steatohepatitis, a subset of NAFLD that may progress to cirrhosis and end-stage liver disease or liver carcinoma. The underlying pathophysiological mechanism of “pediatric” NAFLD remains unclear, although it is strongly associated with obesity and insulin resistance. In this review we provide a general overview on the current understanding of NAFLD in children and adolescents, which underpins practice, enabling early diagnosis and appropriate therapeutic intervention for this life-threatening liver disease.
Core tip: Much work on nonalcoholic fatty liver disease (NAFLD) has been done, but an accurate understanding of its mechanism remains unclear. Our objective was to examine the current literature to better understand the pathogenesis of NAFLD, thus showing how it evolved from the “two-hit theory” to a “multiple hit model”.
- Citation: Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J Gastroenterol 2018; 24(27): 2974-2983
- URL: https://www.wjgnet.com/1007-9327/full/v24/i27/2974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i27.2974
Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, is the most frequent form of chronic liver disease worldwide. Corresponding to abnormal fat accumulation in hepatocytes, it encompasses a spectrum of chronic liver diseases in the absence of excessive alcohol consumption, which may occur with or without hepatocyte inflammation or fibrosis[1]. Isolated steatosis, defined by abnormal accumulation of fat in more than 5% of hepatocytes, is a relatively benign condition.
In contrast, besides steatosis, non-alcoholic steatohepatitis (NASH) coexists with inflammation, hepatic cell injury, and deposition of collagen fibers[2]. NASH is a dynamic condition that can regress to isolated steatosis or cause progressive fibrosis leading to cirrhosis. The prevalence of NAFLD ranges from 10% to 30% depending on the study population and diagnostic methods used and is thought to be increasing worldwide[3,4]. Recently, a meta-analysis showed that the global prevalence of NAFLD is 25.24% (95%CI: 22.10%-28.65%) with the highest prevalence in the Middle East and South America and the lowest in Africa for the year 2016[5]. The “gold standard” test is liver biopsy, but this is neither feasible nor ethical for epidemiological studies aiming to screen NAFLD in the healthy population, and is also problematic in the clinical diagnosis. As so far, there was only one pediatric study which reported prevalence based on liver histology, and it reported that the prevalence of NAFLD in obese children (aged 2-19 years) was 38% and increased with age[6].
Serum biomarkers such as alanine aminotransferase (ALT) and aspartate aminotransferase, as well as liver imaging (liver ultrasound and magnetic resonance imaging), are currently the most widely used tools for screening. Published guidelines vary about the frequency of ALT screening and use of imaging[7-9]. Ongoing developments of new technologies are improving the diagnosis; however, the specificity and sensitivity for the diagnosis of NAFLD have not yet reached an acceptable level.
As mentioned above, NASH is progressive, so early diagnosis and treatments are critical. However, many aspects of the pathogenesis of NAFLD remain unclear; for example, the mechanism of the progression from steatosis to steatohepatitis. It is also unknown why NAFLD occurs only in a subgroup of obese subjects.
In the past decade, our team has researched the causes, pathogenesis, clinical diagnosis, and treatment of NAFLD[10-14]. In this review, we focus on the pathogenesis of NAFLD and explore new ways for improving both the diagnosis and treatment of NASH. The articles cited were identified based on a search of PubMed done in February 2018 using the criteria “NAFLD and pathogenesis and children and adolescent” with studies in humans and animals.
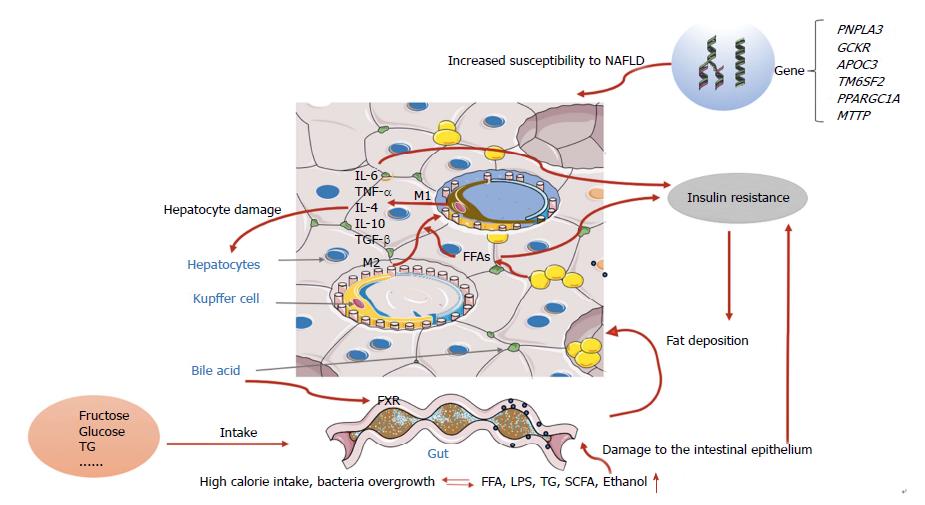
During recent decades, the worldwide prevalence of obesity has increased in the pediatric population and the prevalence of NAFLD has more than doubled during the last 20 years in the United States[14]. The development of NAFLD is strongly influenced by age, sex, and ethnicity, and appears twice as often in boys than in girls[15-18]. NASH can progress to end-stage liver diseases such as hepatic cirrhosis or hepatocellular carcinoma. Conjeevaram PF et al[19] analyzed the database and discovered that as the prevalence of NAFLD increased, the prevalence of NASH also increased, however, compared to adult the prevalence of liver fibrosis in children remained low, which indicated a possibly less aggressive NAFLD phenotype in children. Although the prevalence of NAFLD is increasing, most affected patients present with isolated steatosis with only a minority of cases progressing to liver cirrhosis in children, and it is not clear whether pediatric and adult NAFLD are two different pathologic entities or just age-dependent manifestations of the same disease, which implies that the pathogenesis of NAFLD may be related to the interplay among genetic, environmental, and individual factors. Early theories of the pathogenesis of NAFLD and NASH were described in terms of the “two hit hypothesis”[20]. At the onset of disease, the “first hit” is represented by an increase in liver fat, characterized by hepatic triglyceride accumulation and insulin resistance, and corresponding to hepatic steatosis once the accumulation of hepatic fat is more than 5%. Children, especially pre-pubertal boys, have a pattern of type 2 NAFLD characterized by a zone 1 distribution of steatosis, inflammation and fibrosis[21]. Liver fat accumulation is associated with a hypercaloric diet, sedentary lifestyle, and is perhaps genetically predisposed. Our team successfully established an in vivo NAFLD animal model induced by a high-fat diet, and reported that lifestyle interventions have an effect on NAFLD in obese children[22,23]. Subsequently, the “second hit” emerges, which includes inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress. As the fatty liver is more susceptible to this “second hit”, necroinflammation and fibrosis can develop and ultimately lead to cirrhosis[24,25]. However, with the development of new technology and further research, this view appears too simplistic for recapitulating the complexity of human NAFLD.
Now, the widely accepted theory is the “multiple-hit model”, involving more widespread metabolic dysfunction because of the interaction of genetic and environmental factors as well as changes in crosstalk between different organs and tissues, including adipose tissue, the pancreas, gut, and liver[24-27]. However, liver fat accumulation, caused by obesity and insulin resistance, still seem to represent the “first hits”.
Fat accumulates in the liver of patients with NAFLD mainly in the form of triglycerides, which derive from the esterification of glycerol and free fatty acids (FFAs)[28]. Triglyceride accumulation is not hepatotoxic, in contrast with the excess of FFAs that undergo acetyl coenzyme A (acyl-CoA) synthase activity and form fatty acyl-CoAs which may trigger esterification or β-oxidation pathways[29]. Mitochondrial dysfunction, which consists of oxidative stress and production of reactive oxygen species and endoplasmic reticulum stress-associated mechanisms, also results from NAFLD[30,31].
Physiologically, insulin controls hepatic glucose production by regulating lipolysis of adipocytes, leading to decreased fatty acid flux in the liver[32]. Consequently, the availability of hepatic acetyl coenzyme A (acyl-CoA) concentrations and the activity of pyruvate carboxylase are reduced, resulting in the decreased conversion of pyruvate to glucose (Figure 1).
Insulin resistance (IR) refers to a defective metabolic response to the effect of the hormone in the target cell (e.g., muscle cell, hepatocyte, and adipocyte) or at the whole organism level. Systemic IR means that the ability of insulin to lower the serum glucose concentration to the appropriate level is hampered due to disrupted translocation of the GLUT4 receptor at the surface membrane of the muscle cell. As a result, glucose uptake (which depends on insulin) decreases. Hepatic IR consists of disturbed insulin mediated suppression of hepatic glucose production, but in the presence of preserved stimulation of lipogenesis[33]. In the adipose system, insulin resistance means that insulin is unable to suppress lipolysis. In humans, when the availability of lipids exceeds the lipid accumulation capacity, systemic IR and hepatic IR are likely to progress[34].
Increased FFA levels can cause lipotoxicity and insulin resistance, and together with other factors (such as gut-derived endotoxins), activate the release of proinflammatory cytokines systemically and also locally in the liver. There are two main classical pathways involved in the process of NAFLD inflammation: JNK-AP-1 and IKK-NF-κBD[35]. JNK-AP-1 is a mitogen-activated protein kinase associated with apoptosis and NASH; IKK-NF-κB is a transcription factor regulating inflammatory activation. Previous studies have shown that persistent activation of NF-κB was found in NAFLD animal models as well as in humans with NASH[36,37]. Animal models demonstrated that hepatic exposure to high levels of proinflammatory cytokines could lead to histological changes mimicking NASH[38].
The liver consists of parenchymal cells and nonparenchymal cells (NPCs); NPCs include sinusoidal endothelial cells. Kupffer cells (KCs) and hepatic stellate cells are less numerous than hepatocytes, but play a key role in the immune regulation of the liver, especially through substances released from KCs, which act as antigen presenting cells.
The hypothesis is that when the flow of FFAs or other pathogenic factors (such as endotoxins) from the gut into liver are excessive, KCs phagocytose the factors and present them through pattern recognition receptors (PRRs)[39]. PRRs include toll-like receptors (TLRS) such TLR4, TLR9, and nucleotide oligomerization domain-like receptors (NLRs)[40]. Inflammasomes, through NLR, activate the cascade events which finally generate mature IL-1, IL-8[41], and IL-1, contributing to regulate the activation of the transcription factor NF-κB[42]. KCs per se will differentiate into either the M1 or M2 phenotype, depending on the environmental inducer; the former releasing cytokines like TNF-α, IL-1, and IL-12 and the latter, more heterogeneous, being able to stimulate the secretion of IL-4, IL-10, and TGF-β according to different triggers[43]. IL-6 and TNF-α are the cytokines responsible for NASH progression. Patients with NASH have higher serum TNF-α levels, which play an important role in hepatic fibrosis through KC activation[38].
Therefore, TLR suppression is thought to block the immune response, thereby alleviating liver inflammation. However, to date, despite some animal experiments aiming to reveal the links between TLRs and NAFLD pathogenesis, no investigations on TLR agonists have yet been conducted in humans[44,45].
In summary, hepatocyte damage is an indicator of NASH progression. Different pathogens stimulate cell receptors thus activating the signaling pathway which contributes to cytokine production. Therefore, NASH might be detected at an earlier stage in the future by identifying an appropriate cytokine panel. Future studies should also focus on TLR modulation, which may provide a new target for NAFLD therapy.
In recent years, many studies have been carried out on gut-liver axis (GLA) dysfunction (including intestinal dysbiosis, bacterial overgrowth, and alteration of mucosa permeability) intending to find the possible therapeutic target of NAFLD[46,47]. GLA is characterized by bidirectional traffic. Nutrients and factors derived from the gut lumen reach the liver through the portal circulation; bile acids, produced by hepatocytes, are released in the small intestine through the biliary tract[48]. Two of these components (intestinal barrier and gut microbiota) seem to play a key role in liver damage and its progression[49]. It is well known that trillions of microbes make up the gut microbiota. In normal conditions, only a small amount of bacteria products enter the liver through the portal circulation. However, bacteria dysbiosis or gut barrier alterations will increase the bacteria flow into the liver, thus stimulating inflammation via TLR and other pattern recognition receptor activation in KCs[50]. According to the bidirectional traffic of GLA, bile acid also impacts the gut environment, both directly by causing membrane damage and indirectly via the activation by bile acid metabolitas of special receptors such as the farnesoid X receptor. Gut microbiota (GM) is specific to each individual, but humans share a core functional microbiome[51].
Altered GM associated with NAFLD may occur through several mechanisms as follows: (1) GM digests and ferments the excessive energy into short-chain fatty acids (SCFAs); (2) GM bacteria can produce ethanol that may affect the liver in a similar way to chronic alcoholism; (3) bacteria/endotoxins translocate into the portal circulation and damage the liver via TLR signaling; and (4) disturbed lipid metabolism is mediated by increased bile acid synthesis and decreased choline metabolism[52].
In addition, GM also plays a vital role in maintaining gut barrier integrity and intestinal permeability. GM dysbiosis can damage the intestinal epithelium and destroy tight junction proteins, which is important in preventing harmful substances from the gut such as bacteria, ethanol, and endotoxins from entering portal blood[46,53]. Experiments on mice and humans have confirmed these data[54,55]. A recent study found that E. coli emerges as the predominant bacteria involved in small intestine bacterial overgrowth and that NAFLD may be related to the efficient translocation abilities of these patients[56].
Hepatocyte triacylglycerol (TG) deposition is mainly due to three factors: lipolysis of adipose tissue, de novo lipogenesis, and TG dietary input, with contributions of 59%, 26%, and 15%, respectively. The excessive load of free fatty acid in the liver is the crucial cause of liver steatosis[57].
Carbohydrates can be converted to TG, and fructose is more closely associated with NAFLD compared to glucose. Fructose consumption, largely in the form of high fructose corn syrup (HFCS), a mixture of fructose and glucose monosaccharides, has increased over the past several decades[58]. Recent data suggest that diets high in sugar (sucrose and/or HFCS) not only increase the risk of NAFLD, but also of NASH. Indeed, fructose intake from added sugars in processed foods correlates with the epidemic rise in obesity, metabolic syndrome, and NAFLD. Fructose induced-hepatic fat accumulation involves the stress pathway that results in gluconeogenesis, an increase in fat synthesis, and a decrease in fat oxidation[59-61]. Fructose may modulate the lipogenic enzymes by increasing the expression of sterol regulatory element binding protein-1c (SREBP-1c) and carbohydrate-responsive element-binding protein (ChREBP)[62]. Animal experiments[63] showed that mice exposed to fructose with significant intestinal bacteria growth and increased intestinal permeability, as mentioned above, may trigger inflammation by increasing serum TNF-α.
Chronic fructose consumption induces leptin resistance prior to body weight, which accelerates high-fat induced obesity. Moreover, removal of fructose from this diet reverses leptin resistance and leptin augmentation, favoring a causal relationship[64,65].
Therefore, GLA is involved in the pathogenesis of NALFD, and GM dysbiosis promotes steatosis evolution to NASH. Special bacterial strains translocate more efficiently into the liver portal system. Further multicenter studies are required to test the bacterial genes of the normal population versus obese populations with IR (with and without NAFLD) with the aim of screening high-risk populations. In addition, improving intestinal dysbiosis and determining whether this improvement reduces the risk of NAFLD needs further investigation. These studies may pave the way for improving NAFLD diagnosis and treatment.
Genetic factors are also important in the development of NAFLD. A certain genetic background has been shown to predispose an individual to fatty liver[66,67]. Those genes are involved in inflammation, lipid metabolism, and oxidation, and are associated with progressive liver disease, IR, type 2 diabetes mellitus, and a higher risk for hepatocellular carcinoma.
PNPLA3:PNPLA3 is the most documented NAFLD-related gene. A genome-wide association study (GWAS) showed that the hepatic fat content of PNPLA3 I148M allele carriers was more than 2-fold higher than in non-carriers, and a new variant PNPLA3 S453I allele was identified which was associated with a significantly lower liver fat content particularly in African Americans[68]. Several studies have shown that PNPLA3 I148M increases the risk of NAFLD without a strong effect on metabolic syndrome (MS) components, but abdominal fat (which is closely correlated to MS components) can drive the effect of this polymorphism on liver damage[69,70]. In obese children, weight loss can weaken the effect of this polymorphism[71].
McGeoch et al[72] suggested that patients with PNPLA3 p.I148M showed the greatest response to the fructose-restricted diet, whereas those lacking this variant exhibit minimal or no change from baseline. Wang et al[73] revealed that physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD. These evidences provide new clues to the function of the PNPLA3 gene and are also useful for risk assessment and personalized treatment of NAFLD in the future.
Glucokinase regulator protein: Glucokinase regulator protein (GCKR) is an inhibitor of glucokinase (GCK). GCK regulates glucose storage and disposal in the liver where its activity is regulated by GCKR. The GCKR genotype has been shown to modulate lipogenesis and fibrosis progression in NAFLD[74]. The combined effects of PNPLA3 rs738409 and GCKR rs1260326 polymorphisms account for up to one-third of variability in liver fat content in obese children[75,76].
Apolipoprotein C-III: Apolipoprotein C-III (APOC3) can inhibit the lipoprotein lipase and reduce the clearance of TG. In NAFLD, APOC3 variants may lead to higher plasma concentrations of apolipoprotein C3 ending up in lower clearance. The consequence of the reduced TG clearance is an increase in residual particles of chylomicrons, that will lead to higher levels of circulating chylomicron remnants, which are especially cleared by the liver through a receptor-mediated process[77,78]. However, a recent study of APOC3 transgenic mice suggested that APOC3 dysregulation is not a predisposing factor for linking over-nutrition to NAFLD in obesity[79].
TM6SF2: Transmembrane 6 superfamily 2 (TM6SF2) has been recognized to regulate plasma lipids. On the basis of sequence similarity to Emopamil-binding protein (an enzyme with sterol isomerase activity), TM6SF2 has been hypothesized to play a role in sterol biosynthesis[80,81]. Smagris et al[82] reported that TM6SF2 is involved in the transfer of neutral lipids from cytoplasmic to luminal lipid droplets or very low density lipoprotein (VLDL) particles. Recently, variants of TM6SF2 have been found to influence metabolic traits through alteration of protein stability[83-86].
PPARGC1A: Peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α), expressing the PPARGC1A gene, is involved in the key steps of NAFLD development, such as insulin resistance, mitochondrial biogenesis, and oxidative phosphorylation[87,88]. In hepatocytes, PGC-1a orchestrates broad energy programs, including gluconeogenesis and mitochondrial fatty acid β-oxidation[89]. Moreover, PPARGC1A has been shown to regulate several key genes in hepatic gluconeogenesis (CREB, PPARα, FOXO1, TRB-3)[90-93]. PPARGC1A knockout mice reportedly developed hepatic steatosis due to a combination of reduced mitochondrial respiratory capacity and increased the expression of lipogenic genes[94].
Human microsomal triglyceride transfer protein: The human microsomal triglyceride transfer protein (MTTP) is involved in lipid transfer function and is critical for the assembly and secretion of VLDL to remove lipids from the liver. Thus, genetic polymorphisms in the MTTP gene may contribute to altered lipid metabolism by disrupting the assembly and secretion of lipoproteins, leading to reduced fat export from the involved hepatocytes and to NAFLD. Several genetic polymorphisms in the MTTP gene have been identified; some are related to the pathogenesis of NAFLD while others interact with age, insulin resistance, and BMI and increase the risk for NAFLD[95-99].
Other genes: Recently, Buch et al[100] and Umano et al[101] identified the rs626283 variants in the MBOAT7 gene as risk loci for alcohol-related cirrhosis in adults and obese youth[100,101]. In the Japanese population, the SAMM50 gene (rs738491, rs3761472, and rs2143571), PARVB gene (rs6006473, rs5764455 and rs6006611), and GATAD2A gene (rs4808199) were found to be significantly associated with NAFLD[102,103].
Meanwhile, Chinese children with NAFLD presented a higher prevalence of UCP3 gene rs11235972 GG[104]. Adams et al[105] reported that SNPs in two hepatic genes were associated with NAFLD in adolescents: The group-specific component and the lymphocyte cytosolic protein-1.
The pathogenesis of NAFLD and its progression is a complex process, in which some questions remain unanswered. The initial “two-hit” theory can no longer completely explain the pathogenesis of NAFLD, which involves multiple factors. In recent decades, many experiments have suggested that the gut microbiome plays a key role in NAFLD pathogenesis via the GLA. More recently, with the development of technology (especially GWAS), increasing studies have focused on genetic predispositions and found various gene variants that may alter lipid and sugar metabolism in the liver as well as in other tissues such as adipose tissue. Given the multifactorial nature of the related diseases, it may not be possible to obtain a single indicator that could precisely differentiate NAFLD and NASH. However, data in the future could be more promising in terms of population screening, with the goal to identify individuals at risk for NAFLD.
Hopefully, the “multiple hit model” (once further refined) will pave the way for tailoring therapeutics to genetic predispositions to NAFLD and NASH.
The authors thank Dr. Yu Ming Shiao from the National Health Research Institutes of Taiwan for modifying and improving the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification:
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Maleki I, Riordan JD, Tiribelli C S- Editor: Wang JL L- Editor: Filipodia E- Editor: Huang Y
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3655] [Cited by in F6Publishing: 3609] [Article Influence: 164.0] [Reference Citation Analysis (2)] |
| 2. | Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 586] [Cited by in F6Publishing: 605] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 3. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 4. | Farrell GC, Wong VW, Chitturi S. NAFLD in Asia--as common and important as in the West. Nat Rev Gastroenterol Hepatol. 2013;10:307-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 327] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6270] [Article Influence: 783.8] [Reference Citation Analysis (0)] |
| 6. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | Barlow SE; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 Suppl 4:S164-S192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3035] [Cited by in F6Publishing: 2969] [Article Influence: 174.6] [Reference Citation Analysis (0)] |
| 8. | Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, Durmaz O, Lacaille F, McLin V, Nobili V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 9. | Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, Mouzaki M, Sathya P, Schwimmer JB, Sundaram SS. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. 2017;64:319-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 10. | Zou CC, Liang L, Hong F, Fu JF, Zhao ZY. Serum adiponectin, resistin levels and non-alcoholic fatty liver disease in obese children. Endocr J. 2005;52:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Zou CC, Liang L, Zhao ZY. Factors associated with fasting plasma ghrelin levels in children and adolescents. World J Gastroenterol. 2008;14:790-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wang XM, Jiang YJ, Liang L, Du LZ. Changes of ghrelin following oral glucose tolerance test in obese children with insulin resistance. World J Gastroenterol. 2008;14:1919-1924. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Fu JF, Shi HB, Liu LR, Jiang P, Liang L, Wang CL, Liu XY. Non-alcoholic fatty liver disease: An early mediator predicting metabolic syndrome in obese children? World J Gastroenterol. 2011;17:735-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 60] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Chen LH, Liang L, Fang YL, Wang YM, Zhu WF. Fish oil improves lipid profile in juvenile rats with intrauterine growth retardation by altering the transcriptional expression of lipid-related hepatic genes. J Matern Fetal Neonatal Med. 2016;29:3292-3298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496-500.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 16. | Marzuillo P, Miraglia del Giudice E, Santoro N. Pediatric fatty liver disease: role of ethnicity and genetics. World J Gastroenterol. 2014;20:7347-7355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Giorgio V, Prono F, Graziano F, Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013;13:40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561-e565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Conjeevaram Selvakumar PK, Kabbany MN, Alkhouri N. Nonalcoholic Fatty Liver Disease in Children: Not a Small Matter. Paediatr Drugs. 2018; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 472] [Cited by in F6Publishing: 488] [Article Influence: 34.9] [Reference Citation Analysis (2)] |
| 21. | Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 501] [Article Influence: 26.4] [Reference Citation Analysis (1)] |
| 22. | Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, Lu JR, Wu XM. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598-1602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 88] [Cited by in F6Publishing: 95] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 23. | Fu JF, Fang YL, Liang L, Wang CL, Hong F, Dong GP. A rabbit model of pediatric nonalcoholic steatohepatitis: the role of adiponectin. World J Gastroenterol. 2009;15:912-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 723] [Cited by in F6Publishing: 743] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 25. | Berardis S, Sokal E. Pediatric non-alcoholic fatty liver disease: an increasing public health issue. Eur J Pediatr. 2014;173:131-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Alisi A, Cianfarani S, Manco M, Agostoni C, Nobili V. Non-alcoholic fatty liver disease and metabolic syndrome in adolescents: pathogenetic role of genetic background and intrauterine environment. Ann Med. 2012;44:29-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Ayonrinde OT, Olynyk JK, Marsh JA, Beilin LJ, Mori TA, Oddy WH, Adams LA. Childhood adiposity trajectories and risk of nonalcoholic fatty liver disease in adolescents. J Gastroenterol Hepatol. 2015;30:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res. 2013;52:175-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 29. | Ferramosca A, Zara V. Modulation of hepatic steatosis by dietary fatty acids. World J Gastroenterol. 2014;20:1746-1755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 132] [Cited by in F6Publishing: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 30. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1490] [Cited by in F6Publishing: 1706] [Article Influence: 213.3] [Reference Citation Analysis (1)] |
| 31. | Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol. 2014;25:213-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest. 1996;98:741-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 220] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Petersen MC, Shulman GI. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol Sci. 2017;38:649-665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 225] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 34. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2237-2238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5683] [Cited by in F6Publishing: 5905] [Article Influence: 347.4] [Reference Citation Analysis (1)] |
| 36. | Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1671] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 37. | Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708-1717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 277] [Cited by in F6Publishing: 289] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 38. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 318] [Cited by in F6Publishing: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 39. | Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 547] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 40. | Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tör M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 41. | Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642-654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 365] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 42. | Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 836] [Cited by in F6Publishing: 942] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 43. | Klein I, Cornejo JC, Polakos NK, John B, Wuensch SA, Topham DJ, Pierce RH, Crispe IN. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077-4085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-334.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 558] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 45. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 46. | Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol. 2016;22:8078-8093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 123] [Cited by in F6Publishing: 116] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 47. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 48. | Poeta M, Pierri L, Vajro P. Gut-Liver Axis Derangement in Non-Alcoholic Fatty Liver Disease. Children (Basel). 2017;4:66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 49. | Paolella G, Mandato C, Pierri L, Poeta M, Di Stasi M, Vajro P. Gut-liver axis and probiotics: their role in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15518-15531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 122] [Cited by in F6Publishing: 126] [Article Influence: 12.6] [Reference Citation Analysis (3)] |
| 50. | Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 520] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 51. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3051] [Cited by in F6Publishing: 3194] [Article Influence: 266.2] [Reference Citation Analysis (0)] |
| 52. | Doulberis M, Kotronis G, Gialamprinou D, Kountouras J, Katsinelos P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism. 2017;71:182-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 53. | Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 754] [Cited by in F6Publishing: 776] [Article Influence: 59.7] [Reference Citation Analysis (1)] |
| 54. | Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, Smith T, Neish AS, Li H, Tan S. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151:733-746.e12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 55. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 938] [Cited by in F6Publishing: 982] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 56. | Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, Ray P, Dhiman RK, Chawla Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2112] [Cited by in F6Publishing: 2334] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 58. | Ventura EE, Davis JN, Goran MI. Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring). 2011;19:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 59. | Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732-40744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 470] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 60. | Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, Hunter B, Andrés-Hernando A, Ishimoto T, Sánchez-Lozada LG. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One. 2012;7:e48801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 61. | Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, Hunter B, Rivard CJ, Sautin YY, Gaucher EA. Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: evolutionary implications of the uricase loss in hominids. FASEB J. 2014;28:3339-3350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 62. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 838] [Cited by in F6Publishing: 877] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 63. | Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 403] [Cited by in F6Publishing: 428] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 64. | Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370-R1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 65. | Shapiro A, Tümer N, Gao Y, Cheng KY, Scarpace PJ. Prevention and reversal of diet-induced leptin resistance with a sugar-free diet despite high fat content. Br J Nutr. 2011;106:390-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2633] [Cited by in F6Publishing: 2593] [Article Influence: 129.7] [Reference Citation Analysis (3)] |
| 67. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1127] [Cited by in F6Publishing: 1219] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 68. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2233] [Cited by in F6Publishing: 2311] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 69. | Giudice EM, Grandone A, Cirillo G, Santoro N, Amato A, Brienza C, Savarese P, Marzuillo P, Perrone L. The association of PNPLA3 variants with liver enzymes in childhood obesity is driven by the interaction with abdominal fat. PLoS One. 2011;6:e27933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Speliotes EK, Butler JL, Palmer CD, Voight BF; GIANT Consortium; MIGen Consortium; NASH CRN, Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904-912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 71. | Marzuillo P, Grandone A, Perrone L, del Giudice EM. Weight loss allows the dissection of the interaction between abdominal fat and PNPLA3 (adiponutrin) in the liver damage of obese children. J Hepatol. 2013;59:1143-1144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | McGeoch LJ, Patel PR, Mann JP. PNPLA3: A Determinant of Response to Low-Fructose Diet in Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;154:1207-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Wang S, Song J, Shang X, Chawla N, Yang Y, Meng X, Wang H, Ma J. Physical activity and sedentary behavior can modulate the effect of the PNPLA3 variant on childhood NAFLD: a case-control study in a Chinese population. BMC Med Genet. 2016;17:90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Petta S, Miele L, Bugianesi E, Cammà C, Rosso C, Boccia S, Cabibi D, Di Marco V, Grimaudo S, Grieco A. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS One. 2014;9:e87523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Santoro N, Zhang CK, Zhao H, Pakstis AJ, Kim G, Kursawe R, Dykas DJ, Bale AE, Giannini C, Pierpont B. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 76. | Valenti L, Alisi A, Nobili V. Unraveling the genetics of fatty liver in obese children: additive effect of P446L GCKR and I148M PNPLA3 polymorphisms. Hepatology. 2012;55:661-663. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Windler EE, Greeve J, Daerr WH, Greten H. Binding of rat chylomicrons and their remnants to the hepatic low-density-lipoprotein receptor and its role in remnant removal. Biochem J. 1988;252:553-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 55] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Nagata Y, Chen J, Cooper AD. Role of low density lipoprotein receptor-dependent and -independent sites in binding and uptake of chylomicron remnants in rat liver. J Biol Chem. 1988;263:15151-15158. [PubMed] [Cited in This Article: ] |
| 79. | Cheng X, Yamauchi J, Lee S, Zhang T, Gong Z, Muzumdar R, Qu S, Dong HH. APOC3 Protein Is Not a Predisposing Factor for Fat-induced Nonalcoholic Fatty Liver Disease in Mice. J Biol Chem. 2017;292:3692-3705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Fan Y, Lu H, Guo Y, Zhu T, Garcia-Barrio MT, Jiang Z, Willer CJ, Zhang J, Chen YE. Hepatic Transmembrane 6 Superfamily Member 2 Regulates Cholesterol Metabolism in Mice. Gastroenterology. 2016;150:1208-1218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 81. | Sanchez-Pulido L, Ponting CP. TM6SF2 and MAC30, new enzyme homologs in sterol metabolism and common metabolic disease. Front Genet. 2014;5:439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a Gene Defective in Fatty Liver Disease, Impairs Lipidation but Not Secretion of Very Low Density Lipoproteins. J Biol Chem. 2016;291:10659-10676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 83. | Holmen OL, Zhang H, Fan Y, Hovelson DH, Schmidt EM, Zhou W, Guo Y, Zhang J, Langhammer A, Løchen ML. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat Genet. 2014;46:345-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 84. | Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 817] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 85. | Surakka I, Horikoshi M, Mägi R, Sarin AP, Mahajan A, Lagou V, Marullo L, Ferreira T, Miraglio B, Timonen S. The impact of low-frequency and rare variants on lipid levels. Nat Genet. 2015;47:589-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 86. | Ehrhardt N, Doche ME, Chen S, Mao HZ, Walsh MT, Bedoya C, Guindi M, Xiong W, Ignatius Irudayam J, Iqbal J. Hepatic Tm6sf2 overexpression affects cellular ApoB-trafficking, plasma lipid levels, hepatic steatosis and atherosclerosis. Hum Mol Genet. 2017;26:2719-2731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 87. | Lin YC, Chang PF, Chang MH, Ni YH. A common variant in the peroxisome proliferator-activated receptor-γ coactivator-1α gene is associated with nonalcoholic fatty liver disease in obese children. Am J Clin Nutr. 2013;97:326-331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 88. | Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO, Pirola CJ. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology. 2010;52:1992-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 89. | Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1604] [Cited by in F6Publishing: 1612] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 90. | Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1051] [Cited by in F6Publishing: 1072] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 91. | Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1422] [Cited by in F6Publishing: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 92. | Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1116] [Cited by in F6Publishing: 1125] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 93. | Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 94. | Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 711] [Cited by in F6Publishing: 743] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 95. | Dai D, Wen F, Zhou S, Su Z, Liu G, Wang M, Zhou J, He F. Association of MTTP gene variants with pediatric NAFLD: A candidate-gene-based analysis of single nucleotide variations in obese children. PLoS One. 2017;12:e0185396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Musso G, Gambino R, Cassader M. Lipoprotein metabolism mediates the association of MTP polymorphism with beta-cell dysfunction in healthy subjects and in nondiabetic normolipidemic patients with nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21:834-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Siqueira ER, Oliveira CP, Correa-Giannella ML, Stefano JT, Cavaleiro AM, Fortes MA, Muniz MT, Silva FS, Pereira LM, Carrilho FJ. MTP -493G/T gene polymorphism is associated with steatosis in hepatitis C-infected patients. Braz J Med Biol Res. 2012;45:72-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Peng XE, Wu YL, Lu QQ, Hu ZJ, Lin X. MTTP polymorphisms and susceptibility to non-alcoholic fatty liver disease in a Han Chinese population. Liver Int. 2014;34:118-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Hsiao PJ, Lee MY, Wang YT, Jiang HJ, Lin PC, Yang YH, Kuo KK. MTTP-297H polymorphism reduced serum cholesterol but increased risk of non-alcoholic fatty liver disease-a cross-sectional study. BMC Med Genet. 2015;16:93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 101. | Umano GR, Caprio S, Di Sessa A, Chalasani N, Dykas DJ, Pierpont B, Bale AE, Santoro N. The rs626283 Variant in the MBOAT7 Gene is Associated with Insulin Resistance and Fatty Liver in Caucasian Obese Youth. Am J Gastroenterol. 2018;113:376-383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, Teranishi H, Mizusawa S, Ueno T, Chayama K. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet. 2013;132:783-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 103. | Kawaguchi T, Shima T, Mizuno M, Mitsumoto Y, Umemura A, Kanbara Y, Tanaka S, Sumida Y, Yasui K, Takahashi M. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS One. 2018;13:e0185490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 104. | Xu YP, Liang L, Wang CL, Fu JF, Liu PN, Lv LQ, Zhu YM. Association between UCP3 gene polymorphisms and nonalcoholic fatty liver disease in Chinese children. World J Gastroenterol. 2013;19:5897-5903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 25] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Adams LA, White SW, Marsh JA, Lye SJ, Connor KL, Maganga R, Ayonrinde OT, Olynyk JK, Mori TA, Beilin LJ. Association between liver-specific gene polymorphisms and their expression levels with nonalcoholic fatty liver disease. Hepatology. 2013;57:590-600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |