INTRODUCTION
Aldehyde dehydrogenase 2 (ALDH2), a tetrameric allosteric mitochondrial enzyme, is one of the 19 different ALDH enzymes ubiquitously expressed in all human tissues. It is most abundant in the liver, where it plays an essential role in the ethanol detoxifying pathway[1]. This pathway comprises two enzymatic steps. In the first, ethanol is metabolized by alcohol dehydrogenase (ADH) to form acetaldehyde, which is highly diffusible and crosses biological membranes, then circulates in the blood and is metabolized by ALDH2 to acetic acid[1,2]. ALDH2 is also responsible for the detoxification of other toxic short-term aldehydes, including some aromatic and polycyclic types[1,3]. In addition, ALDH2 oxidizes endogenous aldehydic products from lipid peroxidation of mitochondrial and plasma membranes under oxidative stress conditions, such as 4-hydroxy-2-nonenal (4-HNE) and lipoperoxides (malondialdehyde, MDA)[4,5]. The clearance of these harmful 4-HNE and aldehyde adducts, performed by ALDH2, is crucial for cell survival[1,4-6], since it is well known that 4-HNE affects mitochondrial and membrane integrity and other functions like apoptosis[7,8]. Although ALDH2 was initially known for its role in ethanol metabolism in liver[1], it has also been implicated in several pathologies, such as cardiovascular diseases[1,9], diabetes[1,10], neurologic dysfunctions[1,11] and more recently, in ischemia reperfusion injury (IRI) in organs such as heart[11-14], brain and eyes[15-19], intestine[20,21], kidney[22,23] and spinal cord[24].
IRI, a process inherent in organ tumor resection and transplantation, affects organ quality and transplant outcomes. During the process, the organ deteriorates due to the lack of blood flow and oxygen deprivation (ischemic injury) and subsequently after the restoration of blood flow and oxygen supply (reperfusion injury). While some damage occurs during the ischemia phase, reperfusion by itself triggers a new set of detrimental cellular processes that provoke the energy metabolism breakdown exacerbating the injury and cell death. With the entry of oxygen to the organ, reactive oxygen species (ROS), lipoperoxides and toxic aldehydes are generated, inflammatory mediators are released and cell signaling pathways are activated, extending cell damage[25,26], exacerbating cell death processes, and eventually leading to organ failure.
In this review, we first present some general considerations on the role of ALDH2 in the complex pathophysiology of IRI/oxidative mechanisms, focusing on 4-HNE and its consequences for autophagy/apoptosis processes. Then we discuss the use of therapeutic strategies, such as “surgical preconditioning” (remote ischemic preconditioning and post-conditioning) and “pharmacological preconditioning” (isoflurane, ethanol; nitrite/nitrate strategies and ALDH2 agonists) for the prevention of IRI. In the final section, we offer an update on specific ALDH2 investigations carried out in different organs subjected to I/R such as heart, brain, eyes, intestine and kidney, and assess the possibility of using ALDH2 agonists to prevent cold IRI in the future, with special emphasis on liver transplantation.
ALDH2 AND 4-HNE
The mitochondria are the main source of the ROS generated during reperfusion. ROS interacts with polyunsaturated fatty acids in biological membranes, producing high toxic and reactive molecules such as 4-HNE[4,5], which can trigger the opening of mitochondrial permeability transition pores and inhibit the electron transport chain, thus contributing to the extension of the damage.
The specific electrophilic features of 4-HNE confer on this molecule a high reactivity and capacity to modify enzymes like kinases, belonging to sensitive pathways of cell homeostasis such as mitochondrial bioenergetics, redox balance, ROS formation, autophagy, apoptosis, and so on.
ALDH2 overexpression delays the formation of 4-HNE and toxic aldehyde adducts, and thus preserves the mitochondrial function during IRI[27,28]. Nevertheless, despite the ability of ALDH2 to remove these aldehyde adducts, when 4-HNE is accumulated at high concentrations, it may act as an ALDH2 inhibitor in vitro, countering its beneficial effects and worsening the impact of I/R insult[12,29]. 4-HNE has been shown to inflict organelle damage in a wide variety of cell components, exposing the cell to a high level of stress and finally leading to its death. In this regard, it is clear that 4-HNE may modify essential cell-signaling molecules of survival mechanisms, as it does in autophagy (AMPK) and apoptosis (Akt)[27,28,30].
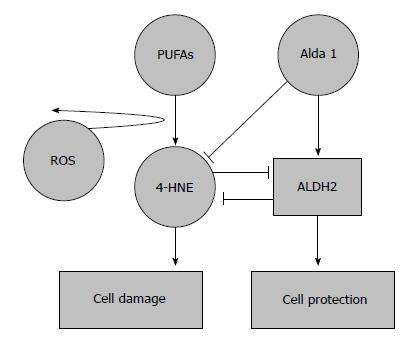
Ultimately, the clearance ratio of 4-HNE-adducts by ALDH2 and the ALDH2-4-HNE balance is crucial in order to prevent the modulation of survival mechanisms by 4-HNE and thus minimize some of the deleterious effects of IRI caused by the formation of these toxic aldehydes (Figure 1).
Figure 1 Protective effects of aldehyde dehydrogenase 2 on 4-hydroxy-2-nonenal accumulation in ischemia reperfusion injury.
4-hydroxy-2-nonenal (4-HNE) is a pivotal marker for cell damage associated with oxidative stress; its accumulation is prevented by aldehyde dehydrogenase 2 (ALDH2) activation and the action of its agonists. An overwhelming 4-HNE accumulation may also inhibit ALDH2 action. 4-HNE: 4-hydroxy-2-nonenal.
ALDH2 AND AUTOPHAGY
Autophagy is believed to play a key role in cell survival, with distinct functions depending on the event the cell is facing. In fact, autophagy is a cellular housekeeping mechanism, which in physiological conditions removes long-lived, aggregated and misfolded proteins, clears damaged organelles and plays an important role in the “survival and death” strategies of cellular stress responses, as occurs in the I/R condition[31,32-34].
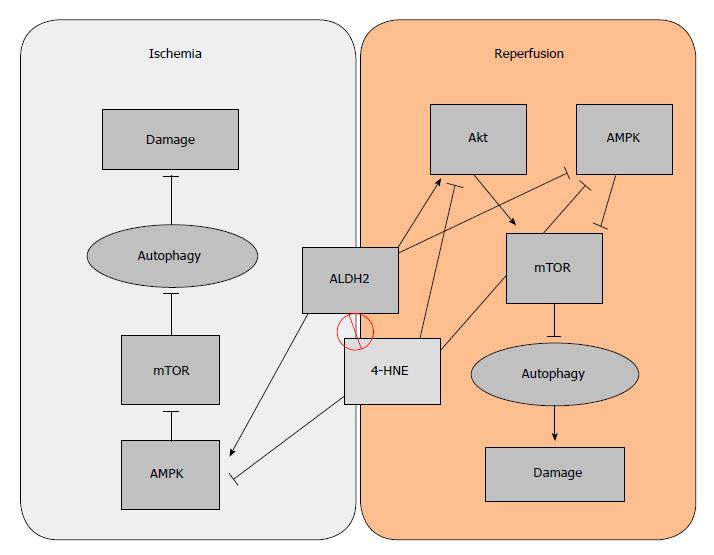
Autophagy has two paradoxically opposite behaviors, depending on the stage of the I/R condition: during ischemia, certain levels of autophagy are cytoprotective[35], but during reperfusion it is detrimental[32,33]. One of the key elements that regulate autophagy is the mammalian target of rapamycin (mTOR), an autophagy inhibitor. The inhibitory effect of mTOR over autophagy can be enhanced by Akt during reperfusion, or inhibited by AMPK (in both ischemia and reperfusion)[31]. Taking into account the dual behavior of autophagy and seeing some of its regulators, the role that ALDH2 plays in I/R is also dual: During ischemia, ALDH2 benefits AMPK activation, thus contributing to the inhibition of mTOR and subsequently increasing cytoprotective autophagy. On the other hand, during the reperfusion phase, cytoprotective autophagy is no longer active. Under reperfusion conditions, ALDH2 inhibits AMPK but also activates Akt, whose phosphorylation is kicked on to activate mTOR, thus resulting in a detrimental Akt-dependent inhibition of autophagy[28]. The ALDH2 dual regulatory effect on autophagy mechanisms through the Akt/AMPK/mTOR pathway could be a useful tool for preventing IRI. Considering that both AMPK and Akt are targets for 4-HNE, the beneficial effects that ALDH2 exerts on autophagy may be due both to its interaction with AMPK and to its capacity to remove 4-HNE (Figure 2).
Figure 2 Dual regulatory effect of aldehyde dehydrogenase 2 (ALDH2) on autophagy.
During ischemia, AMPK is activated to promote “cytoprotective” autophagy due to mTOR inhibition. On the other hand, the activation of Akt during the phase of reperfusion inhibits “deleterious” autophagy, which is associated with apoptosis, thus reducing organ damage.
We have seen that autophagy is a mechanism which, during periods of scarce resources, enables the cell to recycle its own products in order to survive. Most of this survival potential is performed through the preservation of the mitochondria, which are among the organelles most in need of protection. Therefore, mitophagy, the selective process to auto-phagocytize mitochondria, should be carefully regulated[36]. Some IRI-related studies report that after ALDH2 activation, the mitophagy regulators tensin homolog-induced putative kinase 1 (PINK1)/Parkin expression is suppressed, resulting in reduced injury. Even though the specific mechanism of action remains unclear (either via direct inhibition or via 4-HNE cleansing), ALDH2 clearly exerts a beneficial effect on mitophagy[37].
We can conclude that ALDH2 plays a positive role in the modulation of cyto-protective autophagy. It is an especially promising agent for the regulation of the deleterious effects of autophagy in cold IRI processes associated with organ transplantation.
ALDH2: NECROPTOSIS AND APOPTOSIS
Programmed cell death results in either a lytic or a non-lytic morphology, which includes different cell signaling pathways. It can be caused by well-known mechanisms such as autophagy, apoptosis, necrosis and necroptosis[19,38,39], which are determinant for organ preservation/cell survival associated with ischemia reperfusion injury.
Briefly, “necrosis” is one of the most frequent consequences of metabolic injury due to the organ oxygen deprivation during ischemia, leading to ATP depletion. It is characterized by cell swelling, membrane rupture and release of cell contents with the subsequent inflammatory response[40]. By contrast, “apoptosis” is a non-lytic form of silent programmed cell death in which the individual dying cells separate from their neighbors and shrink rather than swell[40]. Cell death by necrosis may also be programmed, as also occurs in apoptosis, and is then called “necroptosis”. In any case, distinct signaling events drive the lytic cell and non-lytic cell death processes (necroptosis and apoptosis, respectively). Thus, necroptosis is similar in nature to necrosis but it is induced by the activation of RIPK1 (receptor-interacting protein kinase 1) and RIPK3 (receptor- interacting protein kinase 1), a caspase-independent form of programmed cell death. Studies by Cheng Shen et al[41] showed that ALDH2 deficiency promotes necroptosis through the activation of the RIPK1/RIPK3/MLKL pathway.
In contrast to necroptosis, there is no inflammation during apoptosis; the process is dependent on caspases 3 and 9. Apoptosis is a process controlled at multiple checkpoints by cell signaling expression, sometimes with opposite actions; examples are Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic). The ratio between the two is decisive for the apoptotic response, which is regulated by an upstream mediator, Akt, which ultimately determines the fate of the cell[42,43].
Due to its cytotoxic characteristics, 4-HNE is an important mediator of oxidative stress-induced apoptosis[42]. As previously mentioned, 4-HNE inhibits a group of a several kinases, one of which is Akt. Under high concentrations of 4-HNE, a dramatic apoptotic response is induced. This effect has been suggested to occur through the inhibition of Akt, which increases the Bax/Bcl-2 ratio and activates a caspase-3 apoptotic cascade[43,44]. This Akt inhibition in the presence of 4-HNE can be totally reversed by using an ALDH2 agonist, such as Alda-1[45]. Further investigations have evidenced that increased ALDH2 expression through Alda-1 treatment protects I/R-induced brain cell necrosis and apoptosis[16,24]. Besides these benefits of ALDH2 observed in the heart and brain, benefits have also been shown for limiting neuronal apoptosis in spinal cord IRI[24]. Recently, Zhong et al[45] reported that low ALDH2 promotes liver apoptosis through the MAPK pathway when ALDH2 agonists are used, in which ALDH2 action is not only based on the 4-HNE clearance ratio, but also on its subsidiary involvement in controlling indirect 4-HNE pathways.
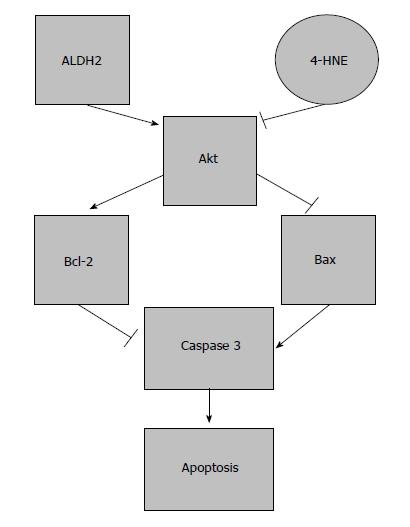
Again, the ratio of 4-HNE formation and its cleansing by ALDH2 is essential. High 4-HNE levels will limit ALDH2 activity, avoiding subsequent 4-HNE clearance and producing a positive 4-HNE feedback that ultimately causes apoptosis and cell death[1,42] (Figure 3).
Figure 3 Effects of aldehyde dehydrogenase 2 on apoptosis in ischemia reperfusion injury.
Protection induced by aldehyde dehydrogenase 2 (ALDH2) on apoptosis. The balance between ALDH2/4-HNE is responsible for the modulation of apoptosis through Akt and caspase 3 expression.
ALDH2 ACTIVATION AND IRI PREVENTION IN DIFFERENT ORGANS: SOME CONSIDERATIONS
In general, ALDH2 activation plays a protective role in the complex physiopathology of IRI, whose beneficial effects have mostly been studied in the heart, inducing cardio-protection as well as a protective role against the I/R insult[12,46]. This protection has been evidenced in other organs such as brain, intestine, kidney and spinal cord[16,20,22,24]. Surprisingly, the role of ALDH2 in hepatic I/R has not been studied in depth, even though the liver is one of the organs where ALDH2 is most abundant[1]. This is probably due to the fact that most studies of ALDH2 in liver have focused on ethanol metabolism disorders.
In the following lines, we describe the most important ongoing research into the role of ALDH2 in IRI in heart, brain, eyes, intestine, and kidney.
ALDH2 and heart
The most important feature of ALDH2 myocardial cardio-protection is the clearance of toxic aldehydes (such as 4-HNE) and its adducts[12,46]; Alda-1, an activator of ALDH2, raises this reactive aldehyde clearance even further, increasing the protection against myocardial IRI disorders[14,15]. Moreover, ALDH2 activation is associated with an improved mitochondrial function and a remodeling of the ventricular function[47,48]. ALDH2-induced cardio-protection (with the subsequent detoxification of toxic aldehydes) against IRI also occurs through a differential regulation of autophagy, involving both AMPK and Akt-mTOR signaling mechanisms, produced during ischemia and reperfusion respectively[28,49]. Guo et al[50] recently described a novel protective ALDH2 mechanism in type I diabetes, which defends against the induced myocardial dysfunction via an AMPK-dependent regulation of autophagy. These benefits are confirmed by the fact that ALDH2 inhibition by O-linked-N-acetylglucosamine (O-GlcNAC) acylation contributes to hyperglycemic exacerbation of myocardial IRI, which is prevented by Alda-1[51]. Even more recently, it has been reported that ALDH2 induces cardio-protection through the regulation of mitophagy by suppressing tensin homolog-induced putative kinase 1 (PINK1)/Parkin expression, thus preventing the accumulation of 4-HNE and other toxic species[37].
ALDH2 in brain, intestine, kidney and eyes
The role of ALDH2 in other organs, such as brain, eyes, intestine, and kidney, has been poorly investigated. In brain and intestine, ALDH2 activation contributes to the clearance of the 4-HNE accumulation and MDA generation against IRI. Alda-1, an ALDH2 agonist, increases the 4-HNE adduct clearance, subsequently promoting protection against brain IRI and preventing the deleterious effects of apoptosis[15,16]. In kidney, after bilateral ischemia, ALDH2 activation is involved in the protection induced by ethanol at physiological levels[23]. Interestingly, an increased ALDH2 expression also has a protective effect, reducing renal cell apoptosis by inhibiting the MAPK pathway, after using hypothermic machine perfusion[22].
Recent investigations have shown the existence of ALDH2 expression in adult rat retinal tissues, which may be involved in the retina redox balance. However, its role in the regulatory lipoperoxidation mechanisms associated with retinal ischemia reperfusion injury needs to be investigated in depth[52,53].
ALDH2 ACTIVATION: ISCHEMIC PRECONDITIONING
Several therapeutic surgical and pharmacological strategies have been used to protect the organ against I/R insult. As an example, “ischemic preconditioning” (IPC) is based on the application of a previous transient ischemia and/or reperfusion that will prepare the organ prior to a sustained I/R. IPC was initially evidenced in the heart by Murry et al[54] but has also been found to be protective for other organs such as kidney, intestine and liver[55-57]. Another surgical strategy is “remote ischemic preconditioning” (RIPC)[58], which consists in the application of brief ischemia in one organ to confer protection on distant organs as well[59]. The involvement of ALDH2 as an important mediator in the benefits conferred by RIPC has also been discussed by Contractor et al[59].
ALDH2 ACTIVATION: PHARMACOLOGICAL PRECONDITIONING
The use of pharmacological agents to promote organ protection (pharmacological preconditioning) is limited when it is mandatory to avoid their potential undesirable secondary effects. We have assessed the protective role of ALDH2 when using different protective strategies against IRI based on the administration of volatile anesthetics (isoflurane), nitrites/nitrates and ALDH2 agonists (Alda-1).
ALDH2 and isoflurane preconditioning
Recent investigations in the heart and other organs have demonstrated that the use of isoflurane, a volatile anesthetic agent, is an effective preconditioning agent that mimics the protective effects of IPC but avoids some of its disadvantages caused by the reduction of the blood flow, and presents greater ethical acceptability and clinical safety[60-62]. ALDH2 is involved in the cardio-protection induced by isoflurane preconditioning, and this ALDH2/isoflurane-induced cardio-protection is substantially blocked by a PKCε inhibitor, suggesting that mitochondrial PKCε plays an important role in isoflurane-induced protection mechanisms. It has been discussed whether the phosphorylation of ALDH2 increases PKCε mitochondrial translocation, and the inhibition of mitochondrial translocation by other protein kinases such as PKC delta, contribute even more to isoflurane protection against I/R insult[63].
ALDH2 and ethanol preconditioning
Ethanol, at low doses, also acts as a preconditioning agent[51]. Its acute cardio-protective effects are critically modulated by the dose used and by whether it remains present during the ischemic period. In these conditions of ethanol preconditioning, ALDH2 is activated to induce protection against IRI through the mitochondrial translocation of PKCε, which is responsible for the increased metabolism from HNE to HNA, thus limiting the accumulation of HNE protein adducts and improving cardiac function[64,65].
In this context, it has been suggested that the use of ALDH2 activators mimics ethanol cardio-protection. In addition, ALDH2 protection against ethanol toxicity is regulated by Akt and AMPK, and subsequently, autophagy and apoptosis through their downstream substrate mTOR[28].
ALDH2 and NO/nitrite preconditioning
Nitric oxide (NO) is a free-radical gas, considered as a relevant therapeutic tar- get during IRI. Its effects on I/R injury are probably related to the dose or to the conditions during ischemia and reperfusion, usually at nanomolar or low micromolar concentrations[66]. Apart from its role in tissue protection during ischemia, and due to its volatile nature, NO is a fast-response cell signaling molecule and a well-known vasodilator during hypoxic events, also regulating mitochondrial oxygen consumption[66]. There is growing evidence that eNOS-derived NO is a critical component in IPC and APC signal transduction[67,68]. Last but not least, due to its reductive capacity, NO is a potent ROS scavenger, and thus, performs one of the most important functions during reperfusion[69].
As a volatile molecule, the half-life of NO is not very long; this is why there is a need for a pool of nitrogen in a more stable form, i.e., nitrites (NO2-) and nitrates (NO3-). Nitrites are the reduced form of nitrates, and under physiological conditions they can be recycled in blood and tissues, where NO generation from nitrites is linearly dependent on oxygen and pH levels[66,69].
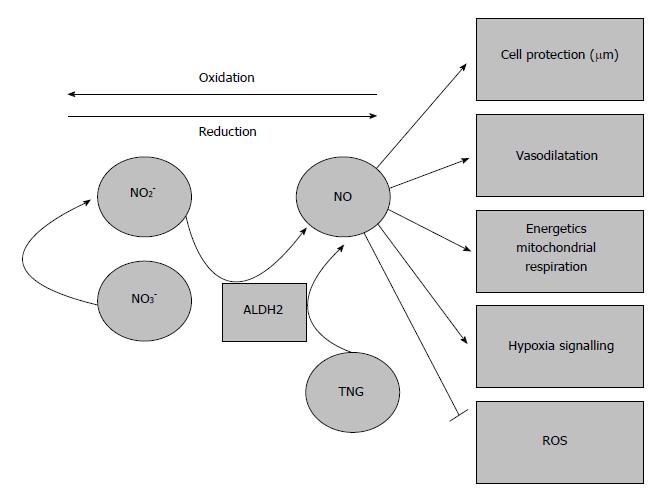
It has been widely shown that ALDH2 has a reductase-dependent activity that produces the bioconversion of nitroglycerin to 1, 2-glyceryl denigrates, resulting in the release of NO[1]. Further experiments have shown that in vitro, ALDH2 is partially responsible for nitrite bioactivation, promoting vasodilation during hypoxic episodes (Figure 4). However, Oelze et al[70] showed that the peroxynitrite generated from NO impairs the enzymatic activity of ALDH2, and therefore the scavenging activity of NO may result in a suicide inhibition of ALDH2 in later stages.
Figure 4 Aldehyde dehydrogenase 2 and vasodilation due to nitrates/nitrites/nitric oxide generation.
The generation of nitric oxide from nitrates/nitrites and its vasodilatory properties may contribute to prevent the deleterious effects of ischemia-reperfusion injury. ALDH2: Aldehyde dehydrogenase 2.
The role of ALDH2 cannot be conclusively confirmed, due to the impossibility of ruling out other non- enzymatic or nitrite reductase species (e.g., eNOS)[71-73]. However, it can be concluded that ALDH2 is closely related to NO and nitrogen metabolism and exerts positive effects[72,73].
ALDH2 agonist preconditioning: Alda-1
With this in mind, it has been proposed that new therapeutic ALDH2 approaches might be based on promoting ALDH2 over-expression by using ALDH2 activators and determining the role of ALDH2 in a variety of human pathologies such as IRI. Here we focus specifically on the use of Alda-1, a selective ALDH2 agonist, in IRI in different organs.
Small molecules called ALDAs have been used as activators of ALDH2. One of the most frequently used is Alda-1. Defined as N-(1,3-benzodioxol-5-ylmethyl)-2,6 di-chloro-benzamide, Alda-1 increases ALDH2 activity in humans twofold with its wild-type and 11-fold with its defective variant ALDH2*2[1].
Experiments have shown that the administration of Alda-1 just before the ischemic insult reduces infarct size by 60%, most likely through ALDH2 activation[12]. Alda-1 also has beneficial effects on the heart and brain by inhibiting 4-HNE and related adducts[15]. A potential therapeutic value of Alda-1 during the progression of post-myocardial infarction cardiomyopathy has also been suggested[29]. Alda-1 may be useful for treating heart failure patients, since ALDH2 activation in heart failure restores mitochondrial function and improves ventricular function and remodeling[48]. It was recently reported that ALDH2 activation regulates mitophagy by preventing 4-HNE, ROS and superoxide dismutase (SOD) accumulation[37]. Along these lines, the recent investigations by Zhu et al[20] have shown that Alda-1 pre-treatment reduces the intestinal injury induced by I/R in mice. Benefits were associated with the prevention of 4-HNE and MDA accumulation, suggesting a potential clinical application in the near future[21].
FUTURE PERSPECTIVES OF ALDH2 IN COLD ISCHEMIA REPERFUSION INJURY/ORGAN TRANSPLANTATION
ROS play a major role in the progression of IRI, which is inherent to organ transplantation. ROS are responsible for the generation of MDA, as well as other toxic aldehydes like 4-HNE and its adducts. During graft reperfusion, ROS are mainly produced in the mitochondria, becoming the main source of lipoperoxides and toxic aldehydes. Therefore, the prevention and clearance of those toxic elements generated during an episode of ischemia-reperfusion is decisive in organ transplantation, and the role of ALDH2 is crucial.
So far, the vast majority of investigations on the role of ALDH2 activation in organ preservation are limited to the heart and kidney[22,74]. Gong et al[74] demonstrated that addition of Alda-1 (ALDH2 activator) to histidine-ketoglutarate-tryptophan (HTK; a solution regularly used in heart surgery) improved cardio-protection through the subsequent ALDH2 activation and toxic aldehyde removal. The fact that Alda-1 ameliorates the quality of cardioplegic solutions such as HTK suggests that ALDH2 activators could be used to improve preservation solutions meant for other organs besides the heart, such as UW, HTK, Celsior and IGL-1, which are used for clinical transplantation in liver, where ALDH2 has an important presence[75].
These findings have a bearing on the consideration of the potential use of ALDH2 activators as additives in organ preservation solutions in order to improve their protective quality. They are also relevant to the assessment of the contribution of various commercial solutions to the preservation of mitochondrial ALDH2 activity during graft cold storage, especially when expanded criteria donors are used.
Recent studies have reported higher mitochondrial ALDH2 activity in liver grafts preserved in IGL-1 (24 h at 4 °C) than in livers preserved in UW or HTK solutions[76]. These results are consistent with the increases reported in renal ALDH2 activity by Zhong et al[22], who subjected kidney grafts to machine perfusion preservation before transplantation.
These results confirm that ALDH2 plays a role in both static and dynamic graft preservation. It exerts a protective effect, during the ischemic phase by tackling ATP breakdown and during the reperfusion phase by reducing oxidative damage, which are inherent conditions of IRI. In this regard, further studies of ALDH2 using machine perfusion strategies in subnormothermic conditions should be carried out in order to improve the subsequent graft outcome.
Finally, although ALDH2 plays a role in distinct organs with well-defined physiological functions (heart, brain, eyes, liver, kidney and intestine), each organ contains multiple different cell types which, in turn, may give specific responses under ALDH2 activation/administration. Few studies have sought to clarify the relationship between ALDH2 and specific cell types or its influence on the substantial cross-talk between the different cells in each organ under ALDH2 administration[77]. Therefore, further investigation of this issue is now warranted.
CONCLUSION
In recent years, the ability of ALDH2 to modify the activity of some key enzymes and essential survival pathways of the cell has been demonstrated (autophagy, apoptosis, necroptosis, etc). Some of its effects are exerted directly through its catalytic center, in a one-to-one interaction with those key enzymes that modifies the whole metabolic pathway. However, it may also regulate the metabolism indirectly, removing and cleansing toxic sub-products resulting from pathologies like IRI, which compromise cell viability, as we have seen in the case of 4-HNE (Figure 5).
Figure 5 Aldehyde dehydrogenase 2 and 4-hydroxy-2-nonenal balance in ischemia reperfusion injury.
Red areas represent damage, whereas green ones depict protection of the cell through the various mechanisms. ALDH2: Aldehyde dehydrogenase 2; 4-HNE: 4-hydroxy-2-nonenal.
As a result, ALDH2 will affect any metabolic pathway in which 4-HNE is involved, and so its importance lies not only in its ability to activate other enzymes, but also in the collateral effects of those metabolic processes regulated by 4-HNE. In these processes, the regulation levels will depend on the balance of several factors such as the aldehyde/ALDH2 production ratio (where ALDH2 agonists take on a major role), the metabolization ratio of 4-HNE by ALDH2 and whether 4-HNE reaches the level of no-return where it inhibits all the ALDH2 present and the balance is definitely broken (Figure 5).
ALDH2 affects a wide range spectrum of mechanisms, among which ethanol metabolism has been the most studied. Yet, its role in important pathophysiological processes, such as IRI, remains unclear. With regard to IRI, the behavior of ALDH2 differs according to the phase (ischemia or reperfusion). Given that a variety of harmful elements such as 4-HNE are produced during an I/R episode, ALDH2 may play a critical role in the liver. This role should be further elucidated in future studies.
However, just as most studies have focused on the role of ALDH2 in ethanol metabolism rather than IRI, studies of ALDH2 in IRI are mainly focused on heart, brain or kidney and have paid hardly any attention to the liver. This is especially surprising in view of the fact that the liver is the organ with the highest ALDH2 concentration recorded so far due its detoxifying role. Just before this review was completed, a study evidencing the protective role of ALDH2 activation by Alda-1 in a model of warm ischemia reperfusion in liver was published[78].
ACKNOWLEDGMENTS
This work was supported by Instituto de Salud Carlos III through FIS project PI 15/00110 co-funded by FEDER from Regional Development European Funds (European Union) and the FOIE GRAS project, which has received funding from the European Union’s Horizon 2020 Research and Innovation programme under the Marie Sklodowska-Curie Grant (Agreement No. 722619).
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abdel-Hamid SMM, Jin J, Katada K S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y