Published online Jan 14, 2018. doi: 10.3748/wjg.v24.i2.211
Peer-review started: November 7, 2017
First decision: November 14, 2017
Revised: November 27, 2017
Accepted: December 5, 2017
Article in press: December 5, 2017
Published online: January 14, 2018
Processing time: 68 Days and 11.8 Hours
To evaluate appropriate and rapid polyglycolic acid sheet (PGAs) covering time using device delivery station system (DDSS).
This pilot basic study was conducted to evaluate the potential of accurate and rapid PGAs delivery using DDSS. Three 11-mo-old female Beagle dogs were used in this study. Two endoscopic submucosal dissections (ESDs) 4cm in diameter were performed in lesser curvature of middle gastric body and greater curvature of antrum (total 6 ESDs performed). DDSS (3 cm length, 12 mm in outer diameter) has 2 chambers which 16 cm2 large 2 PGAs were stored, and DDSS was attached post ESD ulcers, respectively. Beriplast P® (CSL Behring K.K., Tokyo, Japan) (combination of fibrin glue and thrombin) was applied equally to the artificial ulcer, and tight attachment of 2 PGAs with DDSS were completed. The evaluation items were covering times, post ESD bleeding and perforation during ESD.
The covering time of PGAs (defined as the duration from the beginning of endoscope insertion into the mouth to the end of the fibrin glue coating process) was 6.07 (4.86-8.29) min. There was no post-ESD bleeding (1-7 d after ESD), and there was no perforation during ESD.
DDSS was very useful for rapid delivering and tight attachment of PGAs, and has potentials of multi-purpose delivery station system.
Core tip: Although mechanical closures of larger artificial ulcer floor are effective to prevent post-endoscopic submucosal dissection (ESD) bleeding, mechanical closures might create a mucosal bridge which reduces the efficacy of prevention of post-ESD bleeding. Although covering with polyglycolic acid sheet (PGAs) is a more ideal treatment, it is difficult to deliver thin PGAs by pan-endoscope. Therefore, novel concept to deliver various devices and materials were developed.
- Citation: Mori H, Kobara H, Nishiyama N, Masaki T. Novel concept of endoscopic device delivery station system for rapid and tight attachment of polyglycolic acid sheet. World J Gastroenterol 2018; 24(2): 211-215
- URL: https://www.wjgnet.com/1007-9327/full/v24/i2/211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i2.211
There were several reports of treatments for large artificial post-endoscopic submucosal dissection (ESD) ulcers, such as natural ulcer healing, ulcer closure using an Over-The-Scope Clip system (Ovesco Endoscopy GmbH, Tüebingen, Germany)[1] and covering an ulcer with a polyglycolic acid sheet (PGAs) (Gunze Co., Kyoto, Japan). PGAs has been used to prevent post-ESD bleeding[2]. Although mechanical closures of larger artificial ulcer floor are somewhat effective to prevent post-ESD bleeding, mechanical closures might create a mucosal bridge which reduces the efficacy of prevention of post-ESD bleeding. Therefore, covering with PGAs is a more ideal treatment. However, as it is difficult to deliver thin PGAs by pan-endoscope, PGAs delivery is one of the main problems of treating post-ESD ulcers[3,4].
Since the delivery method of such a thin membrane like PGAs into the stomach without getting wet with water has not been reported yet, the efficacy of PGAs covering perforation of gastric ulcer by sealed 2 balloon method [endoscopic injection sclerotherapy (EIS) balloon, TOP co., Tokyo, Japan] (11 mm in diameter, 50 mm length) was already reported as new method[5]. Based on the 2 EIS balloon method, we developed the new concept to deliver various endoscopic devices and materials named device delivery station system (DDSS).
This animal study assessed usefulness and future perspectives of the DDSS.
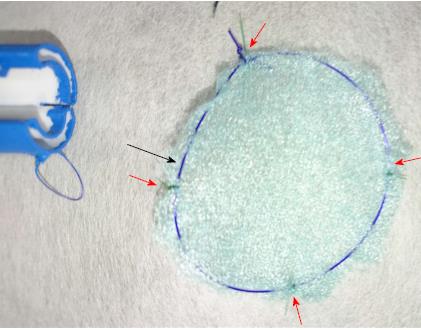
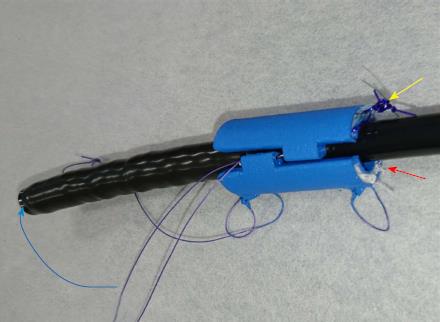
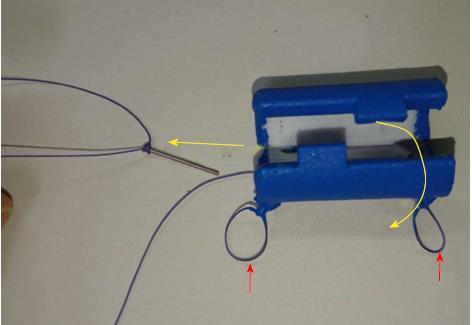
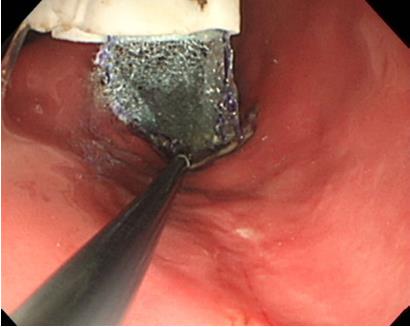
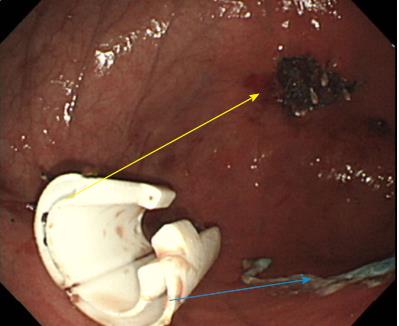
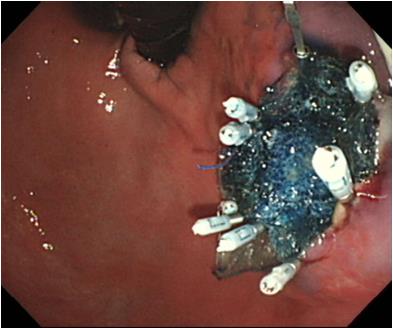
Three 11-mo-old female Beagle dogs were used in this study. This pilot basic study was conducted to evaluate the potential of accurate and rapid PGAs delivery using DDSS. This Animal experiment was approved by Animal Laboratory Regulations of Kagawa University according to the Regulations and Guidelines of animals experiment of Kagawa University. [See the supplement PDF file of Institutional Review Board Approval Form (Approval No: A34) of Kagawa University.] Two endoscopic submucosal dissections (ESDs) 4cm in diameter were performed in lesser curvature of middle gastric body and greater curvature of antrum (total 6 ESDs performed). The evaluation items were covering times, post ESD bleeding and perforation during ESD. DDSS (3 cm length, 12 mm in outer diameter) has 2 chambers which 16 cm2 large 2 PGAs were stored (Figure 1), and DDSS was easily mounted (Figure 2) and detached by pulling the thread connected with lock-pin (Figure 3) (Video). After endoscope mounting DDSS was inserted into dog’s stomach, DDSS was detached by pulling out the lock-pin with grasping forceps which was retrieved through endoscopic channel. Two PGAs were pulled out from each 2 chambers (Figure 4) and attached post ESD ulcers, respectively (Figure 5). Beriplast P® (CSL Behring K.K., Tokyo, Japan) (combination of fibrin glue and thrombin) was applied equally to the artificial ulcer, and tight attachment of 2 PGAs with DDSS were completed. In this study, 0.5 mL Indigo Carmine was mixed with transparent Beriplast P® for visualization of uniformly spraying onto ulcer floor (Figure 6) (Video).
Endoscopes: GIF TYPE Q260J (Olympus Co., Tokyo, Japan). Incisional knife: Dual knife® (KD-650 L), IT knife 2® (KD-611L) (Olympus Co., Tokyo, Japan). Incisional generator device: ERBE VIO300D (Elektromedizin, Tübingen, Germany). CO2 insufflation device: OLYMPUS UCR (Olympus Co., Tokyo, Japan). PGAs (Gunze Co., Kyoto, Japan).
The covering time of PGAs (defined as the duration from the beginning of endoscope insertion into the mouth to the end of the fibrin glue coating process) was 6.07 (4.86-8.29) min. There was no post-ESD bleeding (1-7 d after ESD), and there was no perforation during ESD. DDSS was the most effective way to deliver PGAs to post-ESD ulcer floor accurately and rapidly. There was no adverse event or complications at all during delivery of PGAs by DDSS.
PGAs are widely used in bio-absorbable sutures in the surgical field. In healing process, PGAs have anti-inflammatory effects with rich granulation tissue. PGAs also have the migration effects of epidermal cells. Early anti-inflammatory effects and rich granulation tissue contribute to protecting the ulcer floor[6].
Protection mechanism of PGAs seem to be same that of steroid local injection to prevent stenosis[7,8]. The tight attachment of PGAs to post-ESD ulcer is necessary to obtain a sufficient hemostatic effect. Therefore, DDSS was very useful for rapidly delivery and tightly attachment of PGAs onto post ESD artificial ulcer. Tight attachment of PGAs by DDSS might enable us to decrease post-ESD bleeding[9,10]. Post-ESD bleeding is one of serious adverse events for patients who take antiplatelet and/or anti coagulation agents. There were some reports that post bleeding events occurred within 7- 14 d after ESD. PGAs could prevent the post-ESD bleeding among patients using antithrombotic agents[11], and contribute to reduce the medical cost. In addition, there was no adverse event or complications at all during delivery of PGAs by DDSS.
Moreover, DDSS might be able to deliver much more devices and materials such as gauze, hemoclips and so on. DDSS has potential to considerably change the endoscopic treatment. However, prospective randomized control study is needed to confirm the advantages of DDSS and potential adverse events.
Post-Endoscopic submucosal dissections (ESD) bleeding is one of crucial adverse events for patients who take anti-thrombotic agents.
Although there were several reports with regard to polyglycolic acid sheet (PGAs) to prevent post-ESD bleeding, it is difficult to deliver and cover the post-ESD ulcer floor with PGAs. Developing new measurement or device to deliver PGAs is necessary to perform safer ESD.
To evaluate the efficacy of appropriate and rapid PGAs delivery method using innovative device delivery station system (DDSS).
Beagle dogs were used in this pilot basic study. Two ESDs 4 cm in diameter were performed (total 6 ESDs performed). Covering times of PGAs only by pan-endoscope, post ESD bleedings and perforations during ESD were investigated.
DDSS made it possible to cover post-ESD ulcer rapidly and reduce the post-ESD adverse event (bleeding of 1-7 d after ESD).
DDSS was very useful for rapid delivering and tight attachment of PGAs to control post-ESD bleeding. DDSS can deliver lots of endoscopic devices and surgical materials that can be placed within the digestive tract under sealed conditions.
DDSS has innovative potentials of multi-purpose device delivery measurements.
Multi-center prospective studies are needed to confirm the advantages of DDSS not only for PGAs delivery but also other endoscopic devices and materials.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dogan UB, Dinc T, Shrestha BM S- Editor: Chen K L- Editor: A E- Editor: Ma YJ
| 1. | Maekawa S, Nomura R, Murase T, Ann Y, Harada M. Complete closure of artificial gastric ulcer after endoscopic submucosal dissection by combined use of a single over-the-scope clip and through-the-scope clips (with videos). Surg Endosc. 2015;29:500-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Ono H, Takizawa K, Kakushima N, Tanaka M, Kawata N. Application of polyglycolic acid sheets for delayed perforation after endoscopic submucosal dissection of early gastric cancer. Endoscopy. 2015;47 Suppl 1 UCTN:E18-E19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Tsuji Y, Ohata K, Gunji T, Shozushima M, Hamanaka J, Ohno A, Ito T, Yamamichi N, Fujishiro M, Matsuhashi N. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to cover wounds after colorectal endoscopic submucosal dissection (with video). Gastrointest Endosc. 2014;79:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Mori H, Kobara H, Rahman A, Nishiyama N, Nishiyama A, Suzuki Y, Masaki T. Innovative delivery method using a detachable device to deliver a large polyglycolic acid sheet to a gastric ulcer perforation. Endoscopy. 2017;49:E165-E167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Iizuka T, Kikuchi D, Yamada A, Hoteya S, Kajiyama Y, Kaise M. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy. 2015;47:341-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1389-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Hanaoka N, Ishihara R, Takeuchi Y, Uedo N, Higashino K, Ohta T, Kanzaki H, Hanafusa M, Nagai K, Matsui F. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy. 2012;44:1007-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Shindo Y, Matsumoto S, Miyatani H, Yoshida Y, Mashima H. Risk factors for postoperative bleeding after gastric endoscopic submucosal dissection in patients under antithrombotics. World J Gastrointest Endosc. 2016;8:349-356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Fukuda H, Yasmaguchi N, Isomoto H, Matsushima K, Minami H, Akazawa Y, Ohnita K, Takeshima F, Shikuwa S, Nakao K. Polyglycolic Acid Felt Sealing Method for Prevention of Bleeding Related to Endoscopic Submucosal Dissection in Patients Taking Antithrombotic Agents. Gastroenterol Res Pract. 2016;2016:1457357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |