Copyright
©The Author(s) 2018.
World J Gastroenterol. Apr 28, 2018; 24(16): 1679-1707
Published online Apr 28, 2018. doi: 10.3748/wjg.v24.i16.1679
Published online Apr 28, 2018. doi: 10.3748/wjg.v24.i16.1679
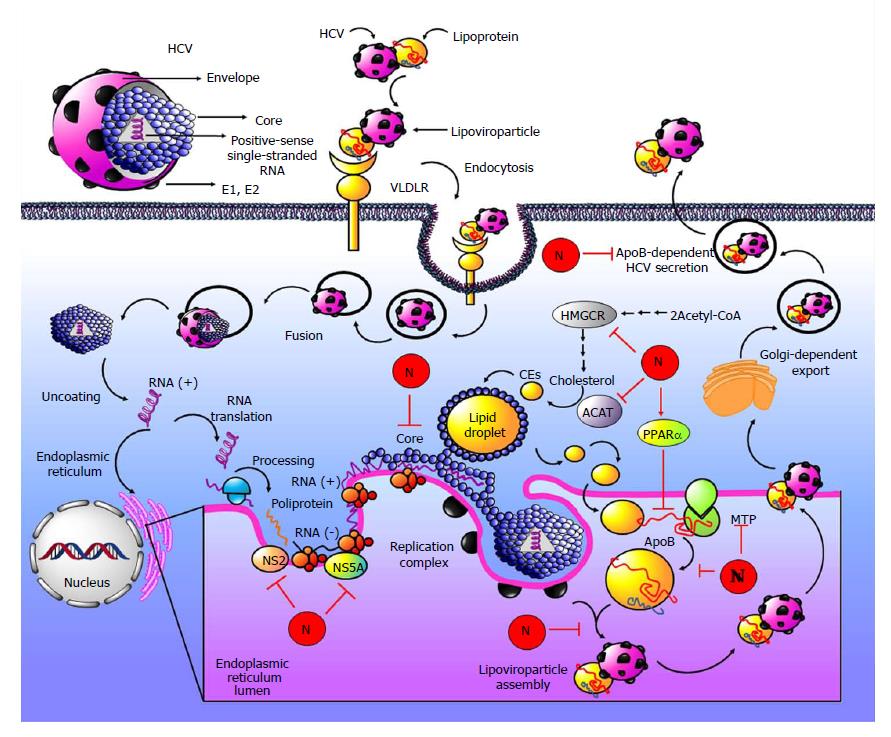
Figure 9 Antiviral properties of naringenin.
The Hepatitis C virus (HCV) genome consists of a positive-sense single-stranded RNA. HCV adopts an icosahedral structure with a lipid envelope and glycoproteins E1 and E2 immersed in the envelope. Underneath the envelope is the nucleocapsid, which is composed of multiple copies of core forming the internal viral coat that encapsulates the genomic RNA. HCV may be associated with lipoproteins such as very-low density lipoprotein (VLDL), forming a lipoprotein complex called lipoviroparticle. Binding of lipoviroparticle to very-low density lipoprotein receptor (VLDLR) results in virus endocytosis; after that, nucleocapsids are deposited into the cytoplasm. Then, nucleocapsids are uncoated, and the RNA is released. The genomic RNA is translated to the endoplasmic reticulum when HCV polyprotein is produced. The positive-stranded RNA genome is used as a template for synthesis of negative-stranded RNA; this new viral RNA is encapsulated within multiple copies of the core to form the nucleocapsid, and then, it acquires envelope and HCV virions, which are exported out of the cell in a Golgi-dependent manner. Naringenin inhibits the secretion and assembly of HCV through regulating lipid metabolism via 3-hydroxy-3-methylglutaryl CoA reductase (HMGCR), and acyl-coenzyme A: cholesterol acyltransferase (ACAT) inhibition. Cholesterol is synthetized in an HMGCR-dependent pathway; it is the rate limiting enzyme for cholesterol synthesis, and then, cholesterol is converted to cholesteryl esters (CEs) by ACAT. CEs are very important to VLDL assembly. In addition, microsomal triglyceride transfer protein (MTP) catalyzes the transfer of lipids to the apolipoprotein (Apo) B-100 ApoB100; if the ApoB-MTP binding is inhibited, VLDL assembly in inhibited. Reduction in the bioavailability of CEs, triglycerides and cholesterol by naringenin reduces MTP activity and apoB-MTP binding. In addition, naringenin decreased intracellular triglycerides through peroxisome proliferator activated receptor alpha (PPARα), a regulator of lipid metabolism. Through these mechanisms, naringenin leads to a reduction in VLDL assembly and to the inhibition of ApoB-dependent HCV secretion. Additionally, naringenin inhibits viral NS5A protein, a multifunctional HCV nonstructural protein. Furthermore, naringenin could be an NS2 protease and core protein inhibitor.
- Citation: Hernández-Aquino E, Muriel P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J Gastroenterol 2018; 24(16): 1679-1707
- URL: https://www.wjgnet.com/1007-9327/full/v24/i16/1679.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i16.1679