Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1310
Peer-review started: December 21, 2016
First decision: January 10, 2017
Revised: January 20, 2017
Accepted: February 7, 2017
Article in press: February 8, 2017
Published online: February 21, 2017
Processing time: 62 Days and 22.5 Hours
To perform a meta-analysis of the related studies to assess whether circulating tumor cells (CTCs) can be used as a prognostic marker of esophageal cancer.
PubMed, Embase, Cochrane Library and references in relevant studies were searched to assess the prognostic relevance of CTCs in patients with esophageal cancer. The primary outcome assessed was overall survival (OS). The meta-analysis was performed using the random effects model, with hazard ratio (HR), risk ratio (RR) and 95% confidence intervals (95%CIs) as effect measures.
Nine eligible studies were included involving a total of 911 esophageal cancer patients. Overall analyses revealed that CTCs-positivity predicted disease progression (HR = 2.77, 95%CI: 1.75-4.40, P < 0.0001) and reduced OS (HR = 2.67, 95%CI: 1.99-3.58, P < 0.00001). Further subgroup analyses demonstrated that CTCs-positive patients also had poor OS in different subsets. Moreover, CTCs-positivity was also significantly associated with TNM stage (RR = 1.48, 95%CI: 1.07-2.06, P = 0.02) and T stage (RR = 1.44, 95%CI: 1.13-1.84, P = 0.003) in esophageal cancer.
Detection of CTCs at baseline indicates poor prognosis in patients with esophageal cancer. However, this finding relies on data from observational studies and is potentially subject to selection bias. Prospective trials are warranted.
Core tip: The clinical validity of circulating tumor cells (CTCs) is still controversial and inconclusive in patients with esophageal cancer. Our meta-analysis provides strong evidence that detection of CTCs in peripheral blood at baseline is an independent prognosticator of poor survival outcomes in esophageal cancer patients.
- Citation: Xu HT, Miao J, Liu JW, Zhang LG, Zhang QG. Prognostic value of circulating tumor cells in esophageal cancer. World J Gastroenterol 2017; 23(7): 1310-1318
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1310
Esophageal cancer (EC) is the eighth most common malignant tumor worldwide[1] and is the sixth leading cause of cancer death[2]. However, the early diagnosis of EC is difficult due to the lack of specific symptoms in the early stages. In most cases, the disease is already at an advanced stage at presentation. EC ranks fourth among cancer-related deaths in China[3]. Surgical resection is the main treatment for EC, with a postoperative 5-year survival rate of only 34%-36%[2]. New treatment strategies including neoadjuvant radiochemotherapy[4,5], preoperative neoadjuvant chemotherapy[6,7], and three-field lymph node dissection[8] are helpful for improving the 5-year survival rate of EC patients. However, the outcomes are still unsatisfactory, and many patients die of local recurrence and distant metastasis[9]. Therefore, biomarkers which can be used to identify the recurrence or metastasis of EC are needed to facilitate timely diagnosis and treatment strategies and thus improve the prognosis of EC patients.
In the early stage of EC metastasis or recurrence, the clinical manifestations are occult and cannot be effectively predicted by routine laboratory tests. In recent years, circulating tumor cells (CTCs) have been recognized as the cause of tumor metastasis or recurrence[10,11]. CTCs are the cells that are shed from a primary or metastatic tumor into the peripheral circulation. Most CTCs will be cleared by the human immune system, whereas a small number of surviving CTCs can reach other parts of the body via the bloodstream, resulting in tumor metastasis[12]. CTCs can remain non-proliferative for a long period of time and can resist the anti-tumor effect of chemotherapy drugs[13,14]. At present, many studies on the correlations between CTC positivity and the prognoses of breast cancer[15], colorectal cancer[16], gastric cancer[17], and lung cancer[18] have shown that CTCs-positivity can indicate poor prognosis.
Although many studies have demonstrated the relationship between CTCs and the prognosis and clinicopathological features of EC, their findings had certain limitations due to differences in CTC detection methods and EC treatment strategies. In addition, the role of CTC detection before surgical or non-surgical treatment in EC patients remains unclear. Therefore, in this meta-analysis, we summarized and analyzed the prognostic value of CTCs in EC patients before and after treatment in a quantitative and comprehensive manner.
A literature search of related studies was conducted using the PubMed, Embase, and Cochrane Library databases. The following search terms were used: (1) “circulating tumor cells” or “CTCs”; (2) “esophageal cancer” using MeSH or free words; and (3) a combination of (1) and (2). The last search was conducted on November 17, 2016.
Two authors, Xu HT and Miao J, independently retrieved the titles and abstracts of the primary studies identified in the electronic search. In addition, references of potentially relevant studies were examined. Duplicate studies were excluded.
The inclusion criteria were as follows: (1) population: patients with esophageal cancer; (2) intervention: CTCs-positivity; (3) comparison: CTCs-negativity; (4) outcome: the primary outcome assessed was overall survival (OS), and clinicopathological characteristics and other prognostic outcomes were assessed as secondary outcomes; (5) design: randomized controlled trials (RCTs) or observational studies; (6) samples used in these studies should be collected from peripheral blood (PB) and at baseline; and (7) sufficient data to calculate hazard ratio (HR) or risk ratio (RR) with 95% confidence intervals (95%CIs) as comparable effect estimates.
Exclusion criteria included the following: (1) review articles, letters, comments and case reports; and (2) studies where it was impossible to retrieve or calculate data of interest.
Data extraction was performed by Xu HT and Miao J independently. The following information was extracted from each study: (1) first author, year of publication, country and study type; (2) number and characteristics of patients in both the CTCs positivity and negativity groups; and (3) outcome data including follow-up period, OS and other prognostic outcomes such as disease-free survival (DFS) or progression-free survival (PFS) or relapse-free survival (RFS).
All relevant texts, tables and figures were reviewed for data extraction, and entered into an Excel file. Furthermore, we included only the most recent or complete studies to avoid duplication of information. Discrepancies between the two reviewers were resolved by discussion and consensus.
The Cochrane risk of bias tool was adopted to assess the risk of bias in RCTs[19]. Observational studies were evaluated using the Newcastle-Ottawa Scale[20].
Analyses were performed using Cochrane RevMan 5.3. For time-to-event data, HR and 95%CIs were obtained directly or indirectly from studies according to the method described by Tierney[21]. RR was used to compare dichotomous variables. Heterogeneity was tested using the I2 statistic. Studies with an I2 statistic of 0%, 25%, 50% and 75% represented no, low, moderate and high heterogeneity[22,23]. The random effect models were used for the analysis, as this model can obtain more conservative results and better fit the multi-center clinical studies due to the existence of heterogeneity[24]. The Generic Inverse Variance method was used to calculate pooled HRs and 95%CIs. The Mantel-Haenszel method was used to calculate pooled RRs and 95%CIs.
Moreover, a sensitivity analysis was conducted by deleting each study individually to evaluate the quality and consistency of the results. Visual inspection of the funnel plot was carried out to assess publication bias.
In addition, subgroup analyses of the studies were conducted according to region (Asia vs non-Asia), curative method (surgery vs non-surgery), and method used to detect CTCs (CellSearch vs RT-PCR vs other methods). The subgroup analyses were performed only for OS.
A total of 306 studies were identified from the initial database search. Fifty-seven studies were excluded due to duplicates, and 230 studies were excluded for various reasons based on the titles and abstracts (reviews, case reports, or clearly irrelevant to the analysis). Ten studies were excluded due to reviews or the lack of an outcome of interest. In total, 9 studies were included in the meta-analysis[25-33]. The selection process is shown in Figure 1.
The main characteristics of the included studies are shown in Table 1. The studies were conducted in three countries (China, Japan and Germany) and published between 2009 and 2016. Of the included studies, none were RCTs, 5 were prospective cohort studies[25,27-29,31], and 4 were cohort studies[25,30,32,33]. The sample size ranged from 38 to 244 (CTCs-positive group, n = 336; CTCs-negative group, n = 575). All studies assessed CTCs at baseline. Of the 9 studies, 7 studies contained data on clinicopathological characteristics[26-31,33], 8 had HRs for OS[25-29,31-33], 2 had HRs for PFS[25,30], 2 had HRs for DFS[26,31], and 2 had HRs for RFS[27,33].
| Ref. | Country | Study design | Number (M/F) | Age, yr (range) | Population | Detection method | Sample time | Rate% (n/N)1 | Cut-off criteria | Curative method | Follow-up | Outcome |
| Li et al[26], 2016 | China | Cohort | 140 (117/23) | 62.8 ± 8.5 (36-78) | ESCC | Fluorescent IHC | Baseline | 44 (62/140) | > 2/5 mL | Surgery | 3 yr | OS, DFS |
| Su et al[25], 2016 | China | Prospective cohort | 57 (55/2) | 54 (36-78) | EC | Flow cytometry | Baseline | 50 (29/57) | ≥ 21/mL | CCRT | 3 yr | OS, PFS |
| Reeh et al[27], 2015 | Germany | Prospective cohort | 100 (77/23) | 66 (32-85) | EC | CellSearch | Baseline | 18 (18/100) | ≥ 1/7.5 mL | Surgery | 37.5 mo (median) | OS, RFS |
| Matsushita et al[28], 2015 | Japan | Prospective cohort | 90 (78/12) | 65 (46-98) | ESCC | CellSearch | Baseline and after treatment | 27 (25/90) | ≥ 1/7.5 mL | Chemotherapy or CRT | 10.3 mo (median), range 0.3-36.4 mo | OS |
| Tanaka et al[29], 2015 | Japan | Prospective cohort | 38 (30/8) | 63 (43-87) | EC | CellSearch | Baseline and after treatment | 50 (19/38) | ≥ 2/7.5 mL | Chemotherapy or CRT | 19 mo (median) | OS |
| Yin et al[30], 2012 | China | Cohort | 72 (54/18) | 63 (46-83) | ESCC | RT-PCR | Baseline and after treatment | 69 (50/72) | Expression of any one of CEA, CK19, survivin | Radiotherapy | 2 yr | PFS |
| Tanaka et al[31], 2010 | Japan | Prospective cohort | 244 (212/32) | 64 (NR) | ESCC | RT-PCR | Baseline and after treatment | 13 (34/244) | Expression of any one of CEA, SCCA | Surgery | 24.3 mo (median) | OS, DFS |
| Hoffmann et al[32], 2010 | Germany | Cohort | 62 (53/9) | 61 (NR) | EC | RT-PCR | Baseline | 77 (48/62) | Expression of survivin | Surgery | 3 yr (median) | OS |
| Gao et al[33], 20092 | China | Cohort | 108 (85/23) | 58.9 (36-82) | ESCC | RT-PCR | Baseline | 47 (51/108) | Expression of survivin | Surgery | 19.5 mo (median), range, 1-33 mo | OS, RFS |
Assessment of risk of bias in the studies is shown in Table 2. Based on the Newcastle-Ottawa Scale to assess the risk of bias in cohort studies, 7 studies were rated as having a total score of > 5[25-29,31,32], and 2 as having a score of ≤ 5, indicating a high risk of bias[30,33].
| Study | Selection | Comparability | Outcome | Totalscore | |||||
| Exposed cohort | Non-exposed cohort | Ascertainment of exposure | Outcome of interest | Assessment of outcome | Length of follow-up | Adequacy of follow-up | |||
| Li et al[26], 2016 | NS | S | S | S | NS | S | S | S | 6 |
| Su et al[25], 2016 | S | S | S | S | NS | S | S | S | 7 |
| Reeh et al[27], 2015 | S | S | S | S | NS | S | S | S | 7 |
| Matsushita et al[28], 2015 | NS | S | S | S | NS | S | S | S | 6 |
| Tanaka et al[29], 2015 | S | S | S | S | NS | S | NS | S | 6 |
| Yin et al[30], 2012 | NS | S | S | S | NS | S | NS | S | 5 |
| Tanaka et al[31], 2010 | NS | S | S | S | NS | S | S | S | 6 |
| Hoffmann et al[32], 2010 | S | S | S | S | NS | S | S | S | 7 |
| Gao et al[33], 2009 | NS | S | S | S | NS | S | NS | NS | 4 |
The HRs for OS were available in 8 studies[25-29,31-33], including 779 EC patients. The pooled results showed that CTCs-positive EC patients had significantly poorer OS than CTCs-negative patients (HR = 2.67, 95%CI: 1.99-3.58, P < 0.00001), and heterogeneity was statistically nonsignificant (I2 = 14%, P = 0.32) (Figure 2).
We performed subgroup analyses to further assess whether CTCs status had prognostic value in different subsets (Table 3). We first evaluated the effects of CTCs status on OS regarding region and found that for both Asians and non-Asians, detection of CTCs predicted a poor prognosis (Asian: HR = 2.46, 95%CI: 1.77-3.40, I2 = 14%, P < 0.00001; non-Asian: HR = 3.74, 95%CI: 1.98-7.05, I2 = 0%, P < 0.0001). We then determined the effects of CTCs status on OS with regard to curative method and discovered that for both surgery and non-surgery, detection of CTCs at baseline indicated an increased risk of poor prognosis (surgery: HR = 2.81, 95%CI: 1.72-4.58, I2 = 50%, P < 0.0001; non-surgery: HR = 2.70, 95%CI: 1.70-4.30, I2 = 0%, P < 0.0001). We also assessed the effects of CTCs status on OS with regard to detection method and found that CTCs detection by CellSearch or RT-PCR or other methods indicated a worse prognosis (CellSearch: HR = 2.91, 95%CI: 1.78-4.74, I2 = 0%, P < 0.0001; RT-PCR: HR = 3.44, 95%CI: 1.42-8.34, I2 = 70%, P = 0.006; other methods: HR = 2.22, 95%CI: 1.38-3.58, I2 = 0%, P = 0.001). In addition, the stratified results showed that compared to CTCs-negative patients, CTCs-positive patients had a higher risk for poor OS in these subgroups.
| No. of studies | No. of patients | HR (95%CI) | P value | Heterogeneity | ||
| I2 | P value | |||||
| Region | ||||||
| Asia | 6 | 637 | 2.46 (1.77-3.40) | < 0.00001 | 14% | 0.33 |
| Non-Asia | 2 | 162 | 3.74 (1.98-7.05) | < 0.0001 | 0% | 0.36 |
| Curative method | ||||||
| Surgery | 5 | 594 | 2.81 (1.72-4.58) | < 0.0001 | 50% | 0.09 |
| Non-surgery | 3 | 185 | 2.70 (1.70-4.30) | < 0.0001 | 0% | 0.95 |
| Detection method | ||||||
| CellSearch | 3 | 228 | 2.91 (1.78-4.74) | < 0.0001 | 0% | 0.93 |
| RT-PCR | 3 | 354 | 3.44 (1.42-8.34) | 0.006 | 70% | 0.04 |
| Other methods | 2 | 197 | 2.22 (1.38-3.58) | 0.001 | 0% | 0.44 |
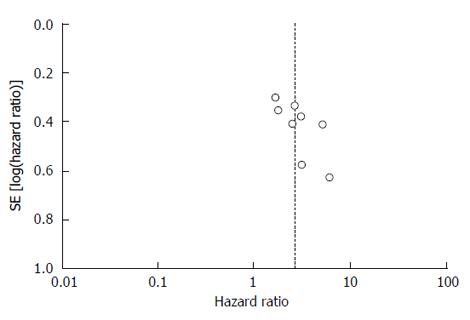
The funnel plot did not show obvious asymmetry (Figure 3). Therefore, no significant publication bias was observed.
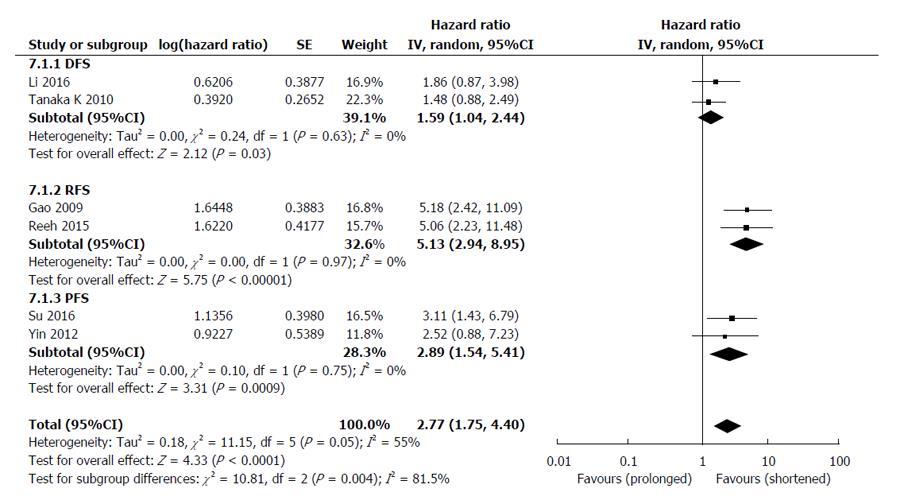
The HRs for disease progression (DFS, RFS and PFS) were available in 6 studies[25-27,30,31,33], involving 661 EC patients. The overall analysis revealed that compared with CTCs-negative EC patients, the CTCs-positive patients had a higher risk of disease progression (HR = 2.77, 95%CI: 1.75-4.40, I2 = 55%, P < 0.0001) (Figure 4). Sensitivity analyses confirmed the stability of our results, and indicated that our results were not obviously affected or dominated by a single study.
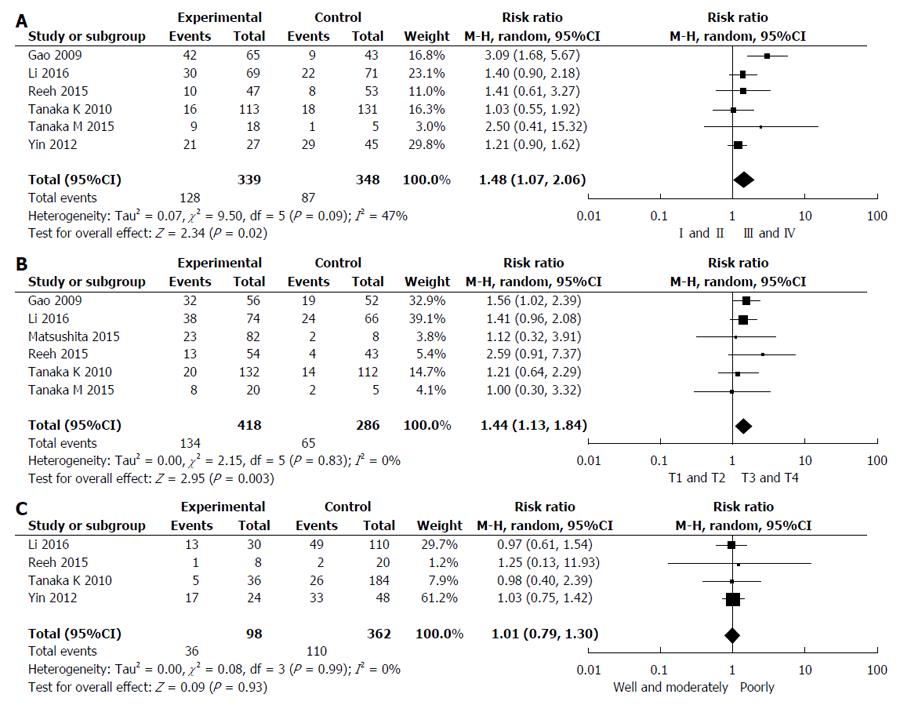
Six studies reported the relationship between CTCs status and TNM stage[26,27,29-31,33]. Pooled analysis showed that CTCs-positivity in stage III and IV was greater than that in I and II (RR = 1.48, 95%CI: 1.07-2.06, I2 = 47%, P = 0.02) as shown in Figure 5A. Studies assessed by pooled analysis showed a significant association between CTCs-positivity and T stage (RR = 1.44, 95%CI: 1.13-1.84, I2 = 0%, P = 0.003) (Figure 5B)[26-29,31,33], but a non-significant association between CTCs-positivity and histological differentiation (RR = 1.01, 95%CI: 0.79-1.30, I2 = 0%, P = 0.93) (Figure 5C)[26,27,30,31]. Six studies assessed the relationship between CTCs-positivity and N stage (RR = 1.47, 95%CI: 1.09-1.98, I2 = 36%, P = 0.01) [26-28,30,31,33]. However, when the Yin 2012 study was removed, no statistical significance in the five remaining studies was observed (RR = 1.51, 95%CI: 0.98-2.32, I2 = 48%, P = 0.06).
Although radical surgery and neoadjuvant radiochemotherapy have been widely used in EC patients, metastasis or recurrence of EC still poses a significant challenge for doctors and patients. Therefore, biomarkers which can be used to identify the recurrence or metastasis of EC are needed to facilitate the timely diagnosis and treatment strategies for EC patients. CTCs, released by primary tumors, are regarded as a key stage of tumorigenesis[34]; their further development leads to metastatic lesions, which can be explained by the “seed and soil” theory[35]. It is generally believed that lymph node metastasis occurs prior to blood-borne metastasis; however, the detection of CTCs in early tumors indicates that blood-borne metastasis can occur even in the early stage of tumorigenesis, i.e., before the occurrence of lymph node metastasis[36]. In one study[31], peripheral venous blood CTCs were detected before and after the surgical treatment of EC; it was found that the preoperative positive expression of CTCs was not correlated with prognosis, whereas the postoperative positive expression of CTCs was significantly correlated with prognosis. However, in another study[27], positive CTCs in peripheral venous blood before treatment were thought to be correlated with prognosis. Thus, the value of CTC detection before surgical or non-surgical treatment in EC patients requires further investigation.
In this meta-analysis, we conducted a comprehensive literature search and demonstrated that detection of peripheral venous blood CTCs in EC patients can predict disease progression and poor prognosis. Compared with a previous study[37], in the present study, we included most recent literature and restricted the CTCs detection time to “before treatment”. It is well known that EC patients who have received surgical treatment or non-surgical treatment had different prognoses. Our subgroup analysis of EC treatment further showed that detection of CTCs before treatment is valuable for predicting the prognosis of EC patients, and the expression of CTCs was not significantly correlated with treatment mode. In addition, considering the differences in treatment mode and observation time, we combined DFS, RFS, and PFS as “disease progression” and carried out a subgroup analysis accordingly.
Regional differences and differences in treatment modes and CTCs examination methods can also result in clinical heterogeneity; in this regard, we also carried out a subgroup analysis. As shown in our study, there was no significant heterogeneity when the relationship between CTCs status and the OS of EC patients was analyzed, and subgroup analysis also showed stable results. However, heterogeneity was found in the relationship between CTC status and disease progression. The sensitivity analysis showed that the exclusion of any of the articles did not affect the outcome, and the heterogeneity was generated from the difference in the clinical observation time points among different studies. Also, different studies came to different conclusions regarding the relationship between CTC status and clinicopathological factors. Li et al[26] and Matsushita et al[28] concluded that positive CTC expression was correlated with TNM stage, but not with T stage, N stage, and the degree of differentiation; Reeh et al[27] and Tanaka et al[31] found that the CTC status was not correlated with TNM stage, T stage, and lymph node metastasis; and other studies[33,38] found that CTC positivity was correlated with N stage. In the present study, we found that CTC status was associated with TNM stage and T stage, but not with lymph node metastasis or degree of differentiation. Because different TNM staging methods were used among studies, and the clinical stages and pathological stages also differed, heterogeneity was inevitable in the present study. The results were unstable during the pooled analysis of the relationship between N stage and CTC status. However, when one article by Yin et al[30] 2012 was removed, the research outcome changed, which may be explained by the fact that the N stage in five other articles[26-28,31,33] was the pathological stage, whereas Yin et al[30] used clinical N stage.
Our meta-analysis had some limitations. First, potential biases such as gender, age, and race could not be avoided or controlled during the pooled analysis,which contains females are less susceptive to this type of cancer,white man in certain countries are more susceptible and in Asia(specifically in China) the squamous esophageal cancer is detected more than esophageal adenocarcinoma etc. Second, although no publication bias was found during analysis of the relationship between CTC positivity and the survival rate of EC patients, all the included articles were published in the English literature, which may have led to the omission of some non-English literature with negative results. In addition, most studies were conducted in Asian countries and areas and hence were less representative. Third, some of the included literature did not explicitly provide HR and 95%CI values, which had to be extracted from the relevant data and curves in the literature. Fourth, the differences in CTC detection methods, EC therapeutic approaches, and EC staging methods could also have affected the judgment of prognosis. Despite these limitations, we still demonstrated the relationships between CTCs and the prognosis and clinicopathological factors of EC.
In conclusion, CTC detection may be a valuable tool for improving the prognosis of EC patients, and it may be possible to carry out individualized therapy based on the results of CTC detection in the future. For postoperative EC patients, CTCs monitoring can provide individualized clinical information during the follow-up. For EC patients requiring radiochemotherapy, CTCs monitoring may help identify the risk of tumor progression and enable some patients to benefit from second-line therapy. However, some problems still need to be addressed before CTC detection is applied in clinical settings. First, a variety of methods have been developed for detecting CTCs[39]; therefore, multi-center studies are required to standardize the CTC detection techniques and define CTCs reference values. Second, the CTC detection results may be negative in peripheral blood samples in some EC patients with dominant metastasis[40,41], which may be because the detection markers for metastatic lesions are not expressed or because the expression levels of these markers are below the detection thresholds. Therefore, efforts should be made to optimize the detection platforms for CTCs to improve the sensitivity of CTC detection. Finally, the value of CTC detection in predicting prognosis and the risk of EC recurrence/metastasis still need to be validated in large multi-center clinical trials.
Circulating tumor cells are cells released from the primary tumor into peripheral blood and are considered to be the main cause of tumor metastasis. The prognostic role of circulating tumor cells in esophageal cancer has been widely investigated.
The clinical validity of circulating tumor cells is still controversial and inconclusive in patients with esophageal cancer. The aim of this meta-analysis was to include available studies to assess whether circulating tumor cells can be used as a prognostic marker in esophageal cancer.
This meta-analysis provides strong evidence to indicate that detection of circulating tumor cells in peripheral blood at baseline is an independent prognosticator of poor survival outcomes in esophageal cancer patients. More convincing results will be obtained by increasing the sample size.
Circulating tumor cells detected in peripheral blood can predict aggressive disease progression and poor overall survival in patients with esophageal cancer.
It is a well written article. Statistical analysis is comprehensive and well presented. The paper will attract attention of clinical scientists and surgeons in the field of esophageal cancer worldwide.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nabissi M, Sukocheva OA, Vinh-Hung V S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21347] [Article Influence: 2134.7] [Reference Citation Analysis (3)] |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1957] [Article Influence: 163.1] [Reference Citation Analysis (5)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13187] [Article Influence: 1465.2] [Reference Citation Analysis (3)] |
| 4. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4048] [Article Influence: 311.4] [Reference Citation Analysis (0)] |
| 5. | Shapiro J, van Lanschot JJ, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, van Laarhoven HW, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1805] [Article Influence: 180.5] [Reference Citation Analysis (0)] |
| 6. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4578] [Article Influence: 240.9] [Reference Citation Analysis (0)] |
| 7. | van der Sluis PC, Ubink I, van der Horst S, Boonstra JJ, Voest EE, Ruurda JP, Borel Rinkes IH, Wiezer MJ, Schipper ME, Siersema PD. Safety, efficacy, and long-term follow-up evaluation of perioperative epirubicin, Cisplatin, and capecitabine chemotherapy in esophageal resection for adenocarcinoma. Ann Surg Oncol. 2015;22:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ma GW, Situ DR, Ma QL, Long H, Zhang LJ, Lin P, Rong TH. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol. 2014;20:18022-18030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 9. | Zhu ZJ, Hu Y, Zhao YF, Chen XZ, Chen LQ, Chen YT. Early recurrence and death after esophagectomy in patients with esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:1502-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med. 1997;337:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 251] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Sclafani F, Smyth E, Cunningham D, Chau I, Turner A, Watkins D. A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer. 2014;13:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Peeters DJ, Brouwer A, Van den Eynden GG, Rutten A, Onstenk W, Sieuwerts AM, Van Laere SJ, Huget P, Pauwels P, Peeters M. Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer. Cancer Lett. 2015;356:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2776] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 14. | Abdallah EA, Fanelli MF, Buim ME, Machado Netto MC, Gasparini Junior JL, Souza E Silva V, Dettino AL, Mingues NB, Romero JV, Ocea LM. Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients. Int J Cancer. 2015;137:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Lu YJ, Wang P, Wang X, Peng J, Zhu YW, Shen N. The significant prognostic value of circulating tumor cells in triple-negative breast cancer: a meta-analysis. Oncotarget. 2016;7:37361-37369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Huang X, Gao P, Sun J, Chen X, Song Y, Zhao J, Xu H, Wang Z. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: a meta-analysis. Int J Cancer. 2015;136:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Wang J, Wang K, Xu J, Huang J, Zhang T. Prognostic significance of circulating tumor cells in non-small-cell lung cancer patients: a meta-analysis. PLoS One. 2013;8:e78070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24605] [Article Influence: 1757.5] [Reference Citation Analysis (3)] |
| 20. | Wells GA, Shea B, O’Connell D, Peterson J, Wekch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Ottawa Hospital Research Institute website. Available from: http: //www.ohri.ca/programs/clinical_epidemiology/oxford.asp.. |
| 21. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4931] [Article Influence: 273.9] [Reference Citation Analysis (0)] |
| 22. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46193] [Article Influence: 2099.7] [Reference Citation Analysis (3)] |
| 23. | Gu WJ, Wang F, Tang L, Liu JC. Single-dose etomidate does not increase mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials and observational studies. Chest. 2015;147:335-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Schmidt FL, Oh IS, Hayes TL. Fixed- versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br J Math Stat Psychol. 2009;62:97-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 25. | Su PJ, Wu MH, Wang HM, Lee CL, Huang WK, Wu CE, Chang HK, Chao YK, Tseng CK, Chiu TK. Circulating Tumour Cells as an Independent Prognostic Factor in Patients with Advanced Oesophageal Squamous Cell Carcinoma Undergoing Chemoradiotherapy. Sci Rep. 2016;6:31423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Li SP, Guan QL, Zhao D, Pei GJ, Su HX, Du LN, He JX, Liu ZC. Detection of Circulating Tumor Cells by Fluorescent Immunohistochemistry in Patients with Esophageal Squamous Cell Carcinoma: Potential Clinical Applications. Med Sci Monit. 2016;22:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Reeh M, Effenberger KE, Koenig AM, Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK, Izbicki JR, Pantel K. Circulating Tumor Cells as a Biomarker for Preoperative Prognostic Staging in Patients With Esophageal Cancer. Ann Surg. 2015;261:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Matsushita D, Uenosono Y, Arigami T, Yanagita S, Nishizono Y, Hagihara T, Hirata M, Haraguchi N, Arima H, Kijima Y. Clinical Significance of Circulating Tumor Cells in Peripheral Blood of Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2015;22:3674-3680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Tanaka M, Takeuchi H, Osaki Y, Hiraiwa K, Nakamura R, Oyama T, Takahashi T, Wada N, Kawakubo H, Saikawa Y. Prognostic significance of circulating tumor cells in patients with advanced esophageal cancer. Esophagus. 2015;12:352-359. [DOI] [Full Text] |
| 30. | Yin XD, Yuan X, Xue JJ, Wang R, Zhang ZR, Tong JD. Clinical significance of carcinoembryonic antigen-, cytokeratin 19-, or survivin-positive circulating tumor cells in the peripheral blood of esophageal squamous cell carcinoma patients treated with radiotherapy. Dis Esophagus. 2012;25:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Tanaka K, Yano M, Motoori M, Kishi K, Miyashiro I, Shingai T, Gotoh K, Noura S, Takahashi H, Ohue M. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol. 2010;17:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Hoffmann AC, Vallböhmer D, Grimminger P, Metzger R, Prenzel KL, Hoelscher AH, Brabender J. Preoperative survivin mRNA detection in peripheral blood is an independent predictor of outcome in esophageal carcinoma. Pharmacogenomics. 2010;11:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Cao M, Yie SM, Wu SM, Chen S, Lou B, He X, Ye SR, Xie K, Rao L, Gao E. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis. 2009;26:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3605] [Article Influence: 257.5] [Reference Citation Analysis (0)] |
| 35. | Fidler IJ, Kripke ML. The challenge of targeting metastasis. Cancer Metastasis Rev. 2015;34:635-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 841] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 37. | Qiao GL, Qi WX, Jiang WH, Chen Y, Ma LJ. Prognostic significance of circulating tumor cells in esophageal carcinoma: a meta-analysis. Onco Targets Ther. 2016;9:1889-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Kaganoi J, Shimada Y, Kano M, Okumura T, Watanabe G, Imamura M. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg. 2004;91:1055-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Bobek V, Matkowski R, Gürlich R, Grabowski K, Szelachowska J, Lischke R, Schützner J, Harustiak T, Pazdro A, Rzechonek A. Cultivation of circulating tumor cells in esophageal cancer. Folia Histochem Cytobiol. 2014;52:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Yamaguchi T, Okumura T, Hirano K, Watanabe T, Nagata T, Shimada Y, Tsukada K. Detection of circulating tumor cells by p75NTR expression in patients with esophageal cancer. World J Surg Oncol. 2016;14:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Li H, Song P, Zou B, Liu M, Cui K, Zhou P, Li S, Zhang B. Circulating Tumor Cell Analyses in Patients With Esophageal Squamous Cell Carcinoma Using Epithelial Marker-Dependent and -Independent Approaches. Medicine (Baltimore). 2015;94:e1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |