Published online Feb 21, 2017. doi: 10.3748/wjg.v23.i7.1125
Peer-review started: October 19, 2016
First decision: December 2, 2016
Revised: December 16, 2016
Accepted: January 4, 2017
Article in press: January 4, 2017
Published online: February 21, 2017
Processing time: 127 Days and 18.7 Hours
Yīn-Chén-Hāo decoction (YCHD) is a traditional Chinese medicine formula composed of capillaris (Artemisia capillaris), gardenia (Gardenia jasminoides), and rhubarb (Rheum rhabarbarum) that is used for the treatment of damp-heat jaundice. In modern clinics, YCHD is mostly used for hepatic diseases. This review summarizes the biological activities of YCHD and its medical applications. The main active compounds of YCHD are chlorogenic acid, rhein, geniposide, emodin, and scoparone. The pharmacological actions of YCHD include inhibition of hepatic steatosis, apoptosis, necrosis, anti-inflammation, and immune regulation. YCHD could be developed as a new therapeutic strategy for the treatment of hepatic diseases.
Core tip: Yīn-Chén-Hāo decoction (YCHD) was a classical prescription for more than 1800 years in China. This review summarizes the efficacy of YCHD in liver disease from clinical trials and its mechanisms of action in vitro and in vivo. Studies indicate that YCHD can modulate various molecular pathways in liver disease. YCHD is widely used in clinical settings for the treatment of liver diseases, and could be a safe and novel therapeutic drug for liver injury worldwide.
- Citation: Li JY, Cao HY, Sun L, Sun RF, Wu C, Bian YQ, Dong S, Liu P, Sun MY. Therapeutic mechanism of Yīn-Chén-Hāo decoction in hepatic diseases. World J Gastroenterol 2017; 23(7): 1125-1138
- URL: https://www.wjgnet.com/1007-9327/full/v23/i7/1125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i7.1125
Yīn-Chén-Hāo decoction (YCHD) was first described in the “Treatise on Febrile Diseases” by Zhong-Jing Zhang during the Eastern Han Dynasty (AD 25-220). It a classic prescription in traditional Chinese medicine (TCM) that is mainly used for internal stasis heat and jaundice arising from Yang Ming disease and malnutrition, with doctors also widely using this prescription for heat jaundice. Modern usage of YCHD includes treatment for acute icteric infectious hepatitis along with other herbal medicines. YCHD is also used for hepatic diseases and has some uses in internal medicine, surgery, and pediatrics (Table 1). This paper discusses studies on YCHD and its mechanisms in the treatment of hepatic diseases.
| Herb or compound | Dose and course of treatment | Combined medication | Case/control | Disease type | Symptoms | Efficacy | Positive control drug | Side effects | Ref. |
| YCHD | From 28th wk of pregnancy, decocted with water, daily, 4 wk | From 36th wk of pregnancy, both groups, + administration of 30 mg phenobarbital, twice a day, orally | 81/76 | Maternal-fetal ABO blood group incompatibility | IgG anti-A/B antibody titer, growth in fetus, neonatal jaundice | Reduced antibody titer, reduced jaundice incidence rate, reduced TBIL | Vitamin C 0.5 g + 100 IU coenzyme A + 40 mg adenosine triphosphate, IVGTT | None | [61] |
| YCHD | Modified YCHD, decocted with water daily; orally, twice a day | 1000 mg transmetil injection, IVGTT | 38/30 | Hyperbilirubinemia induced by viral hepatitis | Clinical symptoms, liver function, bilirubin | Symptoms, bilirubin levels significantly improved due to liver function | Vitamin C, vitamin B6, diammonium | None | [62] |
| Modified YCHD | Decocted with water, daily, 10 d | Routine Western medical treatment | 34/34 | Pathological neonatal jaundice | Time of jaundice disappearance, jaundice degree, TBIL | Time jaundice disappearance is shut down, liver TBIL significantly decreased | Routine Western medicine therapy, etiological treatment, light therapy, phenobarbital, albumin for patients with high bilirubin, gamma globulin for patients with hemolytic disease | None | [63] |
| Modified YCHD | Decocted with water, daily, 14 d | Plasma exchange | 20/20 | Hepatitis B combined with hyperbilirubinemia | Liver function, prothrombin activity | TBIL significantly decreased effective rate of treatment group compared with significantly increased control group | Plasma exchange | None | [64] |
| Modified YCHD | Decocted with water, daily, orally, 28 d | Mask with the function of removing heat and eliminating stasis | 31/29 | Gastrointestinal damp-heat acne | Acne coalescence condition | Increased total effective rate of acne coalescence, average efficacy index statistically significantly decreased | Danshentong capsules for oral use, vitamin E emulsifiable paste for external use | None | [65] |
| Modified YCHD | Decocted with water, daily, orally, 7 d | None | 32/34 | Juvenile bronchial asthma | Asthma symptoms | Curative effect of treatment group significantly better than control group; fade time of cough, sputum, and wheezing in treatment group significantly shorter than control group (P < 0.05) | Normal saline, dexamethasone injection, ambroxol hydrochloride injection, aerosol inhalation, twice a day, 3-7 days, additional antibiotics for infected individual | None | [66] |
| Modified YCHD | Decocted with water, daily, orally | EST + endoscopic stone extraction + ENBD | 24/24 | Choledocholithiasis | TBIL, DBIL, AKP, GGT, ALT, daily bile reflux | Observed indicators improved significantly, daily bile reflux significantly higher than control group (P < 0.05) | Anti-infection, hepatoprotective, prevents fluid and electrolyte imbalance, symptomatic supportive care | None | [67] |
| Yin-Zhi-Huang injection | 10 mL + 20 mL glucose solution, IVGTT, daily, 15 wk | Kadai surgery, antibiotic treatment, hormone treatment, +vitamin K1, +ursodeoxycholic acid | 18/14 | Biliary atresia | TBIL, DBIL, ALT, AST | Observation target significantly improved (P < 0.05) | None | None | [68] |
| Yin-Zhi-Huang granule | 1.0 g, 3 times a day, orally, 7 d | Blue light irradiation, 8 h daily + 105 mg bifid-triple viable capsule, twice a day orally, 7 d | 150/133/141 | Neonatal jaundice | Time of jaundice disappearance, transcutaneous bilirubin | Time of jaundice disappearance significantly reduced, transcutaneous bilirubin level significantly reduced (P < 0.05) | Blue light irradiation | None | [69] |
| Yin-Zhi-Huang granule | 3.3 mL, 3 times a day, orally, 5 d | None | 120/120/120 | Pathological neonatal jaundice | Bilirubin concentration, time of jaundice disappearance | Plasma bilirubin level significantly reduced, total effective rate significantly increased | Tuihuang mixture | None | [70] |
| Yin-Zhi-Huang injection | 5 mL + 10% glucose solution 50 mL, IVGTT, daily, 10 d | ATP, coenzyme A, inosine, vitamin C, glucuronolactone | 46/12 | Infantile hepatitis syndrome | Treatment results (cured, improved, invalid), serum TBIL, ALT value, size of liver and spleen | Cure rate, total effective rate significantly increased, plasma bilirubin level significantly reduced, obvious change to liver and spleen size | ATP, coenzyme A, inosine, vitamin C, glucuronolactone | None | [71] |
| Modified compound Yīn-Chén recipe | Twice a day | None | 215/120 | Maternal-fetal ABO blood group incompatibility-induced neonatal jaundice | Antibody titer, neonatal jaundice incidence, jaundice degree | Antibody titer and neonatal jaundice incidence much lower post-treatment, TCM treatment before delivery failed to reduce jaundice degree | None | None | [72] |
| ICKT | 7.5 g, daily, orally | None | 1 | Acute cholestatic hepatitis | Clinical course of patient | TBIL level began to decrease, liver biopsy showed chronic active hepatitis with mild fibrosis | Predonine | None | [73] |
| ICKT | 7.5 g, daily, orally | None | 50/50 | Persistent hyperbilirubinemia as a symptom of post-operative liver failure after hepatectomy | Choleretic effect | ICKT group showed significant decrease compared with control group in indirect bilirubin levels | None | None | [74] |
| Modified compound Yīn-Chén recipe | Modified compound Yīn-Chén recipe decoction, orally | Routine Western medical treatment | 30/30 | Chronic severe hepatitis | Liver function indices, complication incidences, score of TCM syndromes, clinical effects | Treatment group better than control group (P < 0.05) | Basic Western medical treatment | None | [75] |
| Modified YCHD | Retention enema, daily | Routine comprehensive treatment | 30/30 | Chronic severe hepatitis | Liver function indices, endotoxin, blood ammonia | Treatment group better than control group (P < 0.05) in improvement of clinical symptoms, endotoxin, blood ammonia, ALT, AST, and TBIL | Lactulose medicated by retention enema | None | [76] |
| Modified Artemisiae | Twice a day | Ursodeoxycholic acid | 30/30 | Primary biliary cirrhosis | Clinical effect, biochemistry indicators, immunoglobulin levels | Treatment group better than control group (P < 0.05) in clinical effect and biochemistry indicators, insignificantly decreased immunoglobulin levels (P > 0. 05) | Ursodeoxycholic acid | None | [77] |
| Scopariae decoction | |||||||||
| Modified YCHD | Twice a day | Conventional treatment, phenobarbital (blue light-struck) | 30/29 | Neonatal hyperbilirubinemia | Clinical effect | Treatment group better than control group (P < 0.05) in clinical effect | Conventional treatment (phenobarbital, blue light-struck) | None | [78] |
| Lidan Xiaohuang decoction | 50-100 mL 2-3 times a day, orally | Routine | 35/31 | Obstructive jaundice, post-operative persistent jaundice | Bilirubin | Treatment group better than control group (P < 0.01) | Acupuncture therapy | None | [79] |
| Western medical treatment | |||||||||
| Qing-Re-Li-Shi formula | Twice a day, 90 d | None | 60 | Chronic hepatitis B | Liver function comparison, hepatitis B virus marker comparison | Contents of ALT, AST, AKP, G, TBIL, DBIL improved (P < 0.01); negative conversion rates of 16.7% HbsAg, 21.2% HbeAg, and 35.0% HBV | None | None | [80] |
| Modified compound Yīn-Chén recipe | Twice a day | Triple anti-Hp therapy | 30/30 | Hp-positive rosacea | Clinical effective rate, Hp- positive rate, serum IL-8, TNF-α | Clinical effective rate in treatment group better than control group (P < 0.05); Hp- positive rate, serum IL-8, and TNF-α in treatment group significantly lower than control group (P < 0.01) | Triple anti-Hp therapy | None | [81] |
| Modified compound Yīn-Chén recipe | Twice a day | Routine | 120/90 | Viral hepatitis jaundice | Jaundice disappearance, liver function improvement | Clinical effective rate and liver function in treatment group better than control group (P < 0.05) | Routine | None | [82] |
| Western medical treatment | Western medical treatment | ||||||||
| Obstructive jaundice | Twice a day | Obstructive jaundice | 28/26 | Obstructive jaundice | TBIL, DBIL | More obvious decline of TBIL and DBIL in experimental group (P < 0.05) | Obstructive jaundice | None | [83] |
| Supplement YCHD | Twice a day | Routine | 30/30 | Severe chronic hepatitis B | Liver function, gross effective rate, mortality rate | YCHD significantly improved liver function and gross effective rate, mortality rate lower than control group (P < 0.05) | Routine | None | [84] |
| Western medical treatment for protective liver, plasma exchange | Western medicinal treatment for protective liver, plasma exchange | ||||||||
| YCHD | Twice a day | Routine | 30/30 | Intrahepatic cholestasis of pregnancy | Pruritus score, serum bile acid level, Apgar score, body weight | Pruritus score and serum bile acid level lower than control group (P < 0.05, P < 0.01, respectively); Apgar score and body weight better than control group (P < 0.01) | Routine | None | [85] |
| Western medical treatment for protective liver | Western medical treatment for protective liver |
Classic Chinese formulae include four elements: the monarch drug, the minister drug, the assistant drug, and the servant drug; the monarch drug plays the most important role in the formula, the assistant drug increases the effectiveness of the monarch drug, the minister drug helps the monarch and minister drugs reach their target positions, and the servant drug can reduce the adverse effects or increase the potency of the entire formula[1].
YCHD (Figure 1) is comprised of 12 g capillaris (Artemisia capillaris), 9 g gardenia (Gardenia jasminoides), and 9 g rhubarb (Rheum rhabarbarum). Capillaris is the monarch drug, and can clear heat and dampness and remove jaundice. Gardenia is the minister drug, and can clear heat-fire and damp-heat in the triple burner, and remove pathogenic factors from the urine. Rhubarb is both the assistant and servant drug, and acts to purge heat from the bowels, cool the blood, detoxify, dispel stasis, and dredge the meridians. The three drugs together prevent stasis, promote bowel movement, and guide stagnant heat to be excreted alongside the stool. YCHD is usually used twice daily. In an animal study with rats, total bilirubin (TBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were improved and the blood level of scoparone was higher for longer when YCHD was administered once vs twice or three times daily. The exact mechanism of this effect has yet to be determined[2].
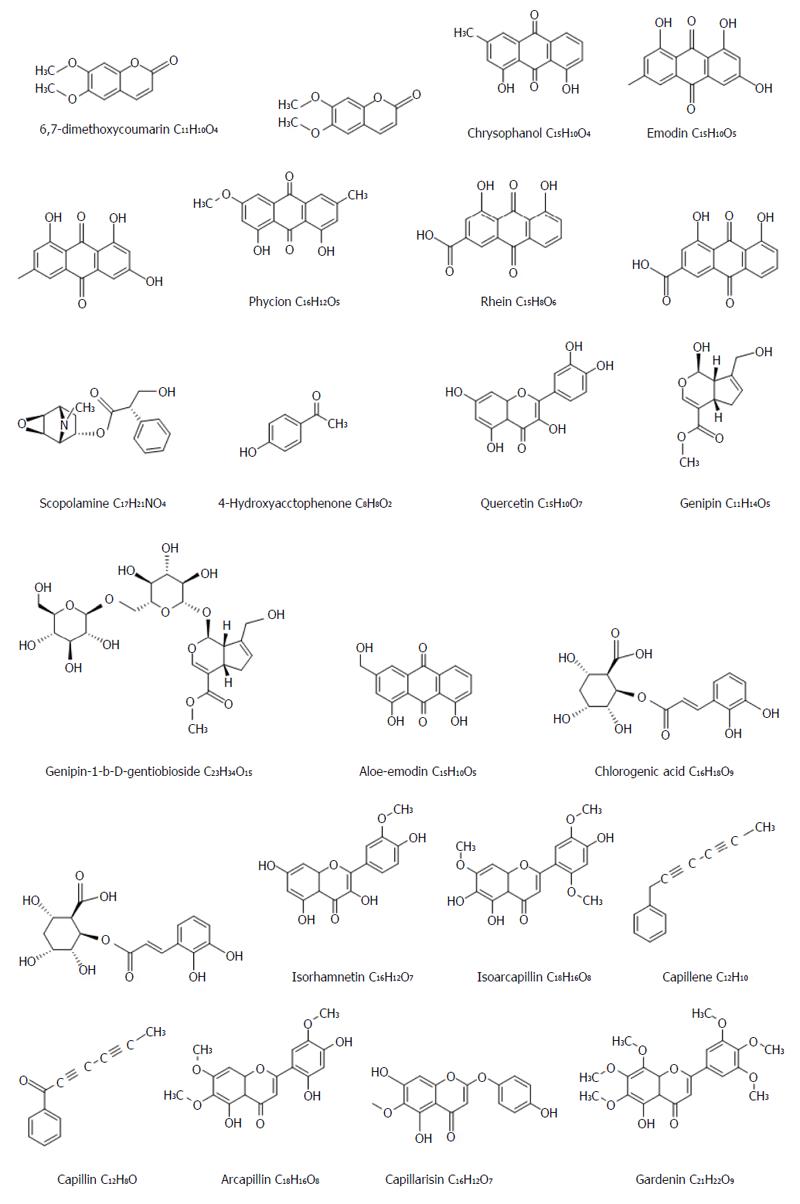
YCHD has broad prospects for application in the field of liver disease, particularly in the treatment of hepatitis with jaundice; for example, it promotes bilirubin metabolism, can prevent liver damage, and inhibits hepatic apoptosis, hepatic stellate cell (HSC) activation, and collagen synthesis[3]. Studies on its chemical composition have determined some effective compounds found in YCHD. Capillaris mainly contains five classes[4] of active compounds: coumarins (e.g., scoparone); flavonoids (e.g., arcapillin, 5,3’,4’,hydroxy-6,7-dimethoxycoumarin, cirsimaritin, 3’-methoxy thistle flavin); chromones (e.g., capillarisn, 7-a methyl wormwood color ketone, 4’-a methyl wormwood color ketone, 1-methoxy 4’ methyl 6 gall color ketone); organic acids (e.g., chlorogenic acid, coumaric acid A and B); and alkaloids (e.g., alkynes, monoterpenes, sesquiterpenes, water-soluble polypeptides). Rhubarb mainly contains five classes of active compounds: anthraquinones (including two types of free anthraquinones like rhein, emodin, aloe emodin, chrysophanol, and physcion; and bound anthraquinones like anthraquinone glycoside and dianthrone glucosides); tannins (e.g., gallic acid, D-catechin); stilbenes (e.g., rhaponticin, piceatannol, resveratrol, 3’,4’,5-hydroxyl stilbene, rhaponticin-2”-O-gallate, rhaponticin-6”-O-gallate); volatile oils (e.g., palmitic acid, hexacosanoic acid, palmitic ethyl ester, dibutyl phthalate); and rhubarb polysaccharides. Gardenia mainly contains five classes of active compounds: gardenosides (e.g., geniposide, hydroxyl gardenoside, caryptoside, gardoside, scandoside methyl ester, geniposidic acid); pigments (e.g., crocin, crocetin); organic acids (e.g., chlorogenic acid, bitter saffron acid, alicyclic acid, 3-oxygen α coffee α mustard acyl α 4-oxygen quinic acid); volatile oils (e.g., linoleic acid, palmitic acid); and gardenia polysaccharides.
One study identified 45 compounds from YCHD, with 21 found in rat blood after oral administration. After studying the influence of different herbs alone compared with the whole YCHD decoction, investigators found eight compounds that are selectively absorbed into the bloodstream only after administration of all herbs from the YCHD decoction. Each compound had a significant effect on protecting the liver and gallbladder[5] (Figure 2).
The biological effects of many ingredients in YCHD reflect the therapy of the entire formula (Table 2). For example, rhein may relieve cellular insult through its anti-inflammatory activity in combination with nitric oxide (NO) from L-arginine[6]. Rhein can improve liver function and remove hepatic fibrosis via anti-inflammatory and antioxidant pathways, and inhibit TGF-α activity and HSC activation[7]. Chlorogenic acid, another critical active ingredient of YCHD, can counteract liver injury at various levels by preventing apoptosis and oxidative stress damage. More specifically, both the glutathione and thioredoxin antioxidant systems and the mitogen-activated protein kinase (MAPK) signaling cascade appear to be engaged in the protective mechanism of chlorogenic acid[8]. Geniposidic acid, the critical active ingredient of gardenia, alleviates liver injury by enhancing the antioxidative defense system and slowing the apoptotic signaling pathways[9].
| Compound | Pharmacological activities | Mechanisms of action | Ref. |
| Chlorogenic acid | Antibacterial, antiviral, antioxidation, | Reversed liver reduced GSH levels and expression of TRX, triggering GSH and TRX antioxidant systems and MAPK signaling cascade | [8] |
| free radical scavenging, | |||
| mutation suppression, anti-tumor | |||
| Scoparone | Anti-inflammatory, analgesia, antioxidant, immunosuppressive, cholagogue, blood pressure, hypolipidemic, anti-asthmatic | Inhibition of protein tyrosine kinase and release of arachidonic acid metabolites, reduced expression of tissue factor at mRNA level and thrombus generation; enhancement of prostacyclin release, protection against endothelium derived relaxing factor inactivation, and activating guanylate cyclase, relaxed bronchial smooth muscle | [86-89] |
| Geniposide | Anti-inflammatory, analgesia, cholagogue, laxness, soft tissue injury repair, gastric acid secretion inhibition, amylopsin secretion reduction | Enhancing antioxidative defense system and reducing apoptotic signaling pathways; regulating adipocytokine release and the expression of PPARα expression | [9,90] |
| Rhein | Antineoplastic, anti-inflammatory, antimicrobial, immunosuppressive, diuresis and purgation, improve glycometabolic | Inhibiting cytochrome P450 enzymes in liver microsomes | [91] |
| Emodin | Antineoplastic, anti-inflammatory, antimicrobial, immunosuppressive, diuresis and purgation | Inhibiting HSC activation | [92] |
One study used high-performance liquid chromatography-UV to test scoparone in rat plasma after intragastric administration of YCHD or water decoctions of capillaris, gardenia, and rhubarb separately. The study found that the scoparone level after YCHD administration was significantly higher than that of water decoctions alone. Moreover, the therapeutic effect of YCHD was found to be better than that of the herbs alone[10]. Another study used scoparone, geniposide, or rhein alone or in combination, and investigated their immunohistochemistry, biochemistry, metabolomics, and proteomics. The results showed that the scoparone, geniposide, and rhein combination exerts a more robust therapeutic effect than any one or two of the three individual compounds in a rat model of hepatic injury. Furthermore, scoparone, geniposide, and rhein synergistically cause intensified dynamic changes in metabolic biomarkers, regulate molecular networks through target proteins, have a synergistic effect, and activate both intrinsic and extrinsic pathways[11].
The liver is a major location for the metabolism and elimination of drugs, as well as being involved in their absorption. Therefore, the liver greatly influences the pharmacokinetics of compounds. Studies demonstrate that liver injury significantly influences the pharmacokinetics of scoparone, not only via changes in intestinal absorption, but also via changes in hepatic metabolism. In one study, the absorption and distribution of scoparone was accelerated in liver-injured rats at the cost of slowed metabolism and elimination. Changes in parameters like metabolism can explain some molecular mechanisms of YCHD in the treatment of liver injury[12].
Research into different drug-induced liver injury forms have been conducted with YCHD. One study explored liver injury induced by α-naphthyl isothiocyanate (ANIT) and carbon tetrachloride (CCl4). ANIT intoxication groups were given ANIT (in corn oil at a ratio of 10:1) at a single dose of 80 mg/kg orally, while CCl4 intoxication groups were given CCl4 (in corn oil at a ratio of 1:4) at a single dose of 1 mL/kg orally. In the ANIT group, the levels of ALT, alkaline phosphatase (AKP), and TBIL were significantly higher than those in the control group. In the CCl4 group, the malondialdehyde content was significantly higher and superoxide dismutase and glutathione peroxidase activities were significantly lower compared with those in the control group. YCHD significantly reduced AST, ALT, and AKP levels in the ANIT group and significantly reduced AST, ALT, AKP, and malondialdehyde levels in the CCl4 group. In addition, YCHD was also found to increase the ratio of liver weight to body weight[13].
Scoparone is an important compound found in capillaris that has been shown to be hepatoprotective, effective in the treatment of liver diseases, and contribute directly to the therapeutic effect of YCHD[14]. The metabonomic characteristics of CCl4-induced hepatotoxicity and intervention with scoparone illustrate that scoparone could have hepatoprotective effects via multiple pathways, including primary bile acid biosynthesis and pyrimidine metabolic pathways[15]. The hepatoprotective effects of scoparone in rat liver injury were associated with regulated expression of six proteins that appear to be involved in antioxidation and signal transduction, energy production, immunity, metabolism, and chaperoning[16].
Network reconstruction techniques were used to study the treatment of alcoholic hepatitis with scoparone. The metabolites after scoparone treatment in rats with alcoholic hepatitis were observed. The results showed that scoparone could regulate dysfunctions in the citrate cycle, sphingolipid metabolism, and taurine and hypotaurine pathways[17]. In addition, scoparone was distributed and eliminated rapidly in rats. Tissue distribution was highest in the liver, followed by the kidney and spleen. There were lower concentrations observed in the muscle, thyroid, and adrenal gland. Scoparone was not detected in the brain, which indicates that it does not cross the blood-brain barrier after oral administration[18].
YCHD combined with Da-Cheng-Qi decoction can reduce Bax and caspase-3 protein expression, increase Bcl-2 protein expression, regulate the balance of Bcl-2/Bax, and treat endotoxin-induced liver cell apoptosis[19].
Recent studies have reported the efficacy of YCHD in reducing hepatic fat accumulation. Enhanced adiponectin and endothelial progenitor cells and upregulation of PPAR-γ might be responsible for the therapeutic effect of YCHD in the treatment of non-alcoholic fatty liver disease (NAFLD). In addition, the antioxidative effect of YCHD might be associated with the inhibition of hepatic-free fatty acid (FFA) concentrations and the elevation of glutathione levels in hepatic tissues. Furthermore, YCHD might promote senescence marker protein-30 metabolism, which increases resistance to hepatic oxidative stress[20].
In steatohepatitis experiments with rats, those treated with YCHD had lower serum ALT activity, tumor necrosis factor (TNF-α) levels, hepatic triglycerides, and FFA levels. Moreover, there was significantly less fat deposition in hepatocytes than in the steatohepatitis rats. Therefore, YCHD has good therapeutic effects on non-alcoholic steatohepatitis, can protect liver function, and can reduce fatty deposition in the liver. These effects are possibly related to reductions in FFA content and inhibition of TNF-α expression[21].
YCHD contains scoparone, geniposide, and rhein, with the three causing dynamic changes in metabolic biomarkers, regulating molecular networks through target proteins, having synergistic effects, and activating both intrinsic and extrinsic pathways to exert a robust therapeutic effect in a rat model of hepatic injury[11]. A meta-analysis was conducted on the role of YCHD in NAFLD. The results showed that YCHD can significantly regulate ALT, AST, gamma-glutamyl transpeptidase (GGT), triglycerides, total cholesterol, low-density lipoprotein cholesterol, and syndrome score. Total effective rate, syndrome effective rate, and B-type ultrasonography effective rate were significantly better in the test group than those in the control group. Therefore, YCHD has a satisfactory therapeutic effect on NAFLD[22].
Hepatitis B is a major global health problem caused by the hepatitis B virus (HBV). Many clinical studies have reported that YCHD can reduce serum transaminase activity, elevate serum albumin, reduce the ratio of albumin and globulin (A/G), improve liver function, and provide satisfactory long-term effects[23,24]. These effects could help in the treatment of hepatitis B.
The pathogenic process of HBV is immune-mediated inflammation. Cytotoxic T lymphocytes (CTL) recognize and destroy target cells by antigen presentation, and cause target-cell apoptosis when fas ligand protein on the membrane surface unites with target-cell Fas antigen. HBV infection activates the immune system to produce and release cytokines, which promote liver inflammation and cause liver damage.
One study found that, in the treatment of chronic hepatitis B, YCHD combined with Western medicine was significantly better than Western medicine treatment alone[25]. In an experiment with concanavalin A-induced acute immune liver injury in mice infected with hepatitis B, YCHD was found to reduce AST, interferon-γ, and Fas gene levels, and increase the level of interleukin (IL)-4 in the liver. YCHD was also able to repair damage to liver cells, decrease the frequency of liver cell apoptosis, and reduce inflammatory response[26].
Drug-induced hepatitis refers to liver damage caused by drugs or their metabolites. In recent years, increased usage of new drugs and drug combinations has increased the incidence of drug-induced hepatitis. YCHD plus glycyrrhizinate or transmetil was found to be more effective than Western medicine in the treatment of drug-induced hepatitis. A control group was treated with glycyrrhizinate capsules and transmetil, while a treatment group received the same drugs plus modified YCHD. The levels of ALT, TBIL, and globulin in the treatment group were lower than those in control group after treatment (P < 0.05). In addition, the level of albumin in the treatment group was higher than that in the control group[27].
Chronic severe hepatitis is another common type of hepatitis. The treatment of chronic severe hepatitis is difficult, and usually results in such complications as ascites, hepatic encephalopathy, gastrointestinal bleeding, and hepatorenal syndrome. Clinical research indicates that YCHD can reduce liver cell inflammation, expand intrahepatic bile capillaries, promote bile secretion, increase bile flow, improve hepatic microcirculation, reduce the absorption of intestinal toxins, and increase the excretion of bilirubin. These effects can reduce symptoms and significantly improve liver function in patients with chronic severe hepatitis[28]. Liver dialysis combined with YCHD is an especially effective therapeutic regimen for severe hepatitis[29].
Fibrosis is a wound-healing response that engages a range of cell types and mediators to encapsulate an injury. During fibrogenesis, pathological factors including inflammation from Kupffer cells (KCs), angiogenesis, and HSC activation lead to collagen deposition[30]. Cirrhosis, an advanced liver disease, is characterized by fibrosis, nodule formation, and inflammation[31]. Despite the high incidence of hepatic fibrosis worldwide, no generally accepted antifibrogenic therapy is available. However, TCM has been widely used for treating chronic liver hepatitis and liver cirrhosis, with said treatments appearing to improve clinical symptoms, liver function, and patient quality of life[32].
YCHD can significantly inhibit apoptosis in liver cells, inhibit HSC activation, and inhibit KC activation, thereby protecting liver function and preventing fibrosis. In a rat model of dimethylnitrosamine (DMN)-induced liver fibrosis, YCHD significantly improved liver function, liver pathology, and reduced collagen content in liver tissue[33]. In addition, a DMN-induced liver fibrosis study in rats found that YCHD could decrease abnormal ALT, AST, TBIL, GGT, hydroxyproline, hyaluronic acid, laminin, collagen type IV, and amino-terminal type III procollagen peptide levels. The study also found that YCHD treatment could improve mRNA and protein levels of α-SMA (a marker of activated HSCs), restore normal albumin levels, and change amino acid metabolism. The molecular mechanism of the anti-fibrotic effects of YCHD might operate via suppression of HSC activation[34,35]. In addition, the therapeutic effect of YCHD is better than that of other classic TCM formulae, such as Yīn-Chén-Si-Ni-Tang[36] and Gan-Lu-Xiao-Du-Dan[37] in the treatment of dampness-heat with liver fibrosis.
KCs are liver macrophages generally believed to be involved in liver damage via inflammation. There are two ways to activate KCs: the classical pathway and the alternative pathway[38,39]. KC activation via the classical pathway releases inflammatory cytokines, which further activate HSCs and lead to a phenotypic transition to myofibroblasts. This transition results in excessive proliferation, as well as the synthesis and secretion of ECM components, which leads to fibrosis. The inhibition of HSC activation can reduce the biosynthesis of collagen, and therefore inhibit fibrosis.
YCHD administration attenuates liver fibrosis partially by inhibiting HSC activation, rather than promoting cell apoptosis of activated HSCs or suppressing the activation of KCs[40]. YCHD reduced the expression of IL-1β, CD68, Tnfrsf14, Tnfrsf9, COL1α2, MMP2, MMP23, TNF-α, and Prkcb, and increased the expression of CD14, Tf, and Igf1. These gene expression changes indicate that YCHD can inhibit liver inflammation, HSC activation, liver sinusoidal endothelial cell activation, and liver parenchymal damage via inhibition and regulation of the classical KC activation pathway. These effects in combination result in anti-fibrotic effects from YCHD[41].
YCHD can also reduce the expression of TNF, FAS, and Prkcb, and regulate the expression of CD14 genes, indicating that it can block the MAPK pathway, which inhibits hepatocyte apoptosis to prevent fibrosis[42].
One study found YCHD plus Huang-Qi decoction reversed liver cirrhosis in rats via a reduction in oxidative stress. YCHD was found to eliminate hepatic lipid peroxide formation and Huang-Qi decoction enhanced the antioxidative ability of the liver[43].
The pathogenesis of DMN-induced liver cirrhosis corresponds to the syndrome of interior dampness-heat with qi deficiency[44], and the pathogenesis of liver cirrhosis corresponds to the syndrome of interior dampness-heat[35].
Cirrhosis with ascites often occurs during chronic hepatitis, with ascites often being observed in end-stage cirrhosis. The clinical manifestations of ascites are tympanites and edema. One clinical study found that YCHD combined with Jijiaolihuang bolus could clear heat and expel dampness, induce diuresis to remove edema, and reduce ascites[45].
Since YCHD can clear heat, promote diuresis, and remove jaundice, it also plays an important role in the treatment of primary liver cancer[46].
Liver failure is caused by extensive liver cell necrosis, which results in severely impaired liver function, while chronic liver failure results from decompensation of the liver during cirrhosis and is accompanied by a poor prognosis and high mortality rate.
HBV is the most common cause of liver failure in China. Although nucleoside analogs can inhibit viral replication in the short-term, they usually result in drug resistance in the long-term. TCM enema can solve issues such as difficulties in oral administration, effectively removing harmful bacteria in the gut, improving intestinal endotoxemia, and promoting the recovery of liver function. In patients with HBV-induced liver failure, an enema of YCHD plus colon lavage was found to result in liver function and symptom improvement, jaundice improvement, TBIL reduction, and increased prothrombin time[47].
One study established a rat model with hepatic failure after 70% liver resection to research the therapeutic effect of capillaris. The results showed that the herb can improve IL-6 levels, increase serum IL-6 levels, and improve survival rate in rats with liver failure after surgery[48].
YCHD-ameliorated alloxan (ALX) induced hyperglycemia in mice and significantly reduced fasting blood glucose (FBG) in normal mice and ALX-diabetes in mellitus model mice and rats. YCHD also improved impaired glucose tolerance in a dexamethasone-induced insulin resistance rat model and reduced 2-h post-prandial blood glucose after an oral glucose tolerance test. This suggests that YCHD has hypoglycemic effects like sulfonylurea or biguanide[49].
Clinical studies indicate that YCHD plus Bai-Tou-Weng decoction and hormones have a better treatment effect than Western medicine in the clinical treatment of Behçet’s disease. In one study, a control group was treated with prednisone, while a treatment group was treated with the same drug plus YCHD and Bai-Tou-Weng decoction. According to the diagnostic criteria of the International Society for Behçet’s Disease, the therapeutic effect in the treatment group was better than in the control group (P < 0.05)[50].
YCHD has also been used clinically in neonatal hyperbilirubinemia[51], neonatal jaundice[52], maternal-fetal ABO blood type incompatibility[53-56], intrahepatic cholestasis of pregnancy[57], newborn pathological jaundice[58], and acne[59].
Compared with insulin and insulin analogs, combined therapy of NovoRapid and modified YCHD is an effective, safe, and economical approach to the treatment of chronic hepatitis B with diabetes[60].
Drugs that include the herbs found in YCHD have been on the market for many years and most have important therapeutic uses. Xiong-Dan-Yīn-Chén Oral Solution® (XDYCKFY, Hei-Bao-Yao-Ye, Hei-Long-Jiang, China) is an effective and safe treatment for chronic liver diseases, and can slow down the progress of cholecystitis and cholelithiasis. Moreover, Ling-Zhi-Yīn-Chén Capsule® (LZYC capsule, Zhong-Long-Yi-Yao, Hei-Long-Jiang, China) and Huang-Dan-Yīn-Chén Granule® (HDYCKL, Fo-Ci-Zhi-Yao, Lan-Zhou, China) are used to improve the symptoms of idiopathic pain, abdominal distension, anorexia, malaise, fatigue, and greasy yellow tongue coating. Yīn-Chén-Tui-Huang Capsule® (YCTH capsule, De Shang Yao Ye, Jilin, China) is often used in the treatment of jaundice caused by acute and chronic liver disease.
This review summarized the efficacy of YCHD in liver disease from clinical trials and its mechanisms of action in vitro and in vivo. Studies indicate that YCHD can modulate various molecular pathways in liver disease. YCHD is widely used in clinical settings for the treatment of liver diseases, and could be a safe and novel therapeutic treatment for liver injury worldwide (Table 3). Future studies on YCHD could help define its various effective constituents, molecular mechanisms, and targets that help prevent inflammation and fibrosis. Although YCHD has been used clinically for thousands of years, most studies on it are basic, and so its material basis and mechanisms remain unclear. At present, there are few multicenter, large, randomized, double-blind, controlled clinical trials on YCHD. Extensive clinical research is warranted to evaluate the safety and efficacy of YCHD alone or in combination with other drugs.
| Patent | Patent number |
| YCHD preparation methods | CN101371882 |
| Damp-proof TCM granule and its preparation method | CN1781499 |
| Composition for improving the composition/kind of composition of crude drug powder containing rhubarb and/or its extract that can improve constipation | JP2005179316 |
| Medical treatment for heterotopic calcification/medicine root in TCM prescription treatment for heterotopic calcification | JP5000961 |
| Kind of formula granules, including YCHD and its preparation and detection methods | CN103230453 |
| Method which can filtrate the material foundation of YCHD efficacy | CN104101674 |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Daliry A, Im SS S- Editor: Yu J L- Editor: Rutherford A E- Editor: Wang CH
| 1. | Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826-4831. [PubMed] |
| 2. | Lv JL, Jin SY, Yuan HL, Han J, Fu SS, Jin SX, Guo JJ, Xiao XH. Rational Daily Administration Times of Yinchenhao Decoction in Rats with Jaundice Based on PD/PK. Zhong Cao Yao. 2012;4:150-156. [DOI] [Full Text] |
| 3. | Mu YP, Liu P, Wang L. Evolution and Modern Research of Yinchenhao Decoction. Zhongguo Shiyan Fangjixue. 2006;12:67-71. [DOI] [Full Text] |
| 4. | Meng S, Xing S. Classical prescription - Chemical Research of Yin-chen-hao Decoction. Yatai Chuantong Yixue. 2009;5:173-175. |
| 5. | Wang X, Zhang B. Elucidation of compatibility principle and scientific value of Chinese medical formulae based on pharmacometabolomics. Zhongguo Zhong Yao Za Zhi. 2010;35:1346-1348. [PubMed] |
| 6. | Zhang YM, Yu F, Dai DZ, Gao J, Cong XD, Dai Y. Hypoxia alters pharmacokinetics of argirein because of mitochondrial dysfunction that is alleviated by apocynin. J Pharm Pharmacol. 2013;65:1360-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Guo MZ, Li XS, Shen DM, Guan XQ, Xu HR, Gao J. Effect of Rhein on the development of hepatic fibrosis in rats. Zhonghua Gan Zang Bing Za Zhi. 2003;11:26-29. [PubMed] |
| 8. | Ji L, Jiang P, Lu B, Sheng Y, Wang X, Wang Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J Nutr Biochem. 2013;24:1911-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Kim SJ, Kim KM, Park J, Kwak JH, Kim YS, Lee SM. Geniposidic acid protects against D-galactosamine and lipopolysaccharide-induced hepatic failure in mice. J Ethnopharmacol. 2013;146:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Yu ZG, Wang Q, Li K, Li YQ, Gao XX. Determination and pharmacokinetics of 6,7-dimethoxycoumarin in rat plasma after intragastric administration of different decoctions of yinchenhao tang. J Chromatogr Sci. 2007;45:544-548. [PubMed] |
| 11. | Wang X, Zhang A, Wang P, Sun H, Wu G, Sun W, Lv H, Jiao G, Xu H, Yuan Y. Metabolomics coupled with proteomics advancing drug discovery toward more agile development of targeted combination therapies. Mol Cell Proteomics. 2013;12:1226-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Lv JL, Li RS, Jin SY, Yuan HL, Fu SS, Han J, Jin SX, Xiao XH. Changes of pharmacokinetics of 6,7-dimethoxycoumarin in a rat model of alpha-naphthylisothiocyanate-induced experimental hepatic injury after Yinchenhao Decoction () treatment. Chin J Integr Med. 2012;18:831-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cao HX, Sun H, Jiang XG, Lu HT, Zhang GM, Wang XJ, Sun WJ, Wu ZM, Wang P, Liu L. Comparative study on the protective effects of Yinchenhao Decoction against liver injury induced by alpha-naphthylisothiocyanate and carbon tetrachloride. Chin J Integr Med. 2009;15:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhang A, Sun H, Yuan Y, Sun W, Jiao G, Wang X. An in vivo analysis of the therapeutic and synergistic properties of Chinese medicinal formula Yin-Chen-Hao-Tang based on its active constituents. Fitoterapia. 2011;82:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Zhang A, Sun H, Dou S, Sun W, Wu X, Wang P, Wang X. Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Analyst. 2013;138:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Zhang A, Sun H, Wu G, Sun W, Yuan Y, Wang X. Proteomics analysis of hepatoprotective effects for scoparone using MALDI-TOF/TOF mass spectrometry with bioinformatics. OMICS. 2013;17:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Zhang A, Sun H, Wang X. Urinary metabolic profiling of rat models revealed protective function of scoparone against alcohol induced hepatotoxicity. Sci Rep. 2014;4:6768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Yin Q, Sun H, Zhang A, Wang X. Pharmacokinetics and tissue distribution study of scoparone in rats by ultraperformance liquid-chromatography with tandem high-definition mass spectrometry. Fitoterapia. 2012;83:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Wang FL, Yang HZ, Li YM, Wu WK, Zou ZC. Prevention and treatment mechanism of qingxia therapy (based on yinchenhao decoction and dachengqi decoction) on hepatocyte apoptosis in rats with acute hepatic injury induced by lipopolysaccharide/D-galactosamine. Zhong Yao Cai. 2014;37:848-852. [PubMed] |
| 20. | Lee TY, Chang HH, Lo WC, Lin HC. Alleviation of hepatic oxidative stress by Chinese herbal medicine Yin-Chen-Hao-Tang in obese mice with steatosis. Int J Mol Med. 2010;25:837-844. [PubMed] |
| 21. | Chen SD, Fan Y, Xu WJ. Effects of yinchenhao decoction (see text) for non-alcoholic steatohepatitis in rats and study of the mechanism. J Tradit Chin Med. 2011;31:220-223. [PubMed] |
| 22. | Liang DZ, Wei W, Yao KW. Yinchenhao decoction adjustment for treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Shijie Huaren Xiaohua Zazhi. 2014;22:2327-2337. |
| 23. | Zhang J, Li HT, Chen P. 48 cases of Modified Yin-Chen-Hao Decoction Treating for Chronic Hepatitis B. Hunan Zhongyi Za Zhi. 2014;30:52-53. |
| 24. | Zhou X. Clinical Observation for 48 cases of Modified Yin-Chen-Hao Decoction Treating for Jaundice Hepatitis B. Taishan Yixueyuan Xuebao. 2014;35:925-926. |
| 25. | Guo SF. 36 cases of Modified Yin-Chen-Hao Decoction Treating for Chronic Hepatitis B. Fujian Zhongyi Zazhi. 2014;45:16-17. |
| 26. | Zhang YQ, Tang XD, Wang FY, Yang B, Liu YL, Guo P, Wang P, Bian LQ, Zhao YP. Effect of ronggan mixture on immunoregulation and hepatocyte apoptosis-related factors in concanavalin A induced acute immunological liver injury mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1500-1506. [PubMed] |
| 27. | Xu XM, Xu N. Clinical observation of Yinchenhao decoction combined with western medicine therapy on the treatment of drug-induced hepatitis. Hebei Zhongyiyao Xuebao. 2014;36:845-854. |
| 28. | Zhu DZ. Observation of the curative effect of Xinjia Yinchenhao Decoction in treating severe chronic hepatitis. Zhong Xi Yi Jie He Xue Bao. 2003;1:77-78. [PubMed] |
| 29. | Zhao AL, Zheng ZG. Clinical Analysis for 50 cases of Modified Yin-Chen-Hao Decoction combine with Plasma exchange Treating for Severe Hepatitis. Beijing Yixue. 2014;36:506-507. |
| 30. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1369] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 31. | Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 32. | Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, Sun M, Liu C, Liu P. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Altern Med. 2012;12:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Bian YQ, Ning BB, Cao HY, Lu Y, Liu C, Chen GF, Liu J, Liu P, Sun MY. Formula-syndrome correlation study of three classical anti-jaundice formulas in inhibition of liver fibrosis induced by dimethylnitrosamine in rats. Zhong Xi Yi Jie He Xue Bao. 2012;10:1405-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Liu C, Sun M, Wang L, Wang G, Chen G, Liu C, Liu P. Effects of Yinchenhao Tang and related decoctions on DMN-induced cirrhosis/fibrosis in rats. Chin Med. 2008;3:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Wang YH, Zhao CX, Chen BM, He M, Liu LQ, Li CY, Chen X. Reverse effect of Yinchenhao decoction in dimethyl nitrosamine-induced hepatic fibrosis in rats. Zhongguo Zhong Yao Za Zhi. 2014;39:1473-1478. [PubMed] |
| 36. | Bian YQ, Liu P, Sun MY. Recipe-syndrome Correlation Study of Yinchenhao Tang and Yinchen Sini Tang on Inhibiton of Liver Fibrosis Induced by Dimethylnitrosamine in Rats. Liaoning Zhongyiyao Daxue Xuebao. 2013;5:48. |
| 37. | Bian YQ, Cao HY, Sun MY. Recipe-syndrome correlation study of Yinchenhao Tang and Ganluxiaodu Dan on inhibition of liver fibrosis induced by dimethylnitrosamine in rats. Zhongguo Zhongyiyao Xuebao. 2013;28:1396-1401. |
| 38. | Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V. Alternative versus classical activation of macrophages. Pathobiology. 1999;67:222-226. [PubMed] |
| 39. | Song E, Ouyang N, Hörbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 340] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Liu C, Sun M, Yan X, Han L, Zhang Y, Liu C, El-Nezami H, Liu P. Inhibition of hepatic stellate cell activation following Yinchenhao decoction administration to dimethylnitrosamine-treated rats. Hepatol Res. 2008;38:919-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Liu C, Liu P, Tao Q. [Recipe-syndrome correlation and pathogenesis mechanism of Yinchenhao Decoction in intervening dimethylnitrosamine induced liver cirrhosis progress in rats]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010;30:845-850. [PubMed] |
| 42. | Cao HY, Li JY, Liu P, Sun MY. Antifibrotic mechanism of action based on the corresponding on Traditional Chinese Medicine formula-syndrome to explore Yin-Chen-Hao Decoction regulation of Kupffer cell function and MAPK pathways. Shijie Zhongyiyao. 2015;2:5-10. [DOI] [Full Text] |
| 43. | Wang L, Liu P, Wang CS. [Effects of 5 classical recipes on anti-oxidative stress in rat liver with cirrhosis]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28:435-439. [PubMed] |
| 44. | Wang L, Liu P, Mu YP, Li FH, Long AH, Gu HT, Chen GF. Study on TCM recipe and syndrome of dimethylnitrosamine-induced hepatic fibrosis in rats. Zhongyi Za Zhi. 2006;47:929-932. [DOI] [Full Text] |
| 45. | Wang XD, Zhang CZ. Clinical Research on the Treatment of Ascites Due to Cirrhosis by Modified Yinchenhao Decoction and Jijiaolihuang Pill. Zhongguo Zhongyao Zazhi. 2014;29:1373-1374. [DOI] [Full Text] |
| 46. | Liu X, Li N. Regularity analysis on clinical treatment in primary liver cancer by traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi. 2012;37:1327-1331. [PubMed] |
| 47. | Guo XJ, Dang D. 36 cases of Modified Yin-Chen-Hao Decoction retention enema combine with Colonic irrigation Treating for Chronic Hepatitis B and hepatic failure. Shanxi Zhongyi Zazhi. 2014;35:858. [DOI] [Full Text] |
| 48. | Li J, Mao DW, Zhang RZ. Artemisia capillaris impact the level of interleukin 6 in Postoperative hepatic failure in rats. Henan Zhongyi. 2014;34:1251-1252. |
| 49. | Pan J, Han C, Liu H, Du J, Li A. Effects of yinchenhao decoction on normal animals and animal models of diabetes mellitus. Zhong Yao Cai. 2001;24:128-131. [PubMed] |
| 50. | Zhang BX, Liu SZ, Peng XH. Clinical Analysis of Yin-Chen-Hao Decoction Combine with Hormone Treating for Behcets disease. Zhongguo Zhongyiyao Keji. 2014;21:412. |
| 51. | Wen HR, Zhang JM, Zhu LJ. 232 cases of Modified Yin-Chen-Hao Decoction Preventive Against Neonatal Hyperbilirubinemia. Zhonguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2014;12:39-40. [DOI] [Full Text] |
| 52. | Wang Y, Ning YT. Effect analysis of modified Virgate wormwood decoction treating infant jaundice in 69 cases. Zhongguo Xiandai Yixue. 2014;21:104-105. |
| 53. | Zhu QY, Zhu WZ. Clinical Observation for 80 cases of Modified Yin-Chen-Hao Decoction Treating for Maternal-fetal ABO Incompatibility. Zhejiang Zhongyi Zazhi. 2014;49:737. |
| 54. | Cai XQ, Cheng HJ. Clinical Observation for 50 cases of Modified Yin-Chen-Hao Decoction Treating for Maternal-fetal ABO Incompatibility. Zhejiang Zhongyi Zazhi. 2014;49:267. |
| 55. | Li H, Yu MM, Zhou HY. Effects of Yinchenhao decoction on TNF-α level of BALB/c mouse immunized with A blood group antigen. Zhongguo Shuxue Zazhi. 2014;2:572-575. |
| 56. | Zhang Y, Zhang YC, Tian M. Clinical Observation for 17 cases of Modified Yin-Chen-Hao Decoction Treating for Maternal-fetal ABO Incompatibility. Nei Mongol Zhongyi Zazhi. 2014;33:11. |
| 57. | Liao YH, Sun RF. Yin-Chen-Hao Decoction Impact on the Balance of Intrahepatic Cholestasis in Patients during Pregnancy. Zhongguo Zhongxiyi Jiehe Zazhi. 2014;34:1017-1018. |
| 58. | Sang YQ, Tian WQ, Feng J. Clinical study on adjuvant treatment of neonatal pathological jaundice by Yinchenhao decoction. Zhongguo Dangdai Yisheng. 2014;52:85-87. |
| 59. | Liu CC, Zhu PH, Sun Y. Clinical Research of Yinchenhao Decoction in Treating Adolescent Female Acnes. Zhongguo Zhongyao Zazhi. 2014;29:1382-1384. |
| 60. | Liu HX, Cheng M, Wang XM. Clinical effect of combination therapy with NovoRapid 30 and modified Yinchenhao decoction in treatment of chronic hepatitis B with diabetes. Linchuang Gandanbing Zazhi. 2014;30:311-313. [DOI] [Full Text] |
| 61. | Xu BH, Li MQ. 81 cases of Modified Yin-Chen-Hao Decoction Treating for Maternal-fetal ABO Incompatibility. Zhongyi Zazhi. 2011;1418-1419. |
| 62. | Gan LM, Li P. Clinical Observation of Modified Yin-Chen-Hao Decoction combine with Transmetil Treating for hyperbilirubinemia induced by viral hepatitis. Xin Zhong Yi. 2009;41:20-21. |
| 63. | Yang FR. Clinical Observation for 68 cases of Modified Yin-Chen-Hao Decoction combine with conventional western treatment Treating treating for pathological jaundice of newborn. Zhongguo Shiyan Fangjixue. 2011;17:260-261. |
| 64. | Yi XD, Luo YW. Clinical Observation of Modified Yin-Chen-Hao Decoction combined with Plasma exchange Treating treating for Hepatitis B merger hyperbilirubinemia. Shandong Yiyao. 2011;51:63-64. |
| 65. | Zhang CY, Gao Z. Clinical Observation for 31 Cases of Modified Yin-Chen-Hao Decoction Combine with Traditional Chinese Medicine Face Pack Treating for Acne with Gastrointestinal Dampness-Heat. Xin Zhong Yi. 2011;43:62-63. |
| 66. | Sang Y, Zhang P. 32 cases of Modified Yin-Chen-Hao Decoction Treating for Children Bronchial Asthma. Zhongyi Zazhi. 2012;53:876-877. |
| 67. | Feng J, Kong D. Clinical research of Traditional Chinese Medicine Combine with Duodenoscopy Treating for Choledocholithiasis. Shizhen Guoyi Guoyao. 2011;22:1533-1534. [DOI] [Full Text] |
| 68. | Huang L, Feng JX, Wei MF, Li MJ. The Adjuvant Therapeutic Effect of Yinzhihuang Injection Before or After Biliary Atresia Surgery in Children. Shiyong Erke Linchuang Zazhi. 2007;11:811-812. |
| 69. | Chen Y, Zheng YN. Clinical Analysis of Yinzhihuang Injection and Bifid-Triple Viable Capsule Combine with Light Therapy Treating for Neonatal Jaundice. Shiyong Erke Linchuang Zazhi. 2012;15:2480-2483. [DOI] [Full Text] |
| 70. | Gao XC, Shen JH, Ni YhYH. Clinical Observation of Yinzhihuang Injection Treating for Pathological Jaundice of Newborn. Zhongguo Yaofang. 2011;22:1872-1873. |
| 71. | Pen H, Dong GQ, Ni YH. Observation of curative effect of yinzhihuang injection for Infantile infantile hepatitis syndromeJ. Zhongyaocai. 2003;26:534-535. |
| 72. | Zhang Y, Song D. Effect of Traditional Chinese Medicine Treatment on Neonatal Jaundice induced by Maternal- Fetal ABO Blood Group Incompatibility. Zhonghua Zhongyiyao Xuekan. 2013;31:1713-1715. |
| 73. | Ohwada S, Kobayashi I, Harasawa N, Tsuda K, Inui Y. Severe acute cholestatic hepatitis of unknown etiology successfully treated with the Chinese herbal medicine Inchinko-to (TJ-135). World J Gastroenterol. 2009;15:2927-2929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Kaiho T, Tsuchiya S, Yanagisawa S, Takeuchi O, Togawa A, Okamoto R, Saigusa N, Miyazaki M. Effect of the herbal medicine Inchin-Ko-To for serum bilirubin in hepatectomized patients. Hepatogastroenterology. 2008;55:150-154. [PubMed] |
| 75. | Ai XY, Fu LC, Tan XH, Zhang AM, Fan HM. Therapeutic Effect of Modified Compound Yinchen Recipe for Chronic Severe Hepatitis. Guangzhou Zhongyiyao Daxue Xuebao. 2013;30:145-148. |
| 76. | Sun JG. Effect of Modified Yinchenhao Decoction Medicated by Retention Enema on Endotoxin and Blood Ammonia of Chronic Severe Hepatitis Patients. Sichuan Zhongyiyao Zazhi. 2013;31:75-77. |
| 77. | Zheng YJ, Tang HH, He JS. Clinical efficacy of ursodeoxycholic acid combined with modified Artemisiae Scopariae decoction for treating 30 cases of primary biliary cirrhosis. Zhongguo Zhongxiyi Jiehe Zazhi. 2012;22:89-91. [DOI] [Full Text] |
| 78. | Li X, Zhu JL. Modified Yinchenhao Decoction in the treatment of 30 cases of neonatal hyperbilirubinemia. Zhongguo Erke Xuebao. 2013;9:42-43. |
| 79. | Jin H, Zhao YF, Dai XH. Lidan Xiaohuang Decoction and Acupuncture Treatment of Obstructive Jaundice Postoperative Persistent Jaundice of 35 cases. Shiyong Zhongyi Neike Zazhi. 2011;25:52-53. [DOI] [Full Text] |
| 80. | Chen XT. Clinical Study of Qingre Lishi Forfula in Treating Chronic Hepatitis B. Zhongguo Zhongyao Zazhi. 2011;26:207-208. |
| 81. | Zhang HY, Chen L, Yu C. Clinical Observation of Integrated Chinese and Western Medicine Sequential Therapy for Treatment of Hp-positive Acne Rosacea. Xin Zhong Yi. 2013;45:81-83. |
| 82. | Li SZ, Xu FM, Li SB. Tarragon Decoction Treat Jaundice Virus Hepatitis. Zhejiang Zhongyiyao Daxue Xuebao. 2012;36:267-269. |
| 83. | Feng W, He L, Dong X. Effects of Capillaris Decoction on Postoperative Bilirubin of Patients with Obstructive Jaundice. Zhongguo Zhongyi Chuangxin. 2012;9:12-13. [DOI] [Full Text] |
| 84. | Cao JL, Wu CM, Zhu XQ, Ou YQ. Enhanced therapeutic effect of Yinchenhao decoction supplemented to plasma exchange in the treatment of severe chronic hepatitis B patients. Zhongguo Bingduxue Zazhi. 2012;2:142-144. |
| 85. | Yang YF, Diao XD, Zhao YZ. Therapeutic Effect of Yinchenhao Decoction for Intrahepatic Cholestasis of Pregnancy: An Analysis of 30 Cases. Xin Zhong Yi. 2013;45:71-74. |
| 86. | Huang HC, Huang YL, Chang JH, Chen CC, Lee YT. Possible mechanism of immunosuppressive effect of scoparone (6,7-dimethoxycoumarin). Eur J Pharmacol. 1992;217:143-148. [PubMed] |
| 87. | Amarnath A, Lenin V, Archunan G. Evaluation of the hypolipidemic activity of 6,7-dimethoxycoumarin on placental tissue factor mRNA expression in experimental anti-phospholipid syndrome. Pharmacogn Mag. 2013;9:264-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Huang HC, Lee CR, Weng YI, Lee MC, Lee YT. Vasodilator effect of scoparone (6,7-dimethoxycoumarin) from a Chinese herb. Eur J Pharmacol. 1992;218:123-128. [PubMed] |
| 89. | Liu YJ, Li Z, Lin ZB. Scoparone’s effects on the airway smooth muscle of asthmatic guinea pigs. Zhongguo Yike Daxue Xuebao. 2001;30 Suppl:12-13. |
| 90. | Ma T, Huang C, Zong G, Zha D, Meng X, Li J, Tang W. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J Pharm Pharmacol. 2011;63:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Tang JC, Yang H, Song XY, Song XH, Yan SL, Shao JQ, Zhang TL, Zhang JN. Inhibition of cytochrome P450 enzymes by rhein in rat liver microsomes. Phytother Res. 2009;23:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Dong MX, Jia Y, Zhang YB, Li CC, Geng YT, Zhou L, Li XY, Liu JC, Niu YC. Emodin protects rat liver from CCl(4)-induced fibrogenesis via inhibition of hepatic stellate cells activation. World J Gastroenterol. 2009;15:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |