Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.853
Peer-review started: August 2, 2016
First decision: October 20, 2016
Revised: November 6, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: February 7, 2017
Processing time: 176 Days and 10.7 Hours
To identify a preoperative blood marker predictive of alveolar echinococcosis (AE) recurrence after hepatectomy.
All consecutive patients who underwent operation for liver AE at the Lausanne University Hospital (CHUV) between January 1992 and December 2015 were included in this retrospective study. Preoperative laboratory values of leukocytes, mean corpuscular volume (MCV), red blood cell distribution width (RDW), thrombocytes, C-reactive protein (CRP) and albumin were collected and analyzed. Univariate and multivariate Cox regression analyses were performed to determine the risk factors for AE recurrence after liver resection. A receiver operating characteristic (ROC) curve was used to define the best discrimination threshold of the blood marker. Moreover, recurrence-free survival curves were calculated using the Kaplan-Meier method.
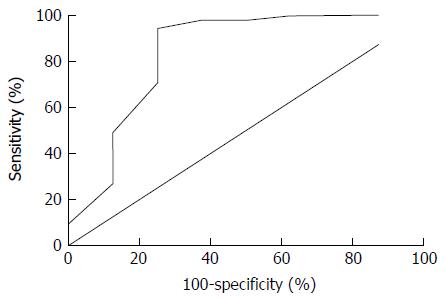
The cohort included 68 adult patients (37 females) with median age of 61 years [interquartile range (IQR): 46-71]. Eight of the patients (12%) presented a recurrence over a median follow-up time of 76 mo (IQR: 34-128). Median time to recurrence was 10 mo (IQR: 6-11). Median preoperative leukocyte, MCV, RDW, thrombocyte and CRP levels were similar between recurrent and non-recurrent cases. Median preoperative albumin level was 43 g/L (IQR: 41-45) for non-recurrent cases and 36 g/L (IQR: 33-42) for recurrent cases (P = 0.005). The area under the ROC curve for preoperative albumin level to predict recurrence was 0.840 (95%CI: 0.642-1, P = 0.002). The cut-off albumin level value was 37.5 g/L for sensitivity of 94.5% and specificity of 75%. In multivariate analysis, preoperative albumin and surgical resection margins were independent predictors of AE recurrence (HR = 0.099, P = 0.007 and HR = 0.182, P = 0.045 respectively).
Low preoperative albumin level was associated with AE recurrence in the present cohort. Thus, preoperative albumin may be a useful biomarker to guide follow-up.
Core tip: This study assessed different blood markers as potential preoperative predictors of recurrence of alveolar echinococcosis (AE) after liver resection. A preoperative serum albumin level of < 37.5 g/L was found to be associated with AE recurrence after liver resection. Preoperative albumin level is easy to obtain and could represent a useful tool to guide postoperative follow-up after hepatectomy for AE.
- Citation: Joliat GR, Labgaa I, Demartines N, Halkic N. Preoperative albumin level is a marker of alveolar echinococcosis recurrence after hepatectomy. World J Gastroenterol 2017; 23(5): 853-858
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/853.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.853
Liver alveolar echinococcosis (AE) is a rare parasitic infection caused by Echinococcus multilocularis[1]. As this infection behaves like a malignant tumor, surgical resection is the treatment of choice when total ablation of the parasitic lesions can be achieved[2]. Even in cases of surgical liver resection with negative histological margins (R0), 2%-5% of the patients will present with recurrence during lifetime[3].
As the global incidence of AE is low and AE is endemic only in certain parts of the world[4], no specific risk factors for recurrence after hepatectomy have been described yet. Furthermore, no preoperative blood markers predictive of AE recurrence have been reported.
Predictive blood markers should be easy to measure and interpret for clinical use[5,6]. Typical markers of inflammation, including leukocytes, thrombocytes and C-reactive protein (CRP), represent such candidate blood markers. Mean corpuscular volume (MCV) and red blood cell distribution width (RDW) are included as well, based upon findings from several recent studies that have shown their levels to be correlated with liver inflammation[7,8]. Serum albumin has also been assessed following new data from liver surgery that have indicated a correlation with inflammation, peri-operative stress and postoperative complications[9-11].
The aim of the present study was to assess potential preoperative biological blood markers to predict recurrence in patients operated for liver AE.
Medical records of all consecutive patients with liver AE who were operated on in the Department of Visceral Surgery, University Hospital CHUV, Lausanne, Switzerland, between January 1992 and December 2015, were retrospectively reviewed for data on demographics, peri-operative details and postoperative outcomes. Recurrent cases were chosen for the analysis. The study was recorded in the Research Registry (UIN: researchregistry1033) and approved by the local ethics committee. Informed consent was obtained from each participant included in the study. The study protocol conforms to the provisions of the Declaration of Helsinki.
All patients underwent thoraco-abdominal computed tomography (CT) scan to assess hepatic involvement and presence of extrahepatic lesions. In case of CT-suspected AE, serology using western blotting and enzyme-linked immunosorbent assay, and liver magnetic resonance imaging (MRI) were performed. In case of preoperative jaundice, percutaneous or endoscopic biliary drainage was placed. If the estimated future remnant liver was < 30% of total liver volume, preoperative portal vein embolization was systematically performed. AE lesions were staged according to the PNM classification of the World Health Organization (WHO)[12]. All patients with liver AE or high suspicion of AE, independent of the lesion size, were candidates for hepatectomy. Anatomical resections were performed. Contra-indications to surgery were Child-Pugh C cirrhosis, comorbidities precluding general anesthesia, or estimated future remnant liver < 30% of total liver volume after portal vein embolization. No surgical strategy change occurred during the study period.
Major hepatectomy was defined as liver resection of ≥ 3 Couinaud’s segments, whereas minor hepatectomy consisted of resection of 1 or 2 segments. Postoperative complications were graded according to the Clavien classification[13]. R0 resection was defined as negative histological margins (> 1 mm) and R1 resection as positive histological margins. During the study period, 3 senior surgeons specialized in AE performed all liver resections.
Preoperative laboratory values of leukocytes, MCV, RDW, thrombocytes, CRP and albumin were determined at the time of hospital admission. These blood tests were performed the day before the operation for all patients.
Benzimidazole was given for 2 years after R0 resections and lifelong after R1 resections, according to WHO guidelines[14]. Patients were seen at the outpatient clinic 1 mo postoperatively. They were also seen twice by the surgeon in a clinical encounter at 6 mo and 1 year after the operation. Moreover, patients had regular (monthly) appointments with the infectiologist for follow-up of the albendazole blood levels and standard serologies. In case of clinical recurrence suspicion or positive serologies, complementary imaging (CT/MRI) was performed. Recurrence was defined as the appearance of new intrahepatic or extrahepatic disease after R0 resection or intrahepatic disease progression after R1 resection.
For continuous variables, a Mann-Whitney U test or Student’s t-test was used depending on the normality of the distribution and homogeneity of the variances. Survival curves were calculated using the Kaplan-Meier method, and the log-rank test was used to compare survivals. To assess the discriminative power of the marker, a receiver operating characteristic (ROC) curve analysis was performed. P < 0.05 was considered significant. Univariate and multivariate analyses were performed using Cox regressions. Only those factors with P < 0.1 (10%) on univariate analysis were included in the multivariate analysis. All statistical analyses were performed using GraphPad Prism 5.0© for Mac OS X and SPSS 19.0© for Mac OS X. The statistical methods of this study were reviewed by Dr. Jocelyn Bellier from the University Hospital CHUV, Lausanne, Switzerland.
During the study period, a total of 68 patients (31 men, 37 women) were diagnosed with AE and underwent liver resection. Between 1992 and 2003, major hepatectomies were performed in 12 patients and minor hepatectomies in 10 patients. Between 2004 and 2015, 27 patients underwent major hepatectomies and 19 patients minor hepatectomies. The median age was 61 years [interquartile range (IQR) 46-71]. Eight patients also had additional extrahepatic lesions. Minor hepatectomy was performed in 29 patients (43%) and major hepatectomy in 39 (57%). Postoperative complications occurred in 23 patients (34%). Minor (grade I-II) and major (grade III-IV) complications were observed in 16 (24%) and 7 patients (10%) respectively. One death (grade V) occurred during the postoperative period and was due to septic shock. The 90-d mortality was 1/68 (1.5%). The median overall survival (OS) was 69 mo for the entire cohort (IQR: 30-111).
Eight of the total 68 patients presented with recurrence over a median follow-up of 76 mo (IQR: 34-128). Median time to recurrence was 10 mo (IQR: 6-11). There were no statistically significant differences between patients with and without recurrence in terms of demographics and preoperative characteristics. For cases with recurrence the median OS was 81 mo (IQR: 34-108), compared to 69 mo (IQR: 30-113, P = 0.932) for cases without recurrence. Regarding the treatments of the 8 patients with recurrence, 4 underwent a repeat hepatectomy, 1 a palliative biliodigestive bypass, 1 a thoracic wedge resection, and 2 pursued long-term medical treatment with albendazole.
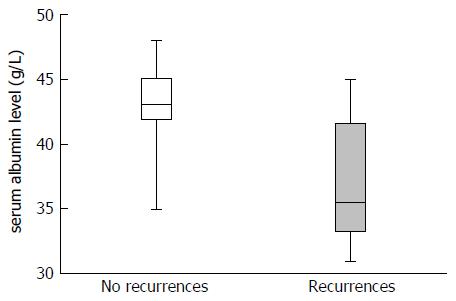
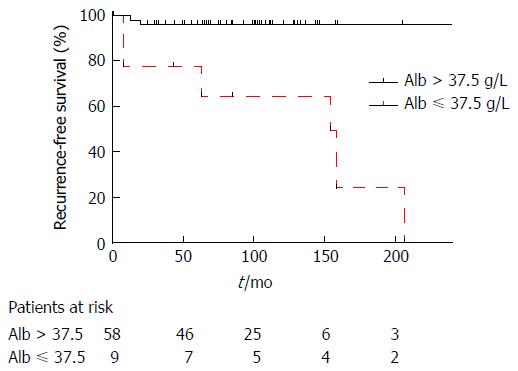
For recurrent and non-recurrent cases, the median laboratory values were: leukocytes: 8.1 G/L (IQR: 4-9) vs 6 G/L (IQR: 5-8, P = 0.554); MCV: 88 fl (IQR: 88-92) vs 89 fl (IQR: 86-92, P = 0.851); RDW: 14% (IQR: 13-15) vs 14% (IQR: 13-15, P = 0.979); thrombocytes: 249 G/L (IQR: 192-266) vs 244 G/L (IQR: 215-302, P = 0.627); CRP: 18 mg/L (IQR: 8-41) vs 4 mg/L (IQR: 2-26, P = 0.118); albumin: 36 g/L (IQR: 33-42) vs 43 g/L (IQR: 41-45, P = 0.005) (Figure 1). The area under the ROC curve for preoperative albumin predicting recurrence (Figure 2) was 0.840 (95%CI: 0.642-1, P = 0.002). The cut-off albumin level for a sensitivity of 94.5%, a specificity of 75%, a positive predictive value of 60%, a negative predictive value of 96% and a likelihood ratio of 3.7 was 37.5 g/L. Recurrence-free survival was better for patients with preoperative serum albumin level > 37.5 g/L (log-rank test: P = 0.0004; Figure 3).
Table 1 summarizes the univariate and multivariate Cox regressions for the preoperative values, surgical resection margins, lesion size and portal vein invasion. Only serum albumin level and surgical resection margins showed significance in the univariate analysis (HR = 0.078, P = 0.003 and HR = 0.137, P = 0.018); these two variables retained significance in the multivariate analysis (Table 1). The disease-free survival of patients according to the different included peri-operative items is presented in Table 2.
| Univariate | Multivariate | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Leukocytes > 9 g/L | 1.572 | 0.171-14.454 | 0.690 | |||
| MCV > 90 fl | 0.298 | 0.034-2.647 | 0.278 | |||
| RDW > 14 % | 0.444 | 0.080-2.461 | 0.353 | |||
| Thrombocytes > 300 g/L | 0.044 | 0.001-68’792 | 0.692 | |||
| CRP > 20 mg/mL | 1.061 | 0.171-6.567 | 0.949 | |||
| Hemoglobin > 130 g/L | 0.789 | 0.132-4.738 | 0.796 | |||
| Bilirubin > 10 μmol/L | 0.242 | 0.040-1.472 | 0.124 | |||
| Albumin > 37.5 g/L | 0.078 | 0.015-0.415 | 0.003 | 0.099 | 0.018-0.530 | 0.007 |
| R0 resection | 0.137 | 0.027-0.711 | 0.018 | 0.182 | 0.034-0.962 | 0.045 |
| Lesion size > 5 cm | 3.342 | 0.385-28.998 | 0.274 | |||
| PV invasion1 | 3.564 | 0.769-16.511 | 0.104 | |||
| Median DFS, mo (IQR) | P value | |
| Leukocytes > 9 g/L | 65 (6-81) | 0.545 |
| Leukocytes ≤ 9 g/L | 58 (23-107) | |
| MCV > 90 fl | 69 (29-121) | 0.116 |
| MCV ≤ 90 fl | 58 (15-92) | |
| RDW > 14% | 68 (28-117) | 0.154 |
| RDW ≤ 14% | 56 (8-81) | |
| Thrombocytes > 300 g/L | 68 (30-128) | 0.510 |
| Thrombocytes ≤ 300 g/L | 58 (18-102) | |
| CRP > 20 mg/mL | 68 (13-105) | 0.325 |
| CRP ≤ 20 mg/mL | 50 (9-74) | |
| Hemoglobin > 130 g/L | 65 (25-111) | 0.440 |
| Hemoglobin ≤ 130 g/L | 41 (13-107) | |
| Bilirubin > 10 μmol/L | 60 (12-129) | 0.488 |
| Bilirubin ≤ 10 μmol/L | 44 (25-81) | |
| Albumin > 37.5 g/L | 56 (26-79) | 0.048 |
| Albumin ≤ 37.5 g/L | 25 (13-57) | |
| R0 resection | 69 (27-129) | 0.024 |
| R1 resection | 41 (12-92) | |
| Lesion size > 5 cm | 65 (8-102) | 0.733 |
| Lesion size ≤ 5 cm | 50 (25-114) | |
| PV invasion1 | 56 (7-130) | 0.933 |
| No PV invasion | 60 (25-105) |
The results of this study suggest that preoperative serum albumin level has a good discriminative power to predict recurrence in patients operated for liver AE. No predictive markers of recurrence after hepatectomy for AE have been reported in the literature to date. The present study identified low preoperative serum albumin as associated with AE recurrence. A recent study by Wang et al[15] showed that a low level of CD44 proteins in AE resected liver was associated with the development of AE metastasis; however, in the multivariate analysis, a low level of CD44 proteins did not correlate with OS. The level of CD44 proteins in a pathological specimen is not easily assessed, and immunohistochemical staining of a resected liver sample is necessary. On the contrary, serum albumin can be determined easily in the preoperative period. Moreover, as a preoperative test, serum albumin can provide important information regarding management and follow-up. Indeed, in the case of preoperative hypoalbuminemia, follow-up can be adapted and tailored (e.g., closer follow-up visits with serologies or regular postoperative imaging examinations) since the patient will be at higher risk for recurrence.
Recently, albumin has been shown to be linked to peri-operative stress[9]. Several studies of liver surgery and other major surgeries have shown that a drop in albumin level correlates with postoperative complications[10]. Moreover, preoperative serum albumin level has been characterized as an independent factor of recurrence and OS after resection of hepatocellular carcinoma[16] and of OS in patients with hilar cholangiocarcinoma[17]. The tumor-like behavior of AE is probably more similar to a cancer such as cholangiocarcinoma than to those cancers frequently related to underlying liver diseases, such as hepatocellular carcinoma which commonly presents with hepatitis and/or cirrhosis.
The findings of the present study suggest that the inflammatory state that accompanies AE likely plays a role in the production of albumin in the liver. It can be postulated that the hepatic parasitic load of Echinococcus multilocularis disturbs the standard albumin production by the hepatocytes. Therefore, albumin level could predict the risk of AE recurrence as a reflection of the inflammatory state of the liver. Moreover, intrahepatic dissemination of the parasites could decrease albumin synthesis, but these explanations are speculative. The underlying pathological mechanisms still need to be investigated. In the present study’s cohort, no patient received intravenous albumin supplementation, even in cases of preoperative hypoalbuminemia. If surgeons routinely use albumin supplementation preoperatively, it can be suggested that the albumin level that would correlate to the recurrence risk would be the serum albumin level before supplementation. As no data exist on albumin supplementation and AE, further studies need to be performed to clarify the role of albumin supplementation preoperatively for patients with AE.
A surgical resection with positive histological margins was also identified as an independent risk factor for AE recurrence in the present study. This finding confirms the importance of a complete resection of the parasitic lesion in order to reduce the risk of recurrence. Although, the best distance for achieving a safe surgical resection margin is not clearly established and remains controversial. The WHO expert consensus has recommended a margin of 2 cm[12]. A previous paper from our group showed that the margin distance recommended for hepatocellular carcinoma (i.e., 1 mm) reached the same long-term postoperative outcomes[3]. The resection surgical margin distance needs to be further studied to confirm the safety of a margin defined as < 2 cm.
The present study has several limitations that must be addressed. First, its retrospective nature has inherent biases related to chart review and missing data. Second, the number of patients was relatively small. However, the rarity of the disease and the low incidence of recurrence have to be taken into consideration. The patient cohort involved in the present study represents one of the largest series of patients operated for AE in the literature. Finally, the long study period can induce a historical bias due to improvement of experience and expertise of the team over the years.
In conclusion, low preoperative serum albumin may be a predictive factor for AE recurrence after primary hepatectomy. This easily obtainable blood marker could help in guiding postoperative follow-up of AE patients and should be a recommended preoperative test.
Alveolar echinococcosis (AE) is a rare parasitic infection, primarily involving the liver. As AE behaves like a malignant tumor (liver parenchyma invasion), surgical resection is the treatment of choice to remove all lesions. The recurrence rate after surgical resection is around 5%. No predictive blood markers for AE recurrence after hepatectomy have been described in the literature to date.
New studies on AE have aimed to describe tailoring of the features of postoperative follow-up for AE patients [e.g., length of albendazole treatment, feasibility of positron emission tomography-computed tomography (PET-CT) scan, or predictive markers of recurrence].
Recent articles on AE have evaluated the feasibility of postoperative PET-CT scan for the follow-up of operated AE patients. No preoperative markers of AE recurrence have yet been described in the literature.
The results of this study can be used in daily clinical practice. Indeed, dosing of preoperative serum albumin level in patients with liver AE could help in guiding the postoperative follow-up since patients with low albumin level are at higher risk for AE recurrence.
AE is a parasitic infection that can disseminate within the liver or give rise to distant extrahepatic lesions. Echinococcus multilocularis is the parasitic agent causing AE.
This is a single-center retrospective study of 68 patients who underwent liver resection for AE. The authors found that preoperative albumin level is a significant risk factor for recurrence of AE. This is an interesting result from a highly experienced European center for AE.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Kokudo T, Wu CC S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004;17:107-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1176] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 2. | Kawamura N, Kamiyama T, Sato N, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamaga S, Matsushita M, Todo S. Long-term results of hepatectomy for patients with alveolar echinococcosis: a single-center experience. J Am Coll Surg. 2011;212:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Joliat GR, Melloul E, Petermann D, Demartines N, Gillet M, Uldry E, Halkic N. Outcomes After Liver Resection for Hepatic Alveolar Echinococcosis: A Single-Center Cohort Study. World J Surg. 2015;39:2529-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 714] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 5. | Rettig TC, Verwijmeren L, Dijkstra IM, Boerma D, van de Garde EM, Noordzij PG. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann Surg. 2016;263:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 6. | Vibert E, Boleslawski E, Cosse C, Adam R, Castaing D, Cherqui D, Naili S, Régimbeau JM, Cunha AS, Truant S. Arterial Lactate Concentration at the End of an Elective Hepatectomy Is an Early Predictor of the Postoperative Course and a Potential Surrogate of Intraoperative Events. Ann Surg. 2015;262:787-792; discussion 792-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Dogan S, Celikbilek M, Zararsiz G, Deniz K, Sivgin S, Guven K, Gursoy S, Ozbakir O, Yucesoy M. Red blood cell distribution width as a non-invasive marker for the assessment of inflammation in non-alcoholic steatohepatitis. Hepatogastroenterology. 2015;62:393-398. [PubMed] |
| 8. | Smirne C, Grossi G, Pinato DJ, Burlone ME, Mauri FA, Januszewski A, Oldani A, Minisini R, Sharma R, Pirisi M. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. 2015;47:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Hübner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schäfer M. Postoperative Albumin Drop Is a Marker for Surgical Stress and a Predictor for Clinical Outcome: A Pilot Study. Gastroenterol Res Pract. 2016;2016:8743187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Labgaa I, Joliat GR, Demartines N, Hübner M. Serum albumin is an early predictor of complications after liver surgery. Dig Liver Dis. 2016;48:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Mantziari S, Hübner M, Coti-Bertrand P, Pralong F, Demartines N, Schäfer M. A Novel Approach to Major Surgery: Tracking Its Pathophysiologic Footprints. World J Surg. 2015;39:2641-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 13. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24514] [Article Influence: 1167.3] [Reference Citation Analysis (0)] |
| 14. | Brunetti E, Kern P, Vuitton DA. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1313] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 15. | Wang H, Lu C, Liu X, Zhang W. Metastatic and prognostic factors in patients with alveolar echinococcosis. Int J Clin Exp Pathol. 2015;8:11192-11198. [PubMed] |
| 16. | Pan QX, Zhang JH, Su ZJ, Wang CR, Ke SY. The Glasgow Prognostic Score is an independent prognostic predictor of hepatocellular carcinoma following radical resection. Oncol Res Treat. 2014;37:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Waghray A, Sobotka A, Marrero CR, Estfan B, Aucejo F, Narayanan Menon KV. Serum albumin predicts survival in patients with hilar cholangiocarcinoma. Gastroenterol Rep (Oxf). 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |