Copyright
©The Author(s) 2017.
World J Gastroenterol. Oct 21, 2017; 23(39): 7139-7149
Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7139
Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7139
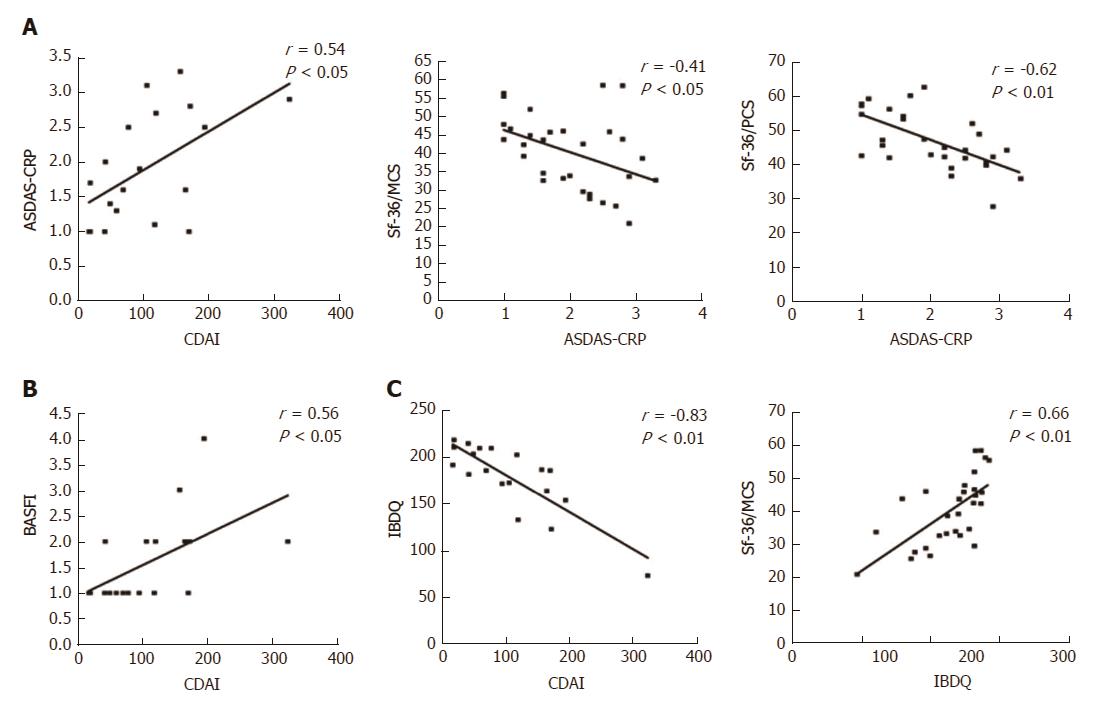
Figure 4 Correlations between articular and gastrointestinal disease activity and HRQoL scores in the ES-AN patient cohort after adalimumab therapy.
A: Evaluation data collected for the 30 ES-AN/Ada patients after 12 mo of therapy, showing correlation between gastrointestinal and articular disease activity in Crohn’s disease patients (assessed by CDAI) and ASDAS-CRP respectively, and between ASDAS-CRP and SF-36 summary scores (PCS and MCS); B: Correlation between CDAI and articular function (assessed by BASFI); C: Correlation between the gastrointestinal quality of life (assessed by IBDQ) and CDAI and SF-36/MCS. ASDAS-CRP: Ankylosing Spondylitis Disease Activity Score-C-Reactive Protein; CDAI: Crohn’s Disease Activity Index; ES-AN: Patients affected by ES in the Ancona’s cohort; ES-AN/Ada: Patients of the ES-AN cohort treated with adalimumab; HRQoL: Health-Related Quality of Life; IBDQ: Inflammatory Bowel Disease Questionnaire; SF-36/MCS: Summary of “Mental Component Score” of the Short Form-36 health survey; SF-36/PCS: Summary of “Physical Component Score” of the Short Form-36 health survey; Sf-36/PCS: Summary of “Physical Component Score” of the short form-36 health survey; PROs: Patient reported outcomes.
- Citation: Luchetti MM, Benfaremo D, Ciccia F, Bolognini L, Ciferri M, Farinelli A, Rossini M, Mosca P, Triolo G, Gabrielli A. Adalimumab efficacy in enteropathic spondyloarthritis: A 12-mo observational multidisciplinary study. World J Gastroenterol 2017; 23(39): 7139-7149
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7139.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7139