Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7191
Peer-review started: July 26, 2017
First decision: August 10, 2017
Revised: August 23, 2017
Accepted: September 5, 2017
Article in press: September 5, 2017
Published online: October 21, 2017
Processing time: 88 Days and 0.7 Hours
Primary biliary cholangitis (PBC) is an idiopathic autoimmune liver disease characterized by chronic cholestasis and destruction of the intrahepatic bile ducts. Similar to other autoimmune diseases, the pathogenesis of PBC is considered to be a complex etiologic phenomenon involving the interaction of genetic and environmental factors. Although a number of common variants associated with PBC have been reported from genome-wide association studies, a precise genetic mechanism underlying PBC has yet to be identified. Here, we describe a family with four sisters who were diagnosed with PBC. After the diagnosis of the index patient who was in an advanced stage of PBC, one sister presented with acute hepatitis, and two sisters were subsequently diagnosed with PBC. Notably, one half-sister with a different mother exhibited no evidence of PBC following clinical investigation. Our report suggests the possibility of a maternal inheritance of PBC susceptibility. Moreover, judging from the high-penetrance of the disease observed in this family, we inferred that a pathogenic genetic variant might be the cause of PBC development. We describe a family that exhibited diverse clinical presentations of PBC that included asymptomatic stages with mildly increased liver enzyme levels and symptomatic stages with acute hepatitis or advanced liver fibrosis. Additional studies are needed to investigate the role of genetic factors in the pathogenesis of this rare autoimmune disease.
Core tip: The precise genetic mechanism underlying primary biliary cholangitis (PBC) has yet to be identified. Here, we describe a family with four siblings who were diagnosed with primary biliary cholangitis. This is the first case report to provide evidence of a maternal inheritance mechanism for PBC based on the identification of a non-PBC half-sibling. This report also highlights the occurrence of all clinical presentations of PBC in one family. Identification of a causal variant is important for a better understanding of the mechanism underlying PBC pathogenesis.
- Citation: Shin S, Moh IH, Woo YS, Jung SW, Kim JB, Park JW, Suk KT, Kim HS, Hong M, Park SH, Lee MS. Evidence from a familial case suggests maternal inheritance of primary biliary cholangitis. World J Gastroenterol 2017; 23(39): 7191-7197
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7191
Primary biliary cholangitis (PBC) is a chronic autoimmune cholestatic liver disease that predominantly affects middle-aged women. PBC is characterized by immune-mediated destruction of the intrahepatic bile ducts that gradually leads to fibrosis, cirrhosis, and eventually liver failure. Most patients are diagnosed when asymptomatic with an elevation of alkaline phosphatase (ALP) or with pruritus and mild elevations on liver biochemical tests. PBC can be diagnosed if two of the three following criteria are met: the presence of anti-mitochondrial antibody (AMA); cholestatic biochemical test results, such as an elevated ALP level; and histologic evidence of non-suppurative cholangitis with destruction of the interlobular bile ducts[1,2].
The current hypothesis regarding the etiology of PBC is that it is a multifactorial disease that occurs due to a combination of genetic, immunologic, and environmental factors[3]. The influence of genetic factors is evidenced by familial clusters and twin studies[4]. Epidemiological studies have demonstrated that family members of patients with PBC are at a higher risk of developing PBC[5-7]. Associations between a number of genetic loci and PBC have been reported in genome-wide association studies (GWAS), which is similar to the cases of many other autoimmune diseases[8-10]. However, no precise genetic mechanism underlying PBC is known.
Herein, we report a family with four sisters who were diagnosed with PBC. The possible implications of a monogenic etiology of PBC are discussed.
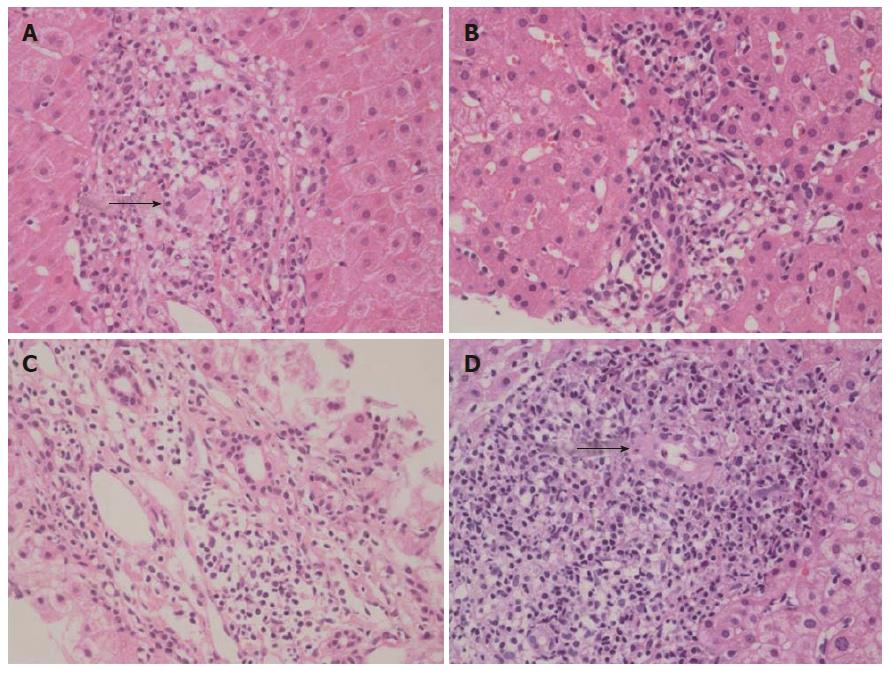
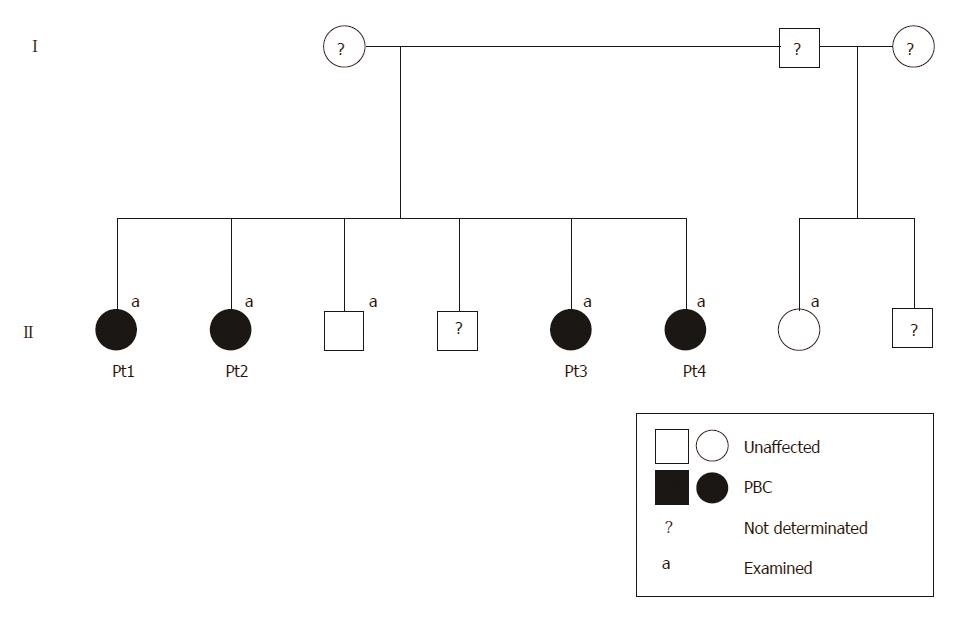
A 56-year-old woman (Table 1, patient 1) was referred to Hallym University Kangnam Sacred Heart Hospital with abnormal liver function test results. Her past history was unremarkable, and she had no history of alcohol or drug abuse. Here, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP, gamma-glutamyltranspeptidase (GGT), and immunoglobulin (Ig) M were elevated, and she was positive for AMA. A liver biopsy revealed non-suppurative destructive cholangitis and granulomatous inflammation with fibrosis (Figure 1A). The patient was diagnosed with PBC, and her family members, including three sisters, one brother, and one half-sister were evaluated (Figure 2).
| Characteristics (reference ranges) | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Brother | Half-sister |
| Sex, age | F, 56 | F, 54 | F, 44 | F, 38 | M, 52 | F, 48 |
| Symptom | Fatigue, pruritus | (-) | Fatigue, nausea | (-) | (-) | (-) |
| Underlying disease | (-) | Thyroid cancer | (-) | (-) | (-) | (-) |
| AST, IU/L (9-39) | 127 | 29 | 311 | 63 | 16 | 17 |
| ALT, IU/L (6-45) | 160 | 24 | 509 | 90 | 11 | 13 |
| Total/direct bilirubin, mg/dL (0.4-1.3/0.1-0.4) | 1.0 / 0.3 | 0.8 /0.2 | 1.3 / 0.7 | 0.6 / 0.2 | 0.5 / 0.2 | 0.9 / 0.3 |
| ALP, IU/L (35-104) | 132 | 122 | 122 | 101 | 83 | 58 |
| GGT, IU/L (8-35) | 169 | 63 | 188 | 112 | 14 | 15 |
| HBs antigen/antibody | (-/+) | (-/+) | (-/-) | (-/+) | (-/+) | Not done |
| Anti-HCV antibody | (-) | (-) | (-) | (-) | (-) | Not done |
| Anti-mitochondrial antibody, titer | (+, > 1:1280) | (+, 1:640) | (+, > 1:1280) | (+, 1:640) | (-) | (-) |
| Anti-smooth muscle antibody | (-) | (-) | (-) | (-) | (-) | (-) |
| Anti-liver-kidney microsome antibody | (-) | (-) | (-) | (-) | Not done | Not done |
| Antinuclear antibody | (-) | (-) | (-) | (-) | (-) | (-) |
| IgG, mg/dL (700-1600) | 1630 | 1150 | 1790 | 2070 | 1130 | 1210 |
| IgA, mg/dL (70-400) | 316 | 226 | 362 | 268 | 287 | 177 |
| IgM, mg/dL (40-230) | 421 | 289 | 542 | 542 | 247 | 94.4 |
Patient 2 had no symptoms but exhibited a mild elevation of ALP. She also exhibited positivity for AMA and non-suppurative cholangitis on a liver biopsy (Figure 1B) and was diagnosed with early-stage PBC.
Patient 3 was admitted to an outside university hospital with acute hepatitis of undetermined etiology. Here, liver enzymes were markedly elevated at the time of admission, i.e., her AST was 744 IU/L, and her ALT was 1273 IU/L. Viral markers of acute hepatitis pathogens, including hepatitis A, hepatitis E, herpes simplex virus, Epstein-Barr virus, and cytomegalovirus, were negative. She then presented to our hospital, where laboratory tests revealed AMA positivity, an elevated IgM level, and liver histologic findings consistent with PBC (Figure 1C).
Patient 4 had no symptoms but exhibited mild elevations of the serum levels of AST, ALT, and GGT. She was AMA-positive, and her IgG and IgM levels were elevated. A subsequent liver biopsy revealed advanced histologic findings consistent with PBC, including portal inflammation, non-suppurative cholangitis, granuloma, lymphoplasmacytic infiltrates, and peri-portal fibrosis (Figure 1D).
One brother (52 years old) with the same mother and one half-sister (48 years old) with a different mother were clinically evaluated for PBC; however, they both exhibited normal liver biochemistry and negative AMA results (Table 1).
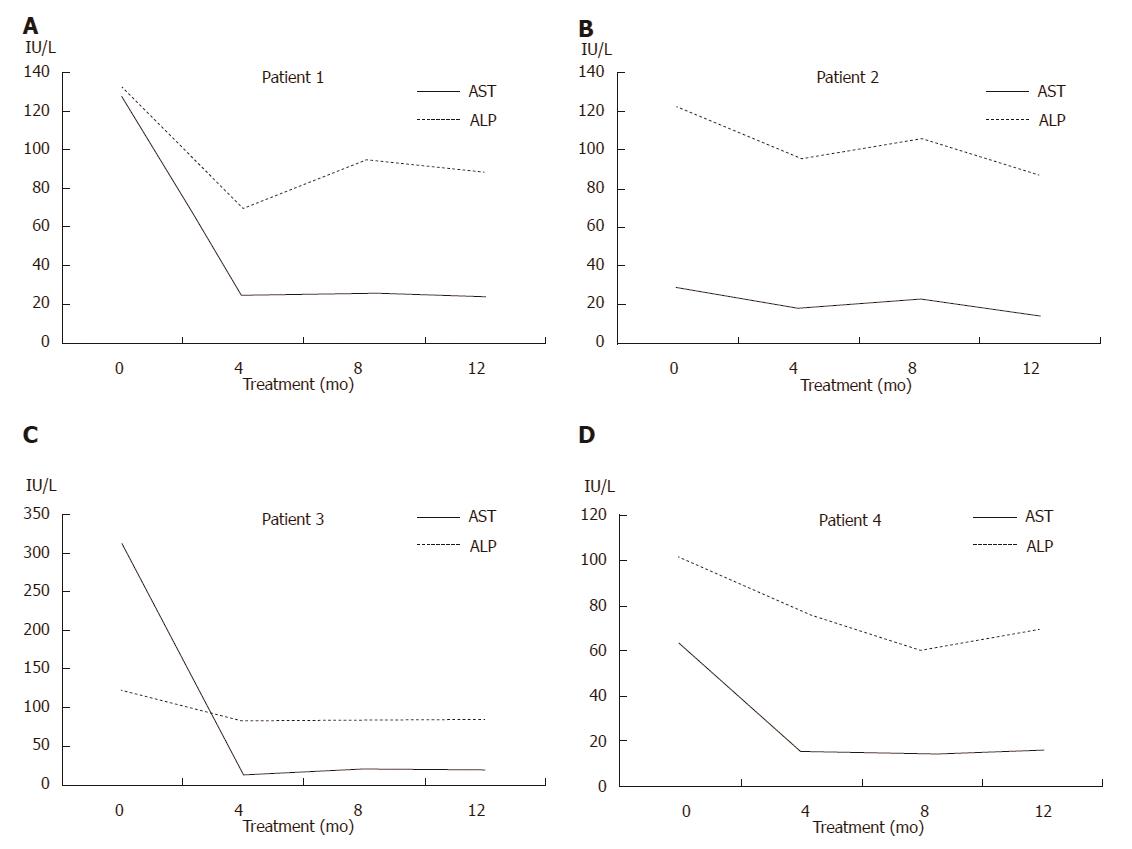
The four patients were treated with ursodeoxycholic acid (UDCA; 15 mg/kg·d), and liver biochemistry results indicated good responses (Figure 3). The patients have been followed and maintained on UDCA therapy without complications.
Here, we report a family that displayed evidence of maternal inheritance and exhibited all possible clinical presentations of PBC. The index patient (patient 1) was diagnosed in a fairly advanced stage of the disease and with early cirrhosis. In contrast, two patients were diagnosed in asymptomatic stages with mildly increased liver biochemistry results (patients 2 and 4). Interestingly, one patient (patient 3) presented with an acute hepatitis-like condition with markedly elevated serum aminotransferases. In PBC, acute presentations have very rarely been reported[11]. Because acute presentations of an overlapping syndrome, i.e., autoimmune hepatitis, are common, it has previously been suggested that autoimmune hepatitis might exhibit overlap with the early stage of PBC in PBC patients with acute presentations[12]. However, we were unable to find any relevant evidence of autoimmune hepatitis in patient 3, including in examinations of liver histology, auto-antibody studies, the serum IgG level, and the treatment response to UDCA[13,14].
There are some factors that are known to trigger PBC symptoms or signs that include adverse drug reactions, pregnancy, and delivery[12]. The mechanisms by which these factors affect disease state are thought to be related to an immunological influence[12]. However, our patient with the acute presentation was not pregnant and had no history of medication use. Our findings suggest that PBC should be considered in the differential diagnosis of acute hepatitis of unknown etiology. Further study is needed to identify possible undiagnosed cases and to investigate the mechanisms that trigger the acute phase of PBC.
Previous GWA studies have contributed to our understanding of the genetic architecture of PBC[8-10]. A number of susceptibility loci are located in genes with known immunologic functions, such as human leukocyte antigen (HLA), interleukin 12 receptor subunit beta 2 (IL12RB2), interleukin 12A (IL12A), C-X-C motif chemokine receptor 2 (CXCR2), and the CD80 molecule (CD80)[8-10]. However, the identified polymorphisms are also found in the general population at high frequencies and could partially explain the disease heritability. Based on the existence of familial cases with many affected members, as we report here[5,15-17], we infer the contribution of a rare, disease-causing variant to the development of PBC. Although the previous literature also reports a high prevalence of PBC in siblings of patients (four or more cases in 6-10 siblings)[5,15,16], the unique and interesting feature in our case is that PBC was diagnosed in all four sisters but not in one half-sister with a different mother. This finding suggests the possibility of maternal inheritance mechanism and a genetic pattern, such as X chromosome-linked or mitochondrial inheritance.
With rapid progression of next-generation sequencing (NGS) technology, the cost and time required to sequence data have substantially declined[18]. Using high-throughput NGS technology, it is technically feasible to quickly and efficiently investigate rare variants that cannot be identified by GWAS[18]. Therefore, we plan to detect the causal genetic variant in this family using whole-exome sequencing. The identification of a rare pathogenic variant is important for a better understanding of the mechanism underlying the pathogenesis of PBC and for identifying novel therapeutic targets.
The early detection of PBC is important because UDCA treatment before the development of late-stage disease may normalize the life expectancy[19-21]. Long-term observational studies have demonstrated the benefits of UDCA on serum liver tests, histologic features, and improved survival[22,23]. The efficacy of a novel bile acid analogue, obeticholic acid, has also recently been demonstrated in patients who exhibit inadequate responses to UDCA[24,25]. Luckily, all of our patients responded well to UDCA therapy.
From our case and prior evidence indicating familial clustering of PBC, if one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives.
The genetic etiology of PBC remains elusive despite much effort. To the best of our knowledge, this is the first case to provide evidence of a maternal inheritance mechanism for PBC based on the identification of a non-PBC half-sibling. This report also highlights the occurrence of all clinical presentations of PBC in one family. Additional studies are needed to identify a causal genetic variant in this family and the exact genetic mechanism that leads to the development of PBC.
Two patients presented with fatigue and nausea, and the other two patients exhibited no symptoms.
Four sisters in a family were diagnosed with primary biliary cholangitis (PBC), although one brother with the same mother and one half-sister with a different mother showed no evidence of PBC.
Drug-induced cholestasis (history for medication), bile duct obstruction (ultrasound for gallstones or malignancy), autoimmune hepatitis [liver histology, auto-antibodies studies, serum immunoglobulin G level, and treatment response to ursodeoxycholic acid (UDCA)].
All four patients showed the presence of anti-mitochondrial antibodies at high titers (≥ 1:640) and elevated serum liver biochemistry results including those for alkaline phosphatase and aspartate aminotransferase.
For all patients, ultrasounds revealed no evidence of biliary obstruction due to gallstones or malignancy.
For all patients, microscopic observations of liver biopsy tissue revealed histologic findings consistent with PBC.
The four patients were treated with UDCA.
To date, many PBC candidate loci have been reported in genome-wide association studies. However, these loci are very heterogeneous, and the exact genetic cause of PBC remains elusive.
Whole-exome sequencing: also called WES or exome sequencing, is a technique for the sequencing of all human protein-coding exons.
From the observations of the presentations of the four siblings diagnosed with PBC, we recommend that PBC occurrence should be considered in family members of any identified patients.
This is an interesting clinical observation that suggests the possibility of a maternal inheritance pattern of PBC. The presented data may provide an incentive for further research.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka H, Reshetnyak VI, Tsoulfas G S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y
| 1. | Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology. 2009;50:291-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 894] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 2. | Reshetnyak VI. Primary biliary cirrhosis: Clinical and laboratory criteria for its diagnosis. World J Gastroenterol. 2015;21:7683-7708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 3. | Smyk D, Cholongitas E, Kriese S, Rigopoulou EI, Bogdanos DP. Primary biliary cirrhosis: family stories. Autoimmune Dis. 2011;2011:189585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, Gordon SC, Wright HI, Zweiban B, Podda M. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485-492. [PubMed] |
| 5. | Yanagisawa M, Takagi H, Takahashi H, Uehara M, Otsuka T, Yuasa K, Hosonuma K, Mori M. Familial clustering and genetic background of primary biliary cirrhosis in Japan. Dig Dis Sci. 2010;55:2651-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Corpechot C, Chrétien Y, Chazouillères O, Poupon R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Zografos TA, Gatselis N, Zachou K, Liaskos C, Gabeta S, Koukoulis GK, Dalekos GN. Primary biliary cirrhosis-specific autoantibodies in first degree relatives of Greek primary biliary cirrhosis patients. World J Gastroenterol. 2012;18:4721-4728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Cordell HJ, Han Y, Mells GF, Li Y, Hirschfield GM, Greene CS, Xie G, Juran BD, Zhu D, Qian DC. International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat Commun. 2015;6:8019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 210] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 9. | Hirschfield GM, Invernizzi P. Progress in the genetics of primary biliary cirrhosis. Semin Liver Dis. 2011;31:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Gulamhusein AF, Juran BD, Lazaridis KN. Genome-Wide Association Studies in Primary Biliary Cirrhosis. Semin Liver Dis. 2015;35:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Sohda T, Shiga H, Nakane H, Nishizawa S, Yoshikane M, Anan A, Suzuki N, Irie M, Iwata K, Watanabe H. Rapid-onset primary biliary cirrhosis resembling drug-induced liver injury. Intern Med. 2005;44:1051-1054. [PubMed] |
| 12. | Nakanuma Y. Is “acute hepatitis-like onset” a hither-to poorly recognized clinical manifestation of primary biliary cirrhosis at an early stage? Intern Med. 2005;44:1023-1024. [PubMed] |
| 13. | Chazouillères O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis-autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 478] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Park Y, Cho Y, Cho EJ, Kim YJ. Retrospective analysis of autoimmune hepatitis-primary biliary cirrhosis overlap syndrome in Korea: characteristics, treatments, and outcomes. Clin Mol Hepatol. 2015;21:150-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Jaup BH, Zettergren LS. Familial occurrence of primary biliary cirrhosis associated with hypergammaglobulinemia in descendants: a family study. Gastroenterology. 1980;78:549-555. [PubMed] |
| 16. | Abu-Mouch S, Selmi C, Benson GD, Kenny TP, Invernizzi P, Zuin M, Podda M, Rossaro L, Gershwin ME. Geographic clusters of primary biliary cirrhosis. Clin Dev Immunol. 2003;10:127-131. [PubMed] |
| 18. | Wu L, Schaid DJ, Sicotte H, Wieben ED, Li H, Petersen GM. Case-only exome sequencing and complex disease susceptibility gene discovery: study design considerations. J Med Genet. 2015;52:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 538] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 20. | Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297-303. [PubMed] |
| 21. | Reshetnyak VI. Concept on the pathogenesis and treatment of primary biliary cirrhosis. World J Gastroenterol. 2006;12:7250-7262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 22. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR; Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 348] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Corpechot C, Abenavoli L, Rabahi N, Chrétien Y, Andréani T, Johanet C, Chazouillères O, Poupon R. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Samur S, Klebanoff M, Banken R, Pratt DS, Chapman R, Ollendorf DA, Loos AM, Corey K, Hur C, Chhatwal J. Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis. Hepatology. 2017;65:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 793] [Article Influence: 88.1] [Reference Citation Analysis (0)] |