Published online Sep 21, 2017. doi: 10.3748/wjg.v23.i35.6420
Peer-review started: May 16, 2017
First decision: June 5, 2017
Revised: June 25, 2017
Accepted: August 1, 2017
Article in press: August 2, 2017
Published online: September 21, 2017
Processing time: 132 Days and 6 Hours
To analyze pancreatic cancer patients who developed metachronous pulmonary metastases (MPM) as a first site of recurrence after the curative-intent surgery.
One-hundred-fifty-nine consecutive pancreatic ductal adenocarcinoma (PDAC) patients who underwent radical pancreatic surgery between 2006 and 2013 were included in this retrospective analysis. The clinical data including age, sex, grade, primary tumor location, pTNM stage, lymph node infiltration, microangioinvasion, perineural invasion, lymphovascular invasion, the therapy administered, and follow-up were all obtained from medical records. Further analysis covered only patients with metachronous metastases. Clinical and histopathological data (age, sex, grade, primary tumor location, pTNM stage, lymph node infiltration, microangioinvasion, perineural invasion, lymphovascular invasion, the therapy administered and follow-up) of patients with metachronous non-pulmonary metastases and patients with metachronous pulmonary metastases were statistically assessed. Disease-free survival (DFS) from pancreas resection until metastases onset and overall survival (OS) were calculated. Wilcoxon test, χ2 test and survival functions computed by the Kaplan-Meier method were used. Statistical significance was evaluated by the log-rank test using SPSS. A P-value of less than 0.05 was considered statistically significant.
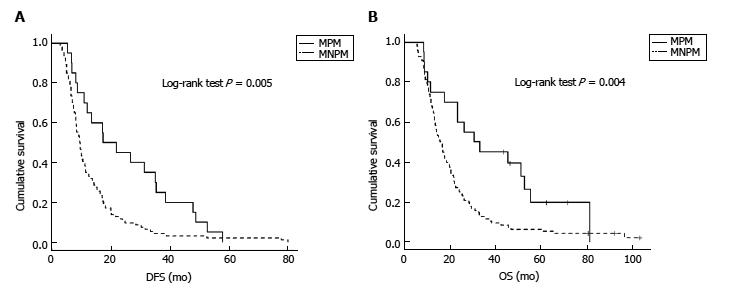
Metachronous pulmonary metastases were observed in 20 (16.9%) and were operable in 3 (2.5%) of PDAC patients after a prior curative-intent surgery. Patients with isolated pulmonary metastases (oligometastases and multiple metastases) had estimated prior DFS and OS of 35.4 and 81.4 mo, respectively, and those with metachronous pulmonary metastases accompanied by other metastases had prior DFS and OS of 17.3 and 23.4 mo, respectively. Patients with non-pulmonary metastases had prior DFS and OS of 9.4 and 15.8 mo, respectively. Different clinical scenarios according to the presentation of MPM were observed and patients could be divided to three subgroups with different prognosis which could be used for the selection of treatment strategy: isolated pulmonary oligometastases, isolated multiple pulmonary metastases and pulmonary metastases accompanied by other metastases.
Surgery should be considered for all patients with isolated pulmonary oligometastases, but the risk of intervention has to be individually weighted for each patient.
Core tip: Metachronous pulmonary metastases represent the most frequent extraabdominal site of recurrence following radical resection for pancreas cancer. Three different clinical scenarios according to the presentations of metachronous pulmonary metastases were observed in our cohort: isolated oligometastases, multiple pulmonary metastases and pulmonary metastases accompanied by other metastases. Patients with isolated pulmonary metastases have the longest disease-free survival and overall survival. Surgery should be considered for all patients with isolated oligometastases.
- Citation: Lovecek M, Skalicky P, Chudacek J, Szkorupa M, Svebisova H, Lemstrova R, Ehrmann J, Melichar B, Yogeswara T, Klos D, Vrba R, Havlik R, Mohelnikova-Duchonova B. Different clinical presentations of metachronous pulmonary metastases after resection of pancreatic ductal adenocarcinoma: Retrospective study and review of the literature. World J Gastroenterol 2017; 23(35): 6420-6428
- URL: https://www.wjgnet.com/1007-9327/full/v23/i35/6420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i35.6420
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignant disease with an increasing incidence[1]. Even though there is a long latency period between the emergence of a malignant cell clone and the death from metastatic disease, the majority of patients still present with either locally advanced or metastatic disease[2]. Only 15%-20% of patients have localized disease which may be considered surgically resectable at the time of diagnosis. An additional 20%-30% of patients have border-line resectable or locally advanced disease among whom one to two thirds could be potential candidates for curative surgery following a neoadjuvant therapy[3,4]. Finally, around 50% of patients have a metastatic disease at the time of diagnosis. The prognosis of PDAC patients after curative surgery is still relatively poor with the 5-year survival rate ranging between 15%-35%[5-7]. Up to 30% of PDAC patients die within the first year after radical surgery due to the disease progression and the mortality rate increases to around 60% by the end of the second year[8,9].
Although the most frequent site of metastases in PDAC is the liver[10], among long-term PDAC survivors the most common site of metastases is the lung[5]. Since the pulmonary metastases usually occur in the context of general disease progression, chemotherapy is considered to represent the standard treatment option. However, with regard of PDAC long-term survivors, there is a subgroup of patients with metachronous pulmonary metastases being the only site of the disease recurrence, presenting an option for surgical treatment. The literature concerning the use of surgical therapy in the treatment of metachronous isolated pulmonary PDAC metastases is still very limited. Isolated late pulmonary metastases in PDAC have been reported as single cases[11-14], case series[15], and retrospective cohort studies[7,16-21]. Katz et al[5] in a study focusing on long-term survivors reported the highest rate (22%) of pulmonary metastases. Most recently, some retrospective analyses tried to define criteria based on the report derived of Thomford et al[22], including a disease-free survival (DFS) of more than 20 mo after the primary surgery, pulmonary metastases ≤ 16 mm, primary stage I, good performance status and a technically operable tumor, for patients in whom the surgical resection of isolated metastases would be meaningful[18,22,23]. However, due to the low prevalence of metachronous pulmonary metastases reliable data regarding the clinical outcome and indications for the optimal surgical or medical treatment are currently limited.
The aim of the present study was to retrospectively analyze a homogeneous cohort of PDAC patients who underwent a surgery with curative intent between the years 2006 and 2013 in our center. The primary objective was the identification of patients with metachronous pulmonary metastases as the first site of a disease recurrence after surgery and the assessment of prior DFS, and an overall survival (OS) in this subgroup of PDAC patients compared to the patients with non-pulmonary metastases. Different clinical scenarios according to a type of pulmonary metastases could also be discerned.
A cohort of 159 consecutive PDAC patients who underwent radical surgery between the years 2006 and 2013 in our center was included in this retrospective analysis. Histopathological diagnosis of PDAC was performed according to the previous standard classification, and all specimens were evaluated before the introduction of the Leeds protocol. The clinical data including age, sex, grade, primary tumor location, pTNM stage, lymph node infiltration, microangioinvasion, perineural invasion, lymphovascular invasion, the therapy administered, and follow-up were all obtained from medical records or from the Czech National Oncological Registry.
Disease free survival (DFS) was defined as the time between the date of surgery and the date of diagnosis of new disease recurrence. Criteria for new recurrence were applied according to previous studies[5,19]. The patients were followed in 3-mo intervals with clinical examination including CA 19-9 level assessment, and 6-mo intervals with cross-sectioning computed tomography (CT), or positron emission tomography (PET/CT) in case elevation CA 19-9 was noted[24,25]. Oligometastatic disease was defined as limited systemic metastatic tumors in which local ablative therapy could be curative[26-30]. As the study was focused to metachronous metastatic PDAC, patients without recurrence, who died in postoperative period (90 d), had R2 resections and those with incomplete data were excluded from DFS analysis. The Thomford criteria for pulmonary metastasectomy including controlled primary lesion, no extrapulmonary metastases present, metastases limited to one lung, patient being able to tolerate surgery were applied[22]. Consensus from a multidisciplinary team had to be obtained before surgery. OS was defined as the time between the date of primary surgery and death. Surviving patients were censored at the last follow-up on 31st December 2016.
Further analysis covered only patients with metachronous metastases. Clinical and histopathological data (age, sex, grade, primary tumor location, pTNM stage, lymph node infiltration, microangioinvasion, perineural invasion, lymphovascular invasion, the therapy administered and follow-up) of patients with metachronous non-pulmonary metastases and patients with metachronous pulmonary metastases (MPM) were statistically assessed. Patients with MPM were initially divided into two groups; metachronous isolated pulmonary metastases, and metachronous pulmonary metastases with metastases to other organs.
Wilcoxon test and χ2 test were used for analysis of age and other parameters. The survival functions were computed by the Kaplan-Meier method and statistical significance was evaluated by the log-rank test using SPSS. A P-value of less than 0.05 was considered statistically significant.
This study has been approved by the institutional Review Board at the University Hospital Olomouc.
The study population consisted of 159 patients with resectable PDAC, i.e., no evidence of clinically detectable metastases, cM0 (Supplementary Table 1). R0 and R1 resection rate was not formally assessed as the samples have been evaluated before the introduction of the Leeds protocol into clinical practice at our institution, and the results concerning R0 resection could have been overestimated.Two patients (1.3%) had neoadjuvant therapy consisting of concomitant radiotherapy with continuous 5-fluorouracil administration. Adjuvant chemotherapy consisting of nucleoside analogues (gemcitabine and 5-fluorouracil) was administered in 111 (69.8%) patients. Forty-one patients (25.8%) were excluded from further analysis due to no recurrence (n = 24), incomplete data (n = 14), postoperative death (n = 2) or R2 resection (n = 1). In total 1-, 2-, 3- and 5-years survival rates in entire cohort were 66.7%, 36.6%, 28.1% and 18.7%, respectively. The perineural invasion and lymph node infiltration were frequently identified (66.7% and 53.5%), while lymphovascular and microangioinvasion were identified less often (32.7% and 18.2%). The median DFS was 10 mo, and the median OS in entire cohort reached 17.6 mo. The most common sites of metastases within the first year included liver (57%), locoregional recurrence (37%), lymph nodes (22%), peritoneum (18%), and lung (10%), with multiple sites involved in 51%. In the second year, most frequent were locoregional (45%), lymph nodes (40%), liver (20%), and lung (20%), with involvement of multiple sites in 40%. In most patients who subsequently developed pulmonary metastases the primary procedures were head resections or total pancreatectomies with the predominant tumor location in pancreatic head (88.7%). Splenic vein obstructions were not identified. Further analysis was focused on patients who developed metachronous metastases.
| n | DFS | OS | |
| Non-pulmonary | 96 | 9.48 | 16.19 |
| Metachronous pulmonary metastases | 20 | 19.58 | 31.81 |
| Isolated pulmonary | 5 | 35.44 | 81.36 |
| Pulmonary and other metastases | 15 | 17.26 | 23.37 |
| Metastatic cohort | 117 | 10.03 | 16.90 |
| Entire cohort | 159 | 17.59 |
Data of patients with metachronous metastases included in the further analysis are summarized in Supplementary Table 2. Most patients with isolated pulmonary metastases were diagnosed using PET/CT scan triggered by CA 19-9 elevation during the follow up. In total 20 patients (17%) had metachronous pulmonary metastases, almost all diagnosed with PET/CT scans. Adjuvant chemotherapy after primary pancreatic resection was used in 85% of MPM patients compared to 74% of non-pulmonary metastases group. Perineural invasion was higher in MPM patients, but the difference was not statistically significant (P = 0.057). Statistically significant differences between patients with metachronous pulmonary and non-pulmonary metastases were evident in the sex (P = 0.012) and survival rate (P = 0.004). Figure 1 displays prior DFS and OS in both subgroups, with statistically significant differences (P = 0.005 and 0.004, respectively).
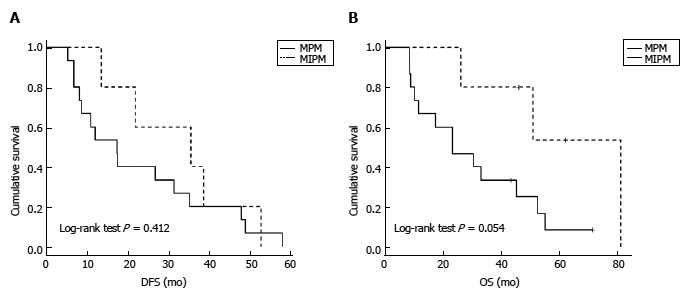
In five patients (25%), all females, metastases were isolated to the lung. Pulmonary metastases along with multiple metastases to other organs were detected in 15 cases (75%). Figure 2 displays DFS and OS in both groups. Analysis is difficult due to very small numbers, but OS prolongation is of borderline statistical significance (P = 0.054). While the estimated median OS is similar in patients with non-pulmonary metastases and the entire cohort, the median OS is double in MPM (Table 1).
In the subgroup of metachronous isolated metastases (n = 5) estimated medians of DFS and OS are 35.4 and 81.4 mo, respectively (Table 1). There were two patients with multiple bilateral lesions and three patients with isolated oligometastatic recurrence.The patients with multiple bilateral lesions were treated with systemic chemotherapy (5-fluorouracil combined with oxaliplatin or gemcitabine). In one patient thoracoscopic biopsy identified PDAC metastases 22 mo after initial pancreatic resection. The lesions were relatively stable and progressed slowly during the sequential lines of systemic chemotherapy, but brain metastases developed 80 mo after primary resection and the patient died 82.5 mo after resection. No intraabdominal recurrence was detected. The second patient was treated with gemcitabine and died of progressive disease 50.5 mo after the initial pancreatic resection.
Patients with metachronous isolated oligometastases (n = 3) could all be considered candidates for surgery. One patient was treated outside of our institution with gemcitabine and subsequently developed liver metastases and died 12.5 mo after diagnosis of MPM. Two patients in this group underwent uncomplicated pulmonary resections for isolated oligometastatic recurrence that manifested 34 and 52 mo after primary pancreatic resections and PDAC metastases were histologically verified. As of December 2016, these patients are still alive without recurrence with a second disease free interval after pulmonary resection of 11+ and 13+ mo, respectively.
In further analysis of patients with pulmonary metastases along with metastases to other organs (n = 15) there were 3 patients with pulmonary metastases and extrapulmonary metastases restricted to retroperitoneal paraaortal lymph nodes. These patients had DFS similar to patients with isolated pulmonary metastases (median 35 mo) and significantly longer OS (median 45 mo) compared to the patients with pulmonary metastases accompanied by metastases to other organs (median DFS and OS of 13.5 mo and 19.6 mo, respectively). However, the data are very limited because of small numbers of patients.
The shortest DFS (9.5 mo) and OS (16.2 mo) were observed in patients with metachronous non-pulmonary metastases where the first location of metastatic disease were liver, peritoneum, locoregional, or lymph nodes.
PDAC may metastasize to any organ[10,26,31-34] by hematogenous, lymphogenic, and perineural route or by direct intracavitary spread[10,26]. Pulmonary metastases as the first site of disease recurrence after resections for PDAC can present as isolated oligometastases, isolated multiple metastases or in association to metastatic spread to other organs. The incidence of PDAC in Central Europe is high, and, in fact, the incidence in the Czech Republic is currently the highest in the world. To the best of our knowledge, the present study is the first large homogenous cohort of PDAC describing these differences on Central European population.
Patients with PDAC after primary radical multidisciplinary treatment (surgical resection followed by adjuvant therapy) have a very high risk of distant recurrence. One-hundred-thirty-two patients (83.1%) in the present cohort developed metastases.
Even though metachronous pulmonary metastases after pancreatic resection are the most frequent extraabdominal site of PDAC recurrence, this presentation of recurrent disease is relatively rare, with the reported incidence of MPM as first site being between 3%-22%[5,7,16-19,21]. The highest rate of 22% was reported by Katz et al[5] in a highly selected cohort of patients of whom 77% had neoadjuvant therapy administered before primary resections. In the present study isolated pulmonary recurrence was observed in 4.2% of all cases of recurrent PDAC and 3.1% of entire cohort, in accordance with 3% rate of MPM reported previously by Arnaoutakis et al[17]. The present study indicates a low rate in unselected patients after radical primary treatment not exceeding 5%. Interestingly, isolated metachronous pulmonary metastases developed only in women, and female predominance was statistically significant in comparison of MPM and non-pulmonary metastatic patients.
Kamisawa et al[10] in an autopsy series in an unselected cohort of patients treated with chemotherapy, radiation, palliative or radical procedures focusing on hematogenous PDAC metastases reported a prevalence of clinically detectable pulmonary metastases in an absence of liver metastases in 12.3%. Hematogenous spread to the lungs was considered mostly for primary location in the tail and splenic vein obstruction. In the present cohort the location of primary tumor in the tail was only in 5% and no case with splenic vein obstruction was noted. In addition, remarkably high rate of perineural invasion in tumor analysis was reported in patients with MPM, but the difference was not statistically significant in comparison with non-pulmonary metastatic patients and, therefore, thus needs to be evaluated in larger cohort or meta-analysis.
Due to the low prevalence of MPM, the clinical course and optimal surgical or medical therapy are currently poorly defined. Early diagnosis of MPM in long-term survivors can bring the best therapeutic result and the highest chance for prolonged OS or even salvage therapy with curative intent. The rate of postoperative systemic therapy after primary resection in patients with pulmonary metastases varies between 85% in the present study to 100%[21]. MPM develop later than intraabdominal metastases and are the most frequent extraabdominal site across the studies[17-20]. In case of isolated pulmonary metastases the age at primary diagnosis is lower than in other groups (44-66 years, median 57).
MPM can present as three different clinical scenarios, in contrast to patients with non-pulmonary metastases as evidenced in the present cohort. Metachronous isolated pulmonary oligometastases with up to 10 small lesions in one lung are eligible for surgical therapy[7,22,23,26,29]. If the patient is fit for pulmonary resection, this represents the best therapeutic option with the potential of cure and longest OS (Table 1). In the present cohort only three cases were operable according to the previously established criteria[7,22,23]. One patient was managed with gemcitabine chemotherapy without surgery. Subsequent liver metastases developed and patient died 12.5 mo after diagnosis of MPM. Only two patients were radically operated and both are currently alive more than one year without recurrence. In cases of metachronous isolated multiple pulmonary metastases local therapy including surgery or radiotherapy has no rationale and systemic therapy is only potential option. Long-term survival was observed in both patients (50.5 and 82.5 mo), but these patients ultimately died of progressive disease or development of subsequent metastases typical for primary pulmonary carcinomas, in one case the brain metastases. The third scenario included metachronous pulmonary with metastases to other organs. In this group a very small subgroup of patients with MPM and retroperitoneal paraaortal lymph node metastases with DFS comparable with group of isolated MPM was identified. Prior DFS and OS in those patients were longer than in patients with local or regional recurrence.
In accordance with prior reports MPM patients in the present study had significantly longer DFS and OS compared to patients with non-pulmonary metastases. In patients with isolated pulmonary metastases median DFS ranges between 10.5 and 52.4 mo and OS between 23 and 92.3 mo[16-18,20,21]. The longest DFS of 156 mo after distal pancreatectomy was reported in one case by Kitasato et al[35]. In the present study we observed median DFS of 34 mo in isolated pulmonary metastatic patients with an estimated median OS of 81.4 mo. Arnaoutakis et al[17], Thomas et al[18] and Downs-Canner et al[20] demonstrated in a total of 21 patients the feasibility and safety of surgical resection for MPM with significantly improved survival in comparison with non-surgical therapy. Reports from Japan include at least 23 cases with resected MPM of PDAC with long-term survival[19,23,35]. The most recent European study by Decoster et al[36] reported 22 MPM patients, 3 of whom were treated surgically, and indicated a female predominance. Our study also showed massive predominance of women in isolated pulmonary metastases. Female predominance in both studies is interesting, but there are currently no evidence based facts to explain this association. An effect of sex hormone could be one of the potential mechanisms, but without support by in vitro or in vivo data remains only speculative. Future studies should investigate whether MPM cases are characterized by specific molecular pathogenesis and biology that could explain this particular metastatic pattern.
The most obvious limitation of the present study is the low number of patients with MPM, but due to the rarity of this presentation, our study is comparable to the earlier published retrospective analyses (Table 2). Even though some of the results cannot be statistically significant because of the small number of patients, together with the previously published data on other small cohorts the findings seem to be highly relevant for clinical practice. Another limitation is the evaluation of resection margins. Because the present study is of retrospective nature, resection margins could not be reevaluated according to the currently used Leeds protocol due to a technically completely different method of resection specimen evaluation. On the other hand, the present study is unique because of the origin of the study population. To date, most studies were published on American PDAC population[16-20], with two studies originating from Japanese PDAC centers (with most of the patients overlapping between both studies)[23,35]. The present study on Central European population together with three similar retrospective analyses from Western Europe[16,21,36] published during last year are important to confirm the results previously reported elsewhere.
| Study | IPM | DFS | MPMR | SMT | SMU | OSPMD |
| Van den Broeck et al[16] RA 1998-2005 | 15 | 17.8 | nr | nr | nr | nr |
| 1Katz et al[5] nr 1990-2002 | 50 | nr | nr | nr | nr | nr |
| 2Arnaoutakis et al[17] RCCS 2000-2009 | 31 | 34 | 9 | 51 | 23 | nr |
| 1Thomas et al[18] RA 1992-2010 | 7 | 52.4 | 7 | nr | nr | nr |
| 2Wangjam et al[7] RA 1998-2007 | 28 | 12.7 | nr | nr | nr | 8.5 |
| Downs-Canner et al[20] RA 2000-2010 | 49 | 14.9 | 5 | 44.4 | 29.9 | nr |
| Yamashita et al[19] RCCS 2003-2012 | 14 | nr | 2 | 70 | nr | nr |
| Kruger et al[21] RA 2002-2015 | 22 | 10.5 | nr | nr | nr | 25.5 |
| Decoster et al[36] RCCS 2007-2013 | 22 | nr | 3 | nr | nr | nr |
| Deeb et al[15] Cserie | 5 | 0 | ||||
| Brieau et al[14] CR | 2 | 2 | ||||
| Kotoulas et al[12] CR | 1 | 1 | ||||
| Moon et al[11] CR | 1 | 1 | ||||
| Sanjeevi et al[13] CR | 1 | 1 | ||||
| Kitasato et al[33] CR + national cases | 1 | 156 | 1+203 | |||
| Nakajima et al[23] CR + national cases | 173 | |||||
| Present study Cserie 2006-2013 | 5 | 34 | 2 | 12+ | nr | 12+ |
MPM, especially cases with isolated pulmonary metastases, are rare, and, obviously, prospective trials addressing the optimal management of these patients would be difficult to organize. Thus, for the foreseeable future retrospective analyses like the present report would remain the only source of information to guide the management of these patients.
Most importantly, in patients with metastatic PDAC only systemic therapy is considered in general practice. Despite some improvements resulting from the introduction of new regimens such as the combination of oxaliplatin, irinotecan, folinic acid and 5-fluorouracil or the combination of gemcitabine with nab-paclitaxel, the efficacy of systemic therapy is still very limited. The results of trials with targeted agents so far have been disappointing[37-39]. Contrary to the prevalent opinion in the medical and surgical oncology community, present data indicate that there is a subgroup of patients with metastatic PDAC who would benefit from surgical therapy. Future studies should identify clinical parameters and molecular biomarkers that would select this population and help with therapeutic decisions.
In conclusion, metachronous pulmonary metastases as first and only site of recurrence after resection for PDAC develop later and have better prognosis than other presentations of metastatic disease. OS is longer than in patients with other sites of metastases. Three different clinical scenarios according to the type of pulmonary oligometases with different prognosis and distinct therapeutic strategy were noted. The resection of pulmonary metastases was safe, with zero mortality and low morbidity. Patients who underwent pulmonary metastasectomy had longer survival compared to patients treated with chemotherapy or symptomatic therapy. Thus, surgery should be considered for all patients with solitary pulmonary metastases, but this has to be carefully weighted for each individual patient. Early diagnosis of recurrence may be viewed as precondition for successful management in these cases. Careful follow up with short (quarterly) interval and flexible PET/CT scanning can lead to early identification of recurrence.
Metachronous pulmonary metastases (MPM) represent the most frequent extraabdominal site of pancreatic ductal adenocarcinoma (PDAC) recurrence. The aim of this study was to analyze PDAC patients who underwent a curative-intent surgery with focus on MPM as a first site of recurrence and to assess disease free survival prior to the diagnosis of metastases and the overall survival.
The manuscript addresses the very rare and clinically important entity of metachronous pulmonary metastases as a first site of pancreatic cancer recurrence with the need of an individualized therapeutic approach as the surgery significantly prolongs overall survival of patients. In particular, the data suggest that surgery should be considered for all patients with isolated pulmonary metastases, but the risk of intervention has to be individually weighted for each patient.
Metachronous pulmonary metastases as first and only site of recurrence after resection for PDAC develop later and have better prognosis than other presentations of metastatic disease. Overall survival is longer than in patients with other sites of metastases. Three different clinical scenarios according to the type of pulmonary oligometases with different prognosis and distinct therapeutic strategy were noted. The resection of pulmonary metastases was safe, with zero mortality and low morbidity. Patients who underwent pulmonary metastasectomy had longer survival compared to patients treated with chemotherapy or symptomatic therapy. Thus, surgery should be considered for all patients with solitary pulmonary metastases, but this has to be carefully weighted for each individual patient.
The data suggested that metachronous pulmonary metastases of pancreatic cancer need an individualized therapeutic approach
Metachronous metastases were defined as those cases in which lesions were observed later on or within 3 mo after the after curative-intent surgery.
The authors studied a special subgroup of PDAC patients who suffered from metachronous pulmonary metastasis after surgery in a Central European population. They identified three different patterns of metachronous pulmonary metastasis and found much better prognosis in such subgroup compared to non-pulmonary metastatic patients. This clinical finding is important and valuable to clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Czech Republic
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kleeff J, Luyer M, Wei DY, Zhang Q
S- Editor: Gong ZM L- Editor: A E- Editor: Huang Y
| 1. | Globocan 2012. Web portal for International Cancer Research. Available from: http://globocan.iarc.fr. |
| 2. | Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2041] [Cited by in RCA: 1930] [Article Influence: 128.7] [Reference Citation Analysis (0)] |
| 3. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 678] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 4. | Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jäger D, Ulrich A, Büchler MW. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg. 2016;264:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 5. | Katz MH, Wang H, Fleming JB, Sun CC, Hwang RF, Wolff RA, Varadhachary G, Abbruzzese JL, Crane CH, Krishnan S. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 383] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 6. | Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, Chang D, Yeo CJ. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Wangjam T, Zhang Z, Zhou XC, Lyer L, Faisal F, Soares KC, Fishman E, Hruban RH, Herman JM, Laheru D. Resected pancreatic ductal adenocarcinomas with recurrence limited in lung have a significantly better prognosis than those with other recurrence patterns. Oncotarget. 2015;6:36903-36910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 8. | Wu W, He J, Cameron JL, Makary M, Soares K, Ahuja N, Rezaee N, Herman J, Zheng L, Laheru D. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. 2014;21:2873-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Lovecek M, Skalicky P, Klos D, Bebarova L, Neoral C, Ehrmann J, Zapletalova J, Svebisova H, Vrba R, Stasek M. Long-term survival after resections for pancreatic ductal adenocarcinoma. Single centre study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Moon JW, Lee HY, Han J, Lee KS. Tree-in-bud sign as a manifestation of localized pulmonary lymphatic metastasis from a pancreas cancer. Intern Med. 2011;50:3027-3029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Kotoulas C, Tsipas P, Patsea E, Kentepozidis N. Lung metastasis and pancreatic carcinoma. Pneeumon. 2013;26:270-272. |
| 13. | Sanjeevi S, Ivanics T, Andrén-Sandberg Å. Long-Term Survival after Resection of Pancreatic Ductal Adenocarcinoma with Late Metachronous Pulmonary Metastasectomy. J Pancreas. 2015;16:623-625. |
| 14. | Brieau B, Barret M, Rouquette A, Dréanic J, Brezault C, Regnard JF, Coriat R. Resection of Late Pulmonary Metastases from Pancreatic Adenocarcinoma: Is Surgery an Option? Cancer Invest. 2015;33:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Deeb A, Haque SU, Olowokure O. Pulmonary metastases in pancreatic cancer, is there a survival influence? J Gastrointest Oncol. 2015;6:E48-E51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 16. | Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 17. | Arnaoutakis GJ, Rangachari D, Laheru DA, Iacobuzio-Donahue CA, Hruban RH, Herman JM, Edil BH, Pawlik TM, Schulick RD, Cameron JL. Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg. 2011;15:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Thomas RM, Truty MJ, Nogueras-Gonzalez GM, Fleming JB, Vauthey JN, Pisters PW, Lee JE, Rice DC, Hofstetter WL, Wolff RA. Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg. 2012;16:1696-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Yamashita K, Miyamoto A, Hama N, Asaoka T, Maeda S, Omiya H, Takami K, Doki Y, Mori M, Nakamori S. Survival Impact of Pulmonary Metastasis as Recurrence of Pancreatic Ductal Adenocarcinoma. Dig Surg. 2015;32:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Downs-Canner S, Zenati M, Boone BA, Varley PR, Steve J, Hogg ME, Zureikat A, Zeh HJ, Lee KK. The indolent nature of pulmonary metastases from ductal adenocarcinoma of the pancreas. J Surg Oncol. 2015;112:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Michl M, Kleespies A, Angele MK, Hartwig W. Isolated pulmonary metastases define a favorable subgroup in metastatic pancreatic cancer. Pancreatology. 2016;16:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg. 1965;49:357-363. [PubMed] |
| 23. | Nakajima M, Ueno T, Suzuki N, Matsui H, Shindo Y, Sakamoto K, Tokuhisa Y, Tokumitsu Y, Takeda S, Yoshino S. Novel Indications for Surgical Resection of Metachronous Lung Metastases From Pancreatic Cancer After Curative Resection. J Clin Gastroenterol. 2016; Epub ahead of print. [PubMed] |
| 24. | Ballehaninna UK, Chamberlain RS. Serum CA 19-9 as a Biomarker for Pancreatic Cancer-A Comprehensive Review. Indian J Surg Oncol. 2011;2:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | Kysucan J, Lovecek M, Klos D, Tozzi I, Koranda P, Buriánková E, Neoral C, Havlík R. [Benefit of PET/CT in the preoperative staging in pancreatic carcinomas]. Rozhl Chir. 2010;89:433-440. [PubMed] |
| 26. | Lu F, Poruk KE, Weiss MJ. Surgery for oligometastasis of pancreatic cancer. Chin J Cancer Res. 2015;27:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 27. | Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1856] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 28. | Kaneda H, Saito Y. Oligometastases: Defined by prognosis and evauated by cure. Cancer Treat Commun. 2015;3:1-6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol. 2010;40:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 30. | Huang F, Wu G, Yang K. Oligometastasis and oligo-recurrence: more than a mirage. Radiat Oncol. 2014;9:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Embuscado EE, Laheru D, Ricci F, Yun KJ, de Boom Witzel S, Seigel A, Flickinger K, Hidalgo M, Bova GS, Iacobuzio-Donahue CA. Immortalizing the complexity of cancer metastasis: genetic features of lethal metastatic pancreatic cancer obtained from rapid autopsy. Cancer Biol Ther. 2005;4:548-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 34. | Mao C, Domenico DR, Kim K, Hanson DJ, Howard JM. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. Findings of 154 autopsies. Arch Surg. 1995;130:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Kitasato Y, Nakayama M, Akasu G, Yoshitomi M, Mikagi K, Maruyama Y, Kawahara R, Ishikawa H, Hisaka T, Yasunaga M. Metastatic pulmonary adenocarcinoma 13 years after curative resection for pancreatic cancer: report of a case and review of Japanese literature. JOP. 2012;13:296-300. [PubMed] |
| 36. | Decoster C, Gilabert M, Autret A, Turrini O, Oziel-Taieb S, Poizat F, Giovannini M, Viens P, Iovanna J, Raoul JL. Heterogeneity of metastatic pancreatic adenocarcinoma: Lung metastasis show better prognosis than liver metastasis-a case control study. Oncotarget. 2016;7:45649-45655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Van Laethem JL, Riess H, Jassem J, Haas M, Martens UM, Weekes C, Peeters M, Ross P, Bridgewater J, Melichar B. Phase I/II Study of Refametinib (BAY 86-9766) in Combination with Gemcitabine in Advanced Pancreatic cancer. Target Oncol. 2017;12:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Deplanque G, Demarchi M, Hebbar M, Flynn P, Melichar B, Atkins J, Nowara E, Moyé L, Piquemal D, Ritter D. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol. 2015;26:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |