Published online Jul 14, 2017. doi: 10.3748/wjg.v23.i26.4724
Peer-review started: February 22, 2017
First decision: April 5, 2017
Revised: April 30, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: July 14, 2017
Processing time: 141 Days and 16.7 Hours
To investigate the underlying effect of Jianpi Qingchang decoction (JQD) regulating intestinal motility of dextran sulfate sodium (DSS)-induced colitis in mice.
C57BL/6 mice were randomly divided into four groups: the control group, the DSS group, the JQD group, and the 5-aminosalicylic acid group. Except for the control group, colitis was induced in other groups by giving distilled water containing 5% DSS. Seven days after modeling, the mice were administered corresponding drugs intragastrically. The mice were sacrificed on the 15th day. The disease activity index, macroscopic and histopathologic lesions, and ultrastructure of colon interstitial cells of Cajal (ICC) were observed. The levels of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-10 and interferon gamma (IFN-γ), the expression of nuclear factor-kappa B (NF-κB) p65, c-kit, microtubule-associated protein 1 light chain 3 (LC3-II) and Beclin-l mRNA, and the colonic smooth muscle tension were assessed.
Acute inflammation occurred in the mice administered DSS. Compared with the control group, the levels of IL-1β, TNF-α, IL-10 and IFN-γ, the expression of LC3-II, Beclin-1 and NF-κB p65 mRNA, and the contractile frequency increased (P < 0.05), the expression of c-kit mRNA and the colonic smooth muscle contractile amplitude decreased in the DSS group (P < 0.05). Compared with the DSS group, the levels of IL-10 and IFN-γ, the expression of c-kit mRNA, and the colonic smooth muscle contractile amplitude increased (P < 0.05), the levels of TNF-α and IL-1β, the expression of LC3-II, Beclin-1 and NF-κB p65 mRNA, and the contractile frequency decreased in the JQD group (P < 0.05).
JQD can regulate the intestinal motility of DSS-induced colitis in mice through suppressing intestinal inflammatory cascade reaction, reducing autophagy of ICC, and regulating the network path of ICC/smooth muscle cells.
Core tip: Interstitial cells of Cajal (ICC) lead to a variety of changes in the physiological properties of the neurons in the related circuitry, which then affects gastrointestinal motility. Therefore, ICC have been accepted as a therapeutic target for gastrointestinal motility disorders. It is demonstrated in the current study that Jianpi Qingchang decoction can regulate the intestinal motility of dextran sulfate sodium-induced colitis in mice through suppressing intestinal inflammatory cascade reaction, reducing autophagy of ICC, and regulating the network path of ICC/smooth muscle cells.
- Citation: Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Wang LJ, Tang ZP. Jianpi Qingchang decoction regulates intestinal motility of dextran sulfate sodium-induced colitis through reducing autophagy of interstitial cells of Cajal. World J Gastroenterol 2017; 23(26): 4724-4734
- URL: https://www.wjgnet.com/1007-9327/full/v23/i26/4724.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i26.4724
Ulcerative colitis (UC) is a chronic intestinal disease, and its clinical manifestations are associated with the pathophysiological aspects of intestinal motility disorders, such as diarrhea and tenesmus[1,2]. Intestinal motility disorders of UC can seriously impact the quality of life (QOL) of patients due to the long disease duration. At present, improving the QOL, inducing and maintaining clinical remission and mucosal healing, and preventing complications are together regarded as the targets of UC treatments[3].
Previous clinical and experimental studies have demonstrated that Traditional Chinese Medicine (TCM) is advantageous in treating UC; its curative effect is reliable, with less side effects and low recurrence rate[4,5]. Jianpi Qingchang decoction (JQD) was made on the basis of the theory in TCM to treat UC. Previous studies found that JQD could be used to treat patients with initial or mild UC, improve their intestinal symptoms, such as diarrhea, mucous bloody stool and tenesmus, and regulate their systemic functional state, such as fatigue, consequently improving their QOL[6,7]. Furthermore, JQD could up-regulate the expression of peripheral blood mononuclear cell glucocorticoid receptor-α to improve hormone-dependent status to treat steroid-dependent UC[8]. Besides, JQD could suppress activation of the nuclear factor-kappa B (NF-κB) signaling pathway and regulate the expression of cytokines in dextran sulfate sodium (DSS)-induced colitis in mice[9].
Recent investigations have demonstrated that inflammation influences the morphology and structure of interstitial cells of Cajal (ICC), leading to a variety of changes in the physiological properties of the neurons in the related circuitry, which then affects gastrointestinal motility[10]. Therefore, ICC have been accepted as a therapeutic target for gastrointestinal motility disorders[11]. Defective autophagy has been strongly linked to UC pathogenesis, with evidence showing that enhancing autophagy may be therapeutically beneficial by regulating inflammation and clearing intestinal pathogens[12,13].
The regulation of autophagy might be a potential strategy for UC, which can be achieved by multi-level and multi-path interference[14]. Therefore, it was essential to study relationships between intestinal motility disorder of UC and autophagy of ICC, and to explore upstream signaling pathway regulation to strengthen or advance the beneficial autophagy response, which might be beneficial in preventing and treating intestinal dysmotility of UC[15]. JQD could improve the clinical symptoms of intestinal dysmotility, such as diarrhea and tenesmus in patients with UC, but the specific mechanisms remain unclear[6,7]. The aim of the present study was to investigate the effects of JQD on intestinal motility of DSS-induced colitis in mice.
DSS (MW 36000-50000; MP Biomedicals, Santa Ana, CA, United States); the nine medicinal herbs of JQD raw powder, as shown in Table 1 (Pharmacy Department, Longhua Hospital affiliated to Shanghai University of TCM, Shanghai, China) dissolved in 0.5% carboxymethylcellulose sodium (CMC) solution; mesalazine [5-aminosalicylic acid (5-ASA); Sunflower Pharmaceutical Group, Jiamusi Lu Ling Pharmaceutical Co., Ltd., Liaoning, China; batch number: 111206], external coat removed, broken down, and dissolved in 0.5% CMC solution; fecal occult blood (FOB) (Huashengyuan Medical Science and Technology Co. Ltd., Beijing, China); tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-10 and interferon gamma (IFN-γ) ELISA kits (eBioscience, San Diego, CA, United States); NF-κB antibody (Cell Signaling, Danvers, MA, United States); primer synthesis kit (Yushen Bio-Technique Co. Ltd., Shanghai, China).
| Chinese name | Latin name | English name | Quantity in g |
| Zhi Huang Qi | Radix astragali | Milkvetch root | 30 |
| Huang Lian | Coptis chinensis | Coptis root | 3 |
| Dang Shen | Codonopsis pilosula | Pilose Asiabell root | 15 |
| Ma Chi Xian | Portulaca oleracea L | herba portulacae | 30 |
| Sheng Di Yu | Radix sanguisorbae | Garden Burnet root | 15 |
| San Qi | Panax notoginseng | Notopterygium root | 6 |
| Bai Ji | Bletillae rhizoma | Tuber Bletillae | 3 |
| Mu Xiang | Radix aucklandiae | Common Vladimiria root | 6 |
| Sheng Gan Cao | Radix glycyrrhizae | Licorice root | 6 |
Specific pathogen-free C57BL/6 6- to 8-wk-old male mice (weight, 25 ± 3 g) were purchased from Shanghai Xierpu-Bikai Bio-Technique Co. Ltd. [Certificate No. SCXK(Hu)2013-0016]. All experiments were approved by the local ethics committee for Animal Research Studies at the Shanghai University of TCM (SZY20151002). Forty-six mice were randomly divided into four groups: the control group (n = 10), the DSS group (n = 12), the JQD group (n = 12), and the 5-ASA group (n = 12). Except for the control group, colitis was induced in the other groups by giving distilled water containing 5% (w/v) DSS[16].
Seven days after modeling, the mice were administered corresponding drugs intragastrically for 7 d. The intragastric administration dose of the mice in each group was calculated according to the conversion factor of experimental animals and clinical administration dose, which was 0.2 mL/(10 g•d) according to the body mass of the mice[17,18]. The control group and the DSS group mice were administered 0.5% CMC solution intragastrically. The JQD group mice were administered JQD solution (17.1 g/kg per day) and the 5-ASA group mice were administrated 5-ASA solution (100 mg/kg per day) intragastrically [19].
The color, activity, feces condition, weight, and eating volume of mice were observed daily during modeling and drug administration. FOB was tested, and the severity of colitis was assessed daily using the disease activity index (DAI), which was calculated by grading on a scale of 0 to 4 using the following parameters: loss of body weight (0: normal; 1: 0%-5%; 2: 5%-10%; 3: 10%-15%; 4: > 15%), stool consistency (0: normal; 2: loose stools; 4: watery diarrhea) and FOB (0: negative; 2: positive; 4: gross bleeding)[20].
After the drug administration for 14 d, the mice in each group were sacrificed and colon tissues (from the ileocecal junction to the anal verge) were collected. The general form of colon tissues was observed, and the length was recorded. The colon tissues were cut into three sections. One part was tiled on the filter paper, fixed at the two ends by pins, and then fixed in 4% liquor formaldehyde for hematoxylin and eosin (H and E) staining and in 2.5% glutaraldehyde for transmission electron microscopic (TEM) observation[21]. One part was cut into smooth muscle strips (2 mm wide and 10 mm long) for determining tension. The other tissues were packaged using aluminized paper, put into liquid nitrogen, and stored at -80 °C before being used for ELISA, western blotting, and mRNA analyses.
Colon tissues were doubly fixed with glutaraldehyde and osmic acid, dehydrated with gradients of ethyl alcohol and acetone, embedded and saturated, sliced into ultrathin sections (thickness, 50-60 nm), and doubly stained with uranyl acetate and lead citrate. The structure of ICC and autophagosomes were observed by TEM (Philips, Eindhoven, Netherlands).
Colons were weighed and homogenized in 1 mL of ice-cold RIPA lysis buffer containing 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. The lysate was centrifuged (15000 × g, 4 °C) for 15 min, and the supernatant was used for TNF-α, IL-1β, IL-10 and IFN-γ analyses. The levels of TNF-α, IL-1β, IL-10 and IFN-γ in colons were measured by commercial ELISA kits following per manufacturer’s instructions, respectively.
The bicinchoninic acid method was utilized to quantify the protein concentrations of colon tissue extracts, which were subsequently separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein spots in the gels were transferred onto polyvinylidene fluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ, United States), which were then blocked by 5% skimmed milk, Tris-buffered saline and Tween 20 at ambient temperature for 2 h. The membranes were incubated with primary antibody against NF-κB p65 (rabbit anti-NF-κB polyclonal antibody, 1:500) overnight at 4 °C and then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies. Finally, the blots were visualized using an enhanced chemiluminescence detection kit (Millipore, Temecula, CA, United States), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Yushen Bio-Technique Co. Ltd., Shanghai, China) was used as a loading control. Three independent replicates were conducted for each experiment.
Total RNA was isolated from mice colon tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) and reverse-transcribed into cDNA using a Reverse Transcription System (Promega, Madison, WI, United States). The thermal cycling conditions were as follows: 95 °C for 3 s, 95 °C for 5 s for 40 cycles, and 60 °C for 30-34 s. The mRNA expression levels of c-kit, microtubule-associated protein 1 light chain 3 (LC3-II) and Beclin-1 were quantitatively analyzed and normalized to GAPDH levels. Three replicates were run for each assay. Table 2 shows the sequences of reverse and forward primers. The relative expression of the target genes was analyzed by the ΔΔCt method.
| Target gene | Primer sequence | Product length in bp |
| c-kit | Forward: AGGCTATCCCTGTTGTGTCTG | 111 |
| Reverse: ACATGGAGTTCACGGATGTAGA | ||
| LC-3II | Forward: TTATAGAGCGATACAAGGGGGAG | 109 |
| Reverse: CGCCGTCTGATTATCTTGATGAG | ||
| Beclin-1 | Forward: ATGGAGGGGTCTAAGGCGTC | 197 |
| Reverse: TCCTCTCCTGAGTTAGCCTCT | ||
| GAPDH | Forward: AGGTCGGTGTGAACGGATTTG | 123 |
| Reverse: TGTAGACCATGTAGTTGAGGTCA |
Colon smooth muscles from mice were perfused with Krebs (mmol/L: NaCl 8 g, KCl 0.2 g, MgSO4•7H2O 0.26 g, NaH2PO4•2H2O 0.065 g, NaHCO3 1 g, CaCl2 0.2 g, glucose 1 g; 37.5 ± 0.5 °C, pH 7.3-7.4) bubbled with 97% O2-3% CO2[22,23]. One end of the smooth muscle strip was fastened to the tension converter in the bath of the perfusion system of isolated tissue, and the other end was fastened to the hook at the bottom of the bath. The load was 1.0 g, and the balance time was 30 min. After the spontaneous contraction of the smooth muscle strips became steady, electrical signals were recorded with a PowerLab 8/30 (ADInstruments, Bella Vista, Australia) and the contractile amplitude and frequency were analyzed every 5 min.
One-way ANOVA was used to analyze the data expressed as mean ± SD for comparison between multiple groups and least significant difference t-test for internal group comparison. All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, United States). The threshold of statistical significance was set to P < 0.05. GraphPad Prism version 5.0 (GraphPad Software Inc., La Jolla, CA, United States) was used to generate histograms.
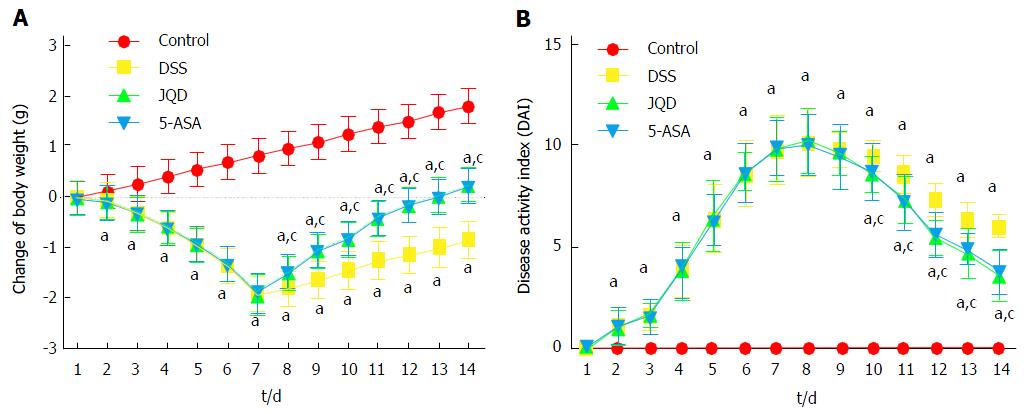
On the 2nd day of modeling, the mice in the model groups began to lose weight. Their stools became sticky, and the FOB test was positive, although the color was yellow. On the 7th day of modeling, the body weight of the model group decreased obviously, and black stool or bloody stool was visible in the anus. During modeling, the DAI scores of mice in the model groups increased gradually. Because of weight loss and bloody stool, two mice died in each of the DSS, JQD and 5-ASA groups.
Following drug intervention for 7 d, two mice died in the DSS group due to obvious hematochezia. One mouse died in each of the JQD and 5-ASA groups due to severe weight loss. In the DSS group, the bloody stool gradually decreased. The stool took shape slightly with soft quality, and the FOB test was positive (6/8). The FOB test turned negative (8/9) in the JQD group and (7/9) in the 5-ASA group. During drug administration, the DAI scores decreased gradually (Figure 1).
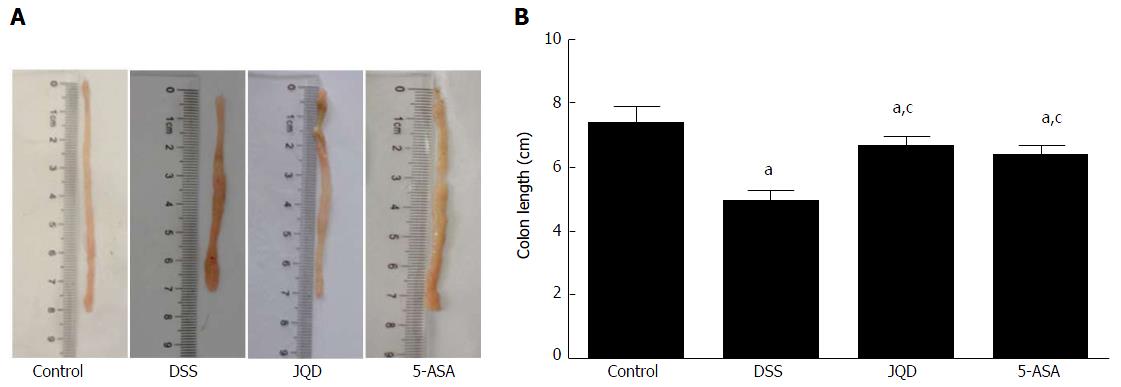
In the control group, the colon had good toughness. It was not easily breakable, and the colonic mucosa was smooth. In the DSS group, the length of the colon was significantly shortened compared with the control group (P < 0.05). The colon had poor toughness, with congestion and edema. Partial colon had adhesion with surrounding tissue, irregular ulcer, and bleeding on the mucosa. In the JQD and 5-ASA groups, the colon had better toughness, with local congestion and edema. Partial colon had a small amount of punctate erosion (Figure 2).
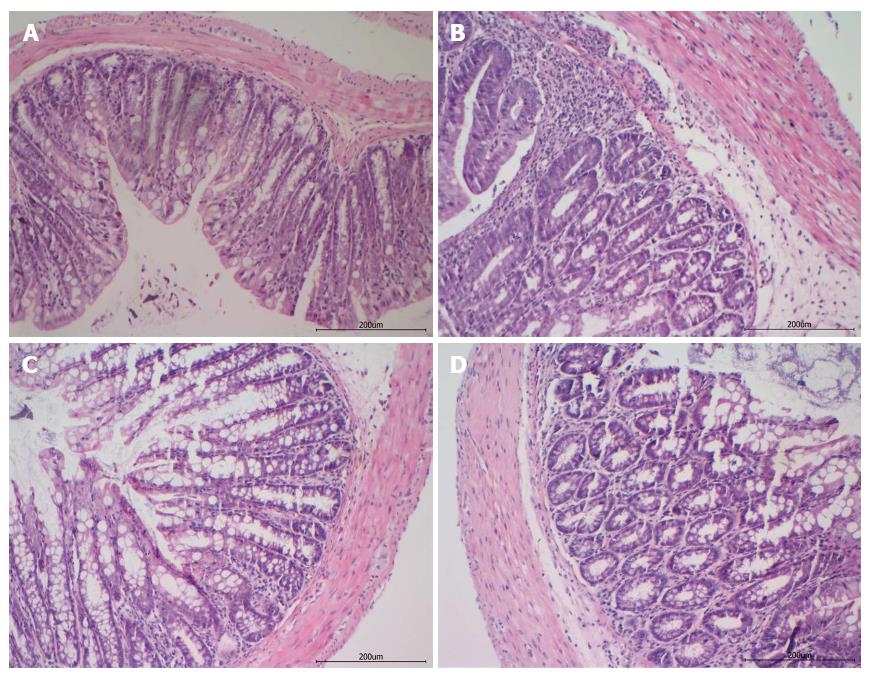
H and E staining showed that in the control group, the colons presented a normal morphology of crypts, abundant goblet cells, a small number of lamina propria mononuclear cells, no signs of mucosal thickening, and complete absence of ulcerations. However, in the DSS group, the colons presented severe epithelial damage with extensive cellular infiltration into the lamina propria and colon mucosa, mucosal thickening, glands arranged irregularly, ulcer and crypt abscess formation, and proliferating granulation tissue. In contrast, in the JQD and 5-ASA groups, the colons presented the migration and repair of epithelial cells on the erosive mucosal surface with infiltration of fewer inflammatory cells and recovery of the glandular structure (Figure 3).
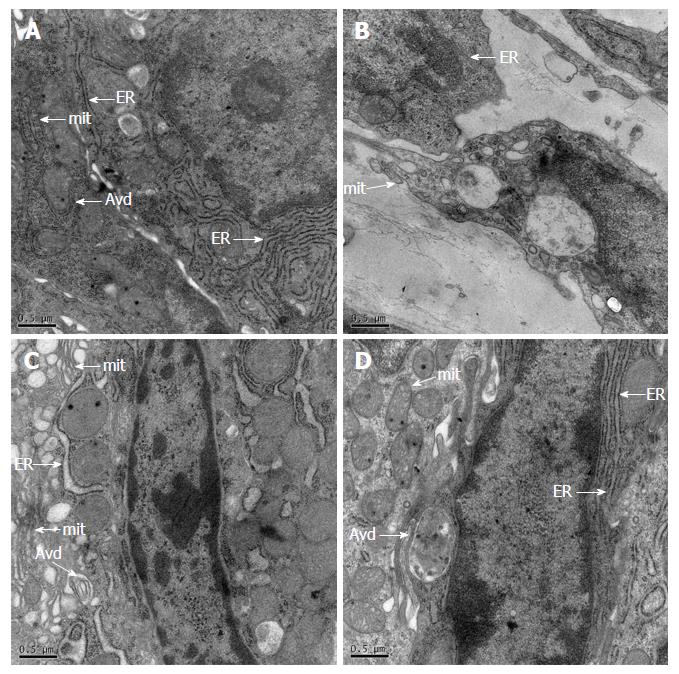
In the control group, ICC was spindle-shaped with a huge ovate nucleolus, caveolae, and numerous free ribosomes. A mass of mitochondria, and smooth and rough endoplasmic reticulum were present in the cytoplasm. ICC was located around the nerve fibers and connected with neighboring smooth muscle cells (SMCs) by intermediate junctions. A few autophagic vacuoles could be observed in ICC. In the DSS group, huge vacuoles, reducing organelles, chromatin margination, cytoplasmic liquefaction and dissolution, and almost invisible autophagic vacuoles could be observed in ICC. In the JQD and 5-ASA groups, the configuration of ICC was normal, with intact connections between cells and the ridge of mitochondria, and a few autophagic vacuoles existed (Figure 4).
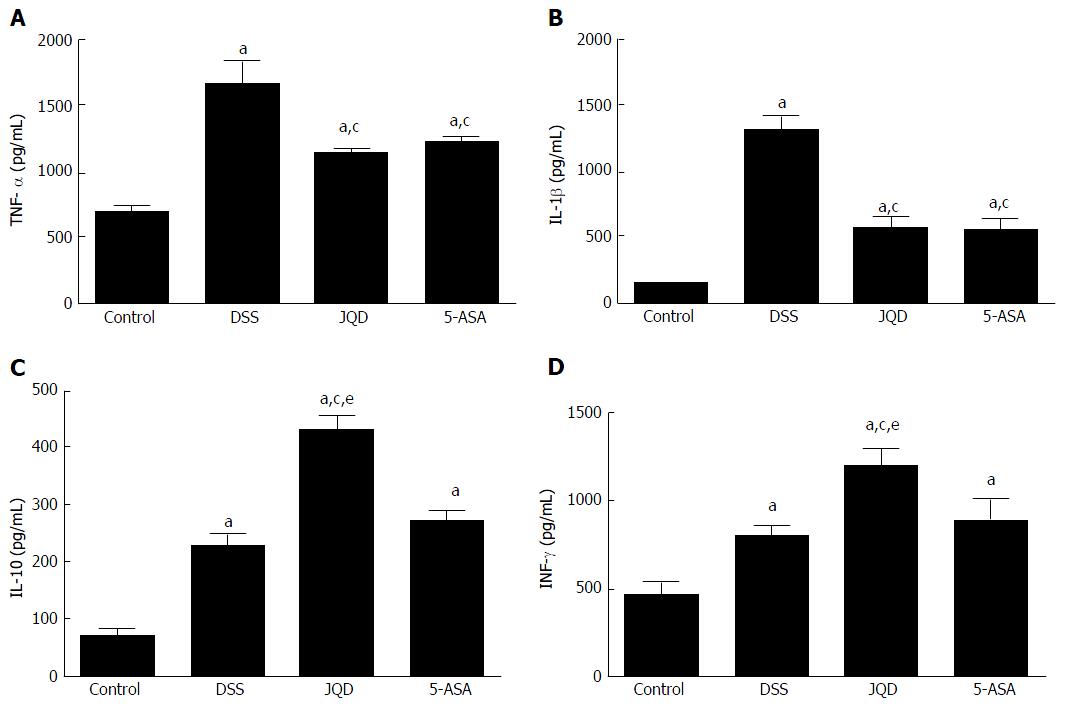
Compared with the control group, the levels of IL-1β, IL-10, IFN-γ and TNF-α increased in the DSS group (P < 0.05). After intervention, compared with the DSS group, the levels of IL-1β and TNF-α decreased in the JQD and 5-ASA groups (P < 0.05). However, no statistically significant difference was found between the two groups (P > 0.05). Compared with the DSS group, the levels of IL-10 and IFN-γ increased in the JQD and 5-ASA groups. A statistically significant difference was found between the JQD and DSS groups (P < 0.05), but no difference was noted between the 5-ASA and DSS groups (P > 0.05) (Figure 5).
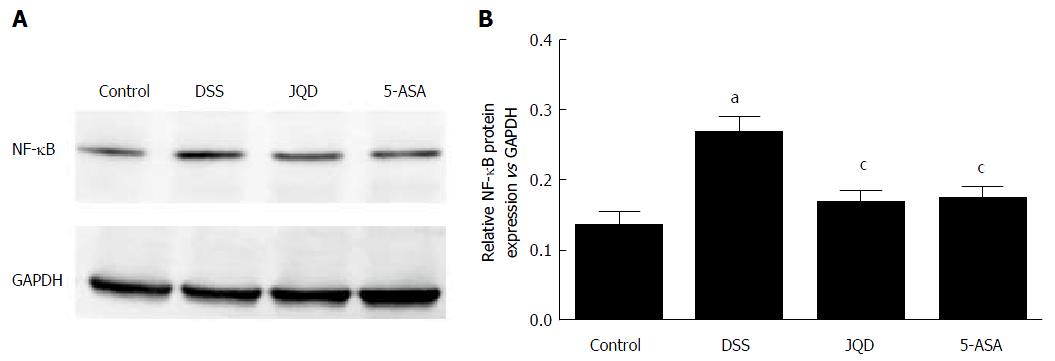
Compared with the DSS group, NF-κB p65 expression increased in the DSS group (P < 0.05). After intervention, compared with the DSS group, NF-κB p65 expression decreased in the JQD and 5-ASA groups (P < 0.05). However, no statistically significant difference was observed between the two groups (P > 0.05) (Figure 6).
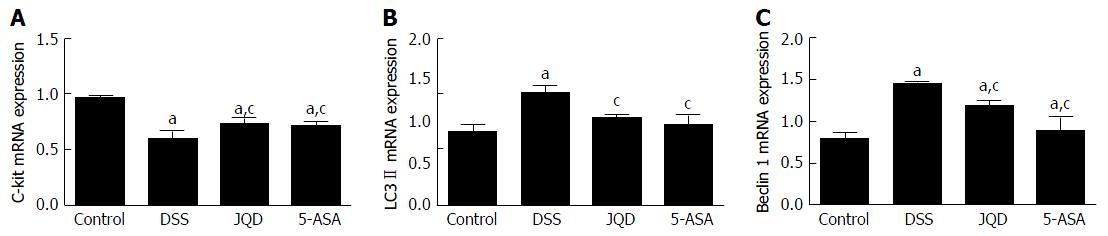
Compared with the control group, c-kit mRNA expression decreased (P < 0.05) and LC-3II mRNA and Beclin-1 mRNA expression increased in the DSS group (P < 0.05). After intervention, compared with the DSS group, c-kit mRNA expression increased in the JQD and 5-ASA groups (P < 0.05), LC-3II mRNA and Beclin-1 mRNA expression decreased in the JQD and 5-ASA groups (P < 0.05). However, no statistically significant difference was found between the two groups (P > 0.05) (Figure 7).
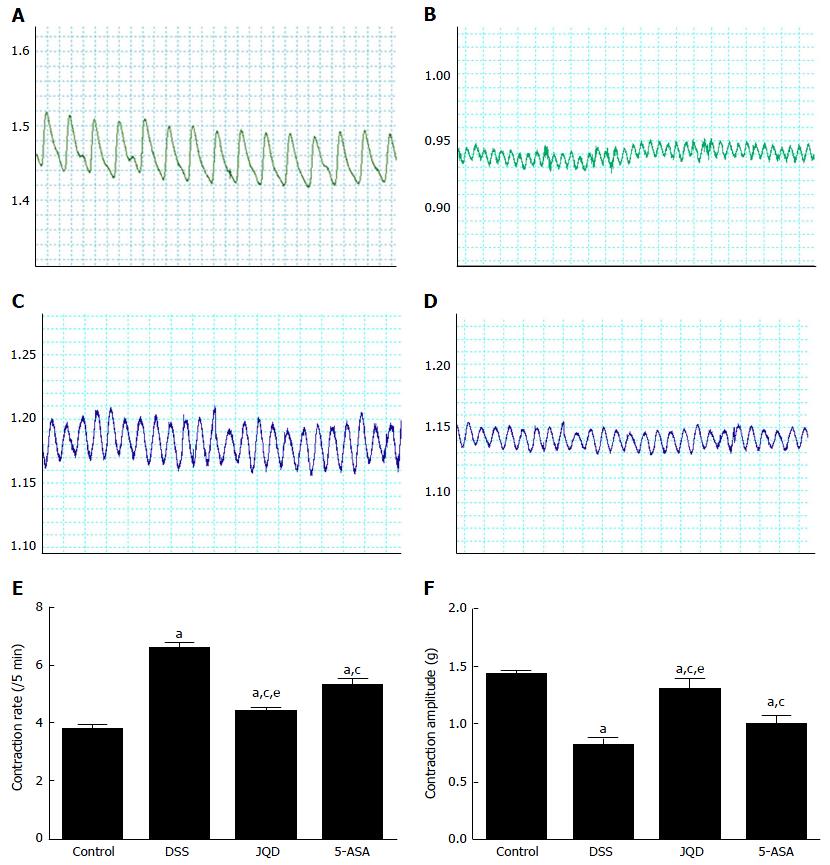
Compared with the control group, the colonic smooth muscle contractile amplitude decreased, while the contractile frequency increased in the DSS group (P < 0.05). After intervention, compared with the DSS group, the colonic smooth muscle contractile amplitude increased, while the contractile frequency decreased in the JQD and 5-ASA groups (P < 0.05). There was statistically significant difference between the two groups (P < 0.05) (Figure 8).
The main clinical manifestations of UC include the intestinal motility disorder symptoms[24]. The abnormal expression of NF-κB/TNF-α pathway and cytokine expression is related to not only the intestinal mucosal inflammation of UC but also the intestinal motility. Research has revealed that the secretion of inflammatory factors increases in UC. TNF-α and IL-1β can inhibit L-type Ca2+ channels in the circular smooth muscle of colon and activate NF-κB. Then, NF-κB enter into the cell nucleus, which inhibits subunit description of L-type Ca2+ channel α1C. Subsequently, the number of Ca2+ channels in the cytomembrane of smooth muscle and Ca2+ entering into cells decrease. This inhibits the recovery of SMC contraction[25]. IL-1β can increase the levels of cytokine IL-6, IL-8 and TNF-α produced by macrophagocytes. Therefore, the neutrophils aggregate near the inflammatory site, which causes a series of pathological changes such as intestinal epithelial cell damage, crypt abscess and small-vessel vasculitis, leading to the abnormal proliferation of SMCs. The proliferation of abnormal SMCs makes them become thin in UC, resulting in motility disorder[26].
This study found that JQD could repair colonic tissues of DSS-induced colitis in mice, and that this effect involved inhibiting the NF-κB/TNF-α pathway, reducing the level of proinflammatory factor IL-1β, increasing the level of anti-inflammatory factor IL-10 and immunomodulatory factor IFN-γ, and suppressing the intestinal inflammatory cascade reaction. The aggravation of inflammatory reaction led to the filtration of a large number of immune cells, which damaged the colonic mucosa. The repair of colonic mucosa might be due to the decrease in immune inflammatory response after JQD treatment.
This study also found that compared with the control group, the levels of IL-10 and IFN-γ increased in the DSS group, which was in accordance with some previous studies[27,28]. This finding reflected that the bodies of mice had self-regulation and self-recovery effects on colitis induced by DSS, but the weak effect was not enough to cope with intestinal inflammation so that the lesions in intestinal mucosa still existed. However, the self-regulation and self-recovery effects were enhanced under the intervention of JQD, and the increasing levels of IL-10 and IFN-γ blocked the secretion of proinflammatory cytokines. Therefore, JQD promoted the recovery of intestinal mucosa.
The ICC/SMC network pathway is the basic functional unit of gastrointestinal motility. It causes effective transmission of nerve impulse to the surrounding smooth muscle, leading to gastrointestinal motility[29]. The abnormal ICC/SMC network pathway has a certain relationship with intestinal motility disorder of UC[30]. ICC often shows multiple secondary lysosomes, large confluent lipid bodies, and disrupted aggregates of vacuolated glycogen clusters. Intermediate filaments show margination and clumping in patients with UC[31].
The intestinal motility disorder in DSS-induced colitis is caused by the decreased expression of sarcoplasmic endoplasmic reticular calcium ATPase 2 and phospholamban in SMCs, and the increased activity of calmodulin kinase II and the level of histone deacetylases 4 in the cytoplasm. In addition, the increased expression of the contractile proteins associated with the actin filaments of SMCs, which inhibits the interaction between the myosin filaments, is also an important factor[32].
This study found that under physiological conditions, moderate autophagy maintained the growth, differentiation, survival and homeostasis of colonic ICC. Under pathological conditions, in a model of DSS-induced colitis, excessive autophagy occurred in ICC, leading to programmed cell death. Pathological microstructure revealed reduced organelles, chromatin margination, cytoplasmic dissolution, changes in mitochondria and vacuoles, and reduced or absent autophagic vacuoles. Findings at the molecular level were reduced c-kit protein expression and increased LC3-II and Beclin-1 protein expression. The colonic smooth muscle contractile amplitude decreased while contractile frequency increased in the model of DSS-induced colitis. The main abnormal manifestations of intestinal motility were reducing muscle tension and increasing unordered propulsion motility. This was similar to the intestinal dysmotility in patients with UC, finally causing diarrhea and tenesmus. JQD can inhibit excessive autophagy in ICC, regulate the ICC/SMC network pathway, increase the contractive amplitude, and decrease the contractive frequency of smooth muscle. Hence, the colonic smooth muscle tends to be normal and regulates intestinal tract dynamics.
JQD reflects the features of TCM treatment for UC on the basis of syndrome differentiation[33]. As a complete decoction, Codonopsis pilosula and Radix astragali could nourish qi, Portulaca oleracea L and Radix sanguisorbae could clear heat and dampness, Panax notoginseng and Bletillae rhizoma could promote blood circulation by removing blood stasis. Modern studies have found that R. astragali containing Astragalus polysaccharide can effectively ameliorate 2, 4, 6-trinitrobenzene sulfonic acid-induced experimental colitis in rats, probably through restoring the number of regulatory T cells, inhibiting IL-17 levels in Peyer’s patches, and regulating the expression of TNF-α and IL-1β[34]. Berberine, the main component of Coptis chinensis, can down-regulate the level of IFN-γ and IL-12, up-regulate the levels of IL-4 and IL-10 in DSS-induced colitis, and relieve inflammatory reaction in intestinal epithelial cells[35,36]. Ginsenoside-Rg1, the main component of P. notoginseng, can prolong the bleeding and clotting time, down-regulate the thromboxane B2 level, and up-regulate the 6-keto-prostaglandin F1a level to improve the hypercoagulable state in DSS-induced colitis in mice[37].
This study found that the NF-κB/TNF-α pathway was activated in DSS-induced colitis in mice. Abnormal expression of cytokines, excessive autophagy of ICC finally resulted in abnormal motility in UC. JQD can repair the colonic tissues in DSS-induced colitis in mice and regulate the intestinal motility through suppressing intestinal inflammatory cascade reaction, reducing excessive autophagy of ICC, and regulating the network path of ICC/SMCs.
We would like to thank our colleagues in the Institute of Digestive Disease affiliated to Shanghai University of Traditional Chinese Medicine for their help and support in this research.
The main clinical manifestations of ulcerative colitis (UC) include the intestinal motility disorder symptoms of abdominal pain, diarrhea and tenesmus, besides bloody stool with mucus and pus. Intestinal motility disorder of UC can seriously impact the quality of life (QOL) of patients.
Interstitial cells of Cajal (ICC) have been accepted as a therapeutic target for gastrointestinal motility disorders. The regulation of autophagy might be a potential strategy for UC, which can be achieved by multi-level and multi-path interference. Therefore, it was essential to study relationships between intestinal motility disorder of UC and autophagy of ICC, and to explore upstream signaling pathway regulation to strengthen or advance the beneficial autophagy response, which might be beneficial in preventing and treating intestinal dysmotility of UC.
Previous studies found that Jianpi Qingchang decoction (JQD) could be used to treat patients with initial or mild UC, improve their intestinal symptoms, such as diarrhea, mucous bloody stool and tenesmus, regulate their systemic functional state, such as fatigue, and consequently improve their QOL. This study provided evidence that JQD can repair colonic tissues of dextran sulfate sodium-induced colitis in mice and regulate the intestinal motility through suppressing intestinal inflammatory cascade reaction, reducing excessive autophagy of ICC, and regulating the network path of ICC/smooth muscle cells.
The present study provides evidence that JQD, a traditional Chinese medicine recipe, regulates intestinal motility in UC.
ICCs serve as electrical pacemakers, active propagation pathways for slow waves, and mediators of enteric motor neurotransmission and are involved in abnormality of intestinal motility. Recent investigations have demonstrated that inflammation influences the morphology and structure of ICC, leads to a variety of changes in the physiological properties of the neurons in this circuitry, and then affects the gastrointestinal motility.
This work focuses on the application of an herbal remedy in an animal model of inflammation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Day AS S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Li D
| 1. | Dai YC, Tang ZP, Li K, Wang ZN. Research progress in intestinal motility during ulcerative colitis. Shijie Huaren Xiaohua Zazhi. 2007;15:721-724. |
| 2. | Dai YC, Tang ZP, Wang ZN. Update on motility of intestinal smooth in inflammatory bowel disease. Guoji Xiaohuabing Zazhi. 2008;2:142-144. |
| 3. | Bressler B, Marshall JK, Bernstein CN, Bitton A, Jones J, Leontiadis GI, Panaccione R, Steinhart AH, Tse F, Feagan B. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035-1058.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Ke F, Yadav PK, Ju LZ. Herbal medicine in the treatment of ulcerative colitis. Saudi J Gastroenterol. 2012;18:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Ng SC, Lam YT, Tsoi KK, Chan FK, Sung JJ, Wu JC. Systematic review: the efficacy of herbal therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Dai YC, Zhang YL, Wang LJ, Guo Q, Yang K, Ye RH, Tang ZP. Clinical presentation and treatment strategies for ulcerative colitis: A retrospective study of 247 inpatients. Chin J Integr Med. 2016;22:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Dai YC, Zheng L, Zhang YL, Chen X, Chen DL, Tang ZP. Effects of Jianpi Qingchang decoction on the quality of life of patients with ulcerative colitis: A randomized controlled trial. Medicine (Baltimore). 2017;96:e6651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Zhang YL, Guo Q, Dai YC. The influence of Jianpiqingchang formula on the expression of GRa in steroid-dependent ulcerative colitis patients with TCM type of spleen-deficiency and retention of damp-heat. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;22:706-709. |
| 9. | Zheng L, Zhang YL, Dai YC, Chen X, Chen DL, Dai YT, Tang ZP. Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation. World J Gastroenterol. 2017;23:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Mawe GM. Colitis-induced neuroplasticity disrupts motility in the inflamed and post-inflamed colon. J Clin Invest. 2015;125:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Negreanu LM, Assor P, Mateescu B, Cirstoiu C. Interstitial cells of Cajal in the gut--a gastroenterologist’s point of view. World J Gastroenterol. 2008;14:6285-6288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hooper KM, Barlow PG, Stevens C, Henderson P. Inflammatory Bowel Disease Drugs: A Focus on Autophagy. J Crohns Colitis. 2017;11:118-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Hosomi S, Kaser A, Blumberg RS. Role of endoplasmic reticulum stress and autophagy as interlinking pathways in the pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2015;31:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Nys K, Agostinis P, Vermeire S. Autophagy: a new target or an old strategy for the treatment of Crohn’s disease? Nat Rev Gastroenterol Hepatol. 2013;10:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Dai YC, Zhang YL, Tang ZP. Regulation of ICC Autophagy -- New Target for Treatment of Intestinal Dysmotility in Ulcerative Colitis. Wei Chang Bing Xue. 2015;20:377-379. [DOI] [Full Text] |
| 16. | Zhou FX, Chen L, Liu XW, Ouyang CH, Wu XP, Wang XH, Wang CL, Lu FG. Lactobacillus crispatus M206119 exacerbates murine DSS-colitis by interfering with inflammatory responses. World J Gastroenterol. 2012;18:2344-2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Chen Q. Research methods in pharmocology of Chinese Materia Medica (the third edition). People’s Medical Publishing House. 2011;1103. |
| 18. | Wen J, Teng B, Yang P, Chen X, Li C, Jing Y, Wei J, Zhang C. The potential mechanism of Bawei Xileisan in the treatment of dextran sulfate sodium-induced ulcerative colitis in mice. J Ethnopharmacol. 2016;188:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Clapper ML, Gary MA, Coudry RA, Litwin S, Chang WC, Devarajan K, Lubet RA, Cooper HS. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2008;14:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | De Fazio L, Cavazza E, Spisni E, Strillacci A, Centanni M, Candela M, Praticò C, Campieri M, Ricci C, Valerii MC. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol. 2014;20:2051-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Nam YS, Kim N, Im KI, Lim JY, Lee ES, Cho SG. Negative impact of bone-marrow-derived mesenchymal stem cells on dextran sulfate sodium-induced colitis. World J Gastroenterol. 2015;21:2030-2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Qureshi S, Song J, Lee HT, Koh SD, Hennig GW, Perrino BA. CaM kinase II in colonic smooth muscle contributes to dysmotility in murine DSS-colitis. Neurogastroenterol Motil. 2010;22:186-195, e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Wang W, Huang H, Hou D, Liu P, Wei H, Fu X, Niu W. Mechanosensitivity of STREX-lacking BKCa channels in the colonic smooth muscle of the mouse. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1231-G1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Bassotti G, Antonelli E, Villanacci V, Salemme M, Coppola M, Annese V. Gastrointestinal motility disorders in inflammatory bowel diseases. World J Gastroenterol. 2014;20:37-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 25. | Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001;120:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Ohama T, Hori M, Momotani E, Elorza M, Gerthoffer WT, Ozaki H. IL-1beta inhibits intestinal smooth muscle proliferation in an organ culture system: involvement of COX-2 and iNOS induction in muscularis resident macrophages. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1315-G1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Tsang SW, Ip SP, Wu JC, Ng SC, Yung KK, Bian ZX. A Chinese medicinal formulation ameliorates dextran sulfate sodium-induced experimental colitis by suppressing the activity of nuclear factor-kappaB signaling. J Ethnopharmacol. 2015;162:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Ledesma-Soto Y, Callejas BE, Terrazas CA, Reyes JL, Espinoza-Jiménez A, González MI, León-Cabrera S, Morales R, Olguín JE, Saavedra R. Extraintestinal Helminth Infection Limits Pathology and Proinflammatory Cytokine Expression during DSS-Induced Ulcerative Colitis: A Role for Alternatively Activated Macrophages and Prostaglandins. Biomed Res Int. 2015;2015:563425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Hirst GD. An additional role for ICC in the control of gastrointestinal motility? J Physiol. 2001;537:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Kinoshita K, Horiguchi K, Fujisawa M, Kobirumaki F, Yamato S, Hori M, Ozaki H. Possible involvement of muscularis resident macrophages in impairment of interstitial cells of Cajal and myenteric nerve systems in rat models of TNBS-induced colitis. Histochem Cell Biol. 2007;127:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 109] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Alkahtani R, Mahavadi S, Al-Shboul O, Alsharari S, Grider JR, Murthy KS. Changes in the expression of smooth muscle contractile proteins in TNBS- and DSS-induced colitis in mice. Inflammation. 2013;36:1304-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Wu PT. What should be kept in mind in the TCM differential treatment for ulcerative colitis? J Tradit Chin Med. 2008;28:308-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Yang M, Lin HB, Gong S, Chen PY, Geng LL, Zeng YM, Li DY. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine. 2014;70:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Hong T, Yang Z, Lv CF, Zhang Y. Suppressive effect of berberine on experimental dextran sulfate sodium-induced colitis. Immunopharmacol Immunotoxicol. 2012;34:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Yan F, Wang L, Shi Y, Cao H, Liu L, Washington MK, Chaturvedi R, Israel DA, Cao H, Wang B. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G504-G514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Hao WW, Wen HZ, Ma GT, Tang ZP, He XY, Li J, LI NN, Liu YT. Ginsenoside-Rg1 regulates blood coagulation in DSS-induced colitis mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;21:238-242. |