Copyright
©The Author(s) 2017.
World J Gastroenterol. Jul 7, 2017; 23(25): 4538-4547
Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4538
Published online Jul 7, 2017. doi: 10.3748/wjg.v23.i25.4538
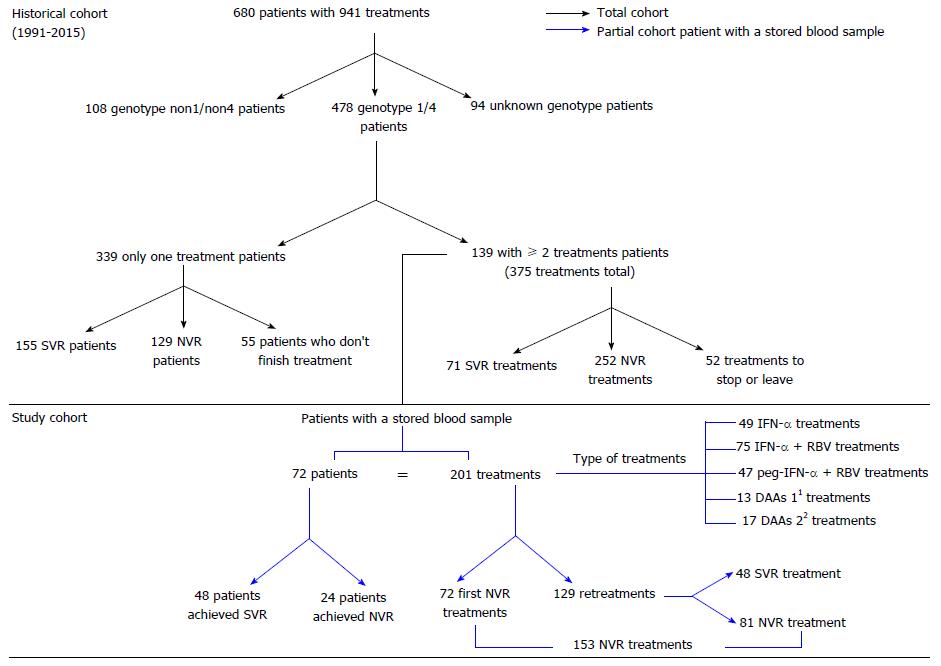
Figure 1 Historical and study chronic hepatitis C patient cohort from 1991 to 2015 in the San Cecilio University Hospital.
1First-generation DAAs (n = 13): peg-IFN-α + RBV + telaprevir (n = 7) and peg-IFN-α + RBV + boceprevir (n = 6); 2Second-generation DAAs (n = 17): peg-IFN-α + RBV + simeprevir (n = 2), simeprevir + sofosbuvir + RBV (n = 2), simeprevir + sofosbuvir (n = 1), sofosbuvir + daclatasvir (n = 1), ledipasvir + sofosbuvir + RBV (n = 4), ledipasvir + sofosbuvir (n = 4) and ombitasvir/paritaprevir/ritonavir + dasabuvir (n = 3). DAAs: Direct-acting antivirals NVR: No virological response; IFN: Interferon; peg-IFN + RBV: Pegylated-interferon + ribavirin; RBV: Ribavirin; SVR: Sustained virological response.
- Citation: Muñoz de Rueda P, Fuentes Rodríguez JM, Quiles Pérez R, Gila Medina A, Martín Álvarez AB, Casado Ruíz J, Ruíz Extremera A, Salmerón J. Hepatitis C virus NS5A region mutation in chronic hepatitis C genotype 1 patients who are non-responders to two or more treatments and its relationship with response to a new treatment. World J Gastroenterol 2017; 23(25): 4538-4547
- URL: https://www.wjgnet.com/1007-9327/full/v23/i25/4538.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i25.4538