Published online Jun 21, 2017. doi: 10.3748/wjg.v23.i23.4252
Peer-review started: February 3, 2017
First decision: February 23, 2017
Revised: April 1, 2017
Accepted: May 19, 2017
Article in press: May 19, 2017
Published online: June 21, 2017
To investigate the range of pathologies treated by pancreas preserving distal duodenectomy (PPDD) and present the outcome of follow-up.
Neoplastic lesions of the duodenum are treated conventionally by pancreaticoduodenectomy. Lesions distal to the major papilla may be suitable for a pancreas-preserving distal duodenectomy, potentially reducing morbidity and mortality. We present our experience with this procedure. Selective intraoperative duodenoscopy assessed the relationship of the papilla to the lesion. After duodenal mobilisation and confirmation of the site of the lesion, the duodenum was transected distal to the papilla and beyond the duodenojejunal flexure and a side-to-side duodeno-jejunal anastomosis was formed. Patients were identified from a prospectively maintained database and outcomes determined from digital health records with a dataset including demographics, co-morbidities, mode of presentation, preoperative imaging and assessment, nutritional support needs, technical operative details, blood transfusion requirements, length of stay, pathology including lymph node yield and lymph node involvement, length of follow-up, complications and outcomes. Related published literature was also reviewed.
Twenty-four patients had surgery with the intent of performing PPDD from 2003 to 2016. Nineteen underwent PPDD successfully. Two patients planned for PPDD proceeded to formal pancreaticoduodenectomy (PD) while three had unresectable disease. Median post-operative follow-up was 32 mo. Pathologies resected included duodenal adenocarcinoma (n = 6), adenomas (n = 5), gastrointestinal stromal tumours (n = 4) and lipoma, bleeding duodenal diverticulum, locally advanced colonic adenocarcinoma and extrinsic compression (n = 1 each). Median postoperative length of stay (LOS) was 8 d and morbidity was low [pain and nausea/vomiting (n = 2), anastomotic stricture (n = 1), pneumonia (n = 1), and overwhelming post-splenectomy sepsis (n = 1, asplenic patient)]. PPDD was associated with a significantly shorter LOS than a contemporaneous PD series [PPDD 8 (6-14) d vs PD 11 (10-16) d, median (IQR), P = 0.026]. The 30-d mortality was zero and 16 of 19 patients are alive to date. One patient died of recurrent duodenal adenocarcinoma 18 mo postoperatively and two died of unrelated disease (at 2 mo and at 8 years respectively).
PPDD is a versatile operation that can provide definitive treatment for a range of duodenal pathologies including adenocarcinoma.
Core tip: Pancreas preserving distal duodenectomy is a versatile operation that can provide definitive treatment for a range of duodenal pathologies including adenocarcinoma. It avoids the morbidity and mortality of a pancreaticoenteric anastomosis and can be undertaken safely with shorter postoperative length of stay than pancreaticoduodenectomy.
- Citation: Mitchell WK, Thomas PF, Zaitoun AM, Brooks AJ, Lobo DN. Pancreas preserving distal duodenectomy: A versatile operation for a range of infra-papillary pathologies. World J Gastroenterol 2017; 23(23): 4252-4261
- URL: https://www.wjgnet.com/1007-9327/full/v23/i23/4252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i23.4252
The duodenum gives rise to more neoplasia, and possibly pathology in general, per unit length, than does any other part of the small bowel[1,2]. The retroperitoneal position of the duodenum, its shared blood supply with the pancreas, and its relationship with the ampulla of Vater and the superior mesenteric vessels ensure that any duodenal resection is potentially a major undertaking. Pancreaticoduodenectomy (PD) constitutes the mainstay of surgical treatment of duodenal lesions[3] and up to 10% of PDs are undertaken for lesions that actually arise in the duodenum[4]. However, PD is associated with significant morbidity and mortality, which is due in part to pancreatic resection and anastomosis. Moreover, it is likely that the actual risks associated with PD are widely underestimated[5].
Duodenal resection with pancreas preservation is possible and has been used in the treatment of a range of duodenal conditions. Pancreas preserving total duodenectomy is an option for the treatment of diffuse non-invasive mucosal disease such as FAP-associated polyposis[6,7] whilst pancreas preserving distal duodenectomy (PPDD) has been described in the treatment of a range of benign and malignant lesions arising distal to the papilla complex[8,9].
We describe how we perform a PPDD and present the long-term results of a series of 19 patients who underwent the procedure in a single centre for a variety of pathologies over a 14-year period and also review the relevant literature.
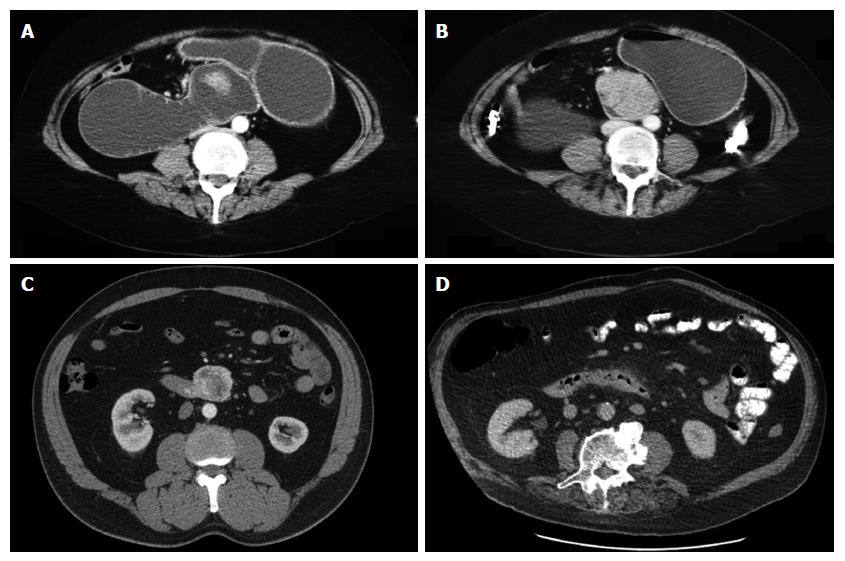
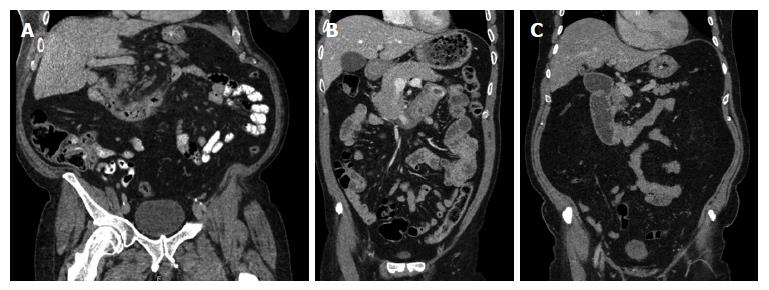
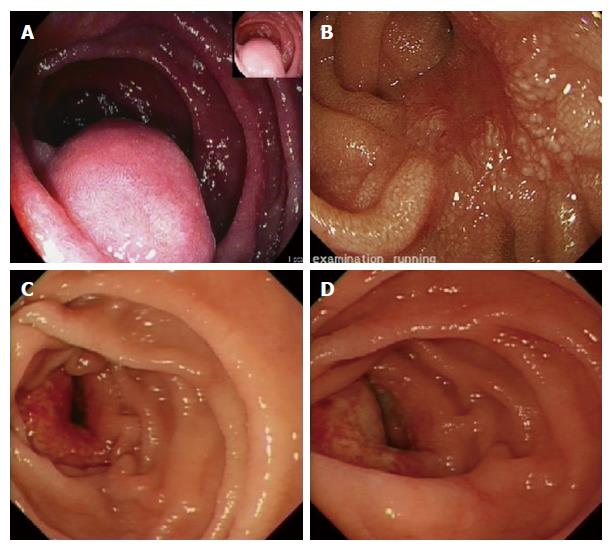
This procedure is usually undertaken in an elective or scheduled capacity for patients with infra-papillary conditions that, in the opinion of the multidisciplinary team, warrant surgical resection. Criteria include M0 duodenal adenocarcinoma; large adenomata or those in positions that prevent effective endoscopic mucosal resection; and gastrointestinal stromal tumours. Patients routinely undergo multi-slice pancreas protocol computed tomography (CT) with occasional fluoroscopic investigations. Most have one or more modalities of endoscopic investigation. Poor nutritional status at presentation is considered an indication for nasojejunal feeding which is commenced 7-14 d preoperatively. Representative CT and endoscopic findings are shown in Figures 1-3. The relationship of the tumour or lesion to the ampulla of Vater on preoperative endoscopy or CT is vital in determining the feasibility of offering the patient a PPDD. Nevertheless, the procedure should only be undertaken in an institution with expertise and facilities to perform a PD, as a small number of patients will not be suitable for a PPDD on surgical exploration because of close proximity of a malignant pathology to the ampulla or involvement of the pancreas by the malignant process. This should be considered in the consent process and we usually obtain consent to perform a PPDD with a view to proceed to a PD or perform a bypass procedure in the event of unresectability.
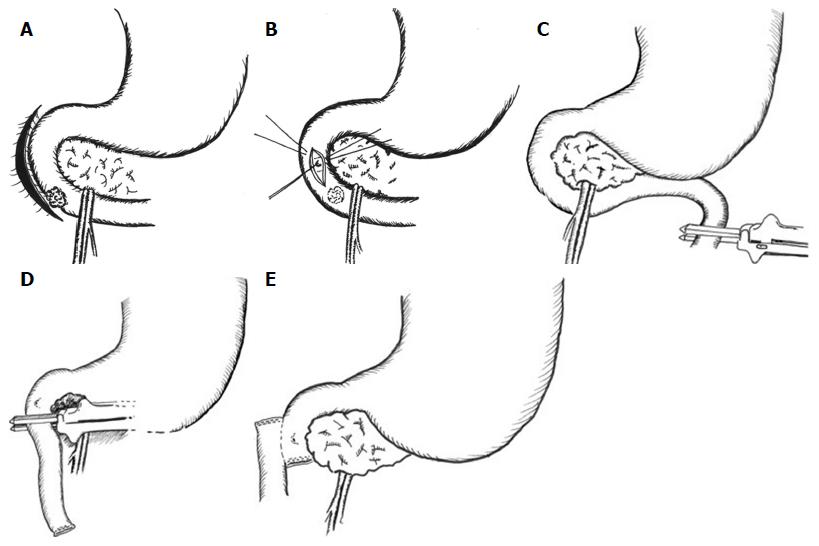
Arterial, central venous, epidural and bladder catheterisations are performed for monitoring and pain relief. Flow-guided intraoperative fluid therapy is used. In cases where there is concern regarding the proximity of the lesion to the ampulla, their relationship is confirmed intraoperatively with side-viewing duodenoscopy. A transverse upper abdominal incision with appropriate fixed table retraction is used. The key operative steps are shown in Figure 4. Wide Kocherisation of the duodenum is undertaken, facilitated by a variable degree of right medial visceral rotation (Cattell-Braasch manoeuver)[10]. Resectability is determined by excluding involvement of the pancreas and peripancreatic vessels and by confirming macroscopic proximal clearance of at least 10 mm with preservation of the major papilla complex. Macroscopic nodal and distant metastatic disease are also excluded. Frozen section biopsies are taken when necessary. After confirming resectability, the proximal jejunum is transected with a transverse linear cutting stapler and its mesentery, along with the ligament of Treitz and the peritoneal attachments of the duodenojejunal junction, are divided to permit delivery of the proximal jejunum behind the superior mesenteric vessels into the supracolic compartment. The third part of the duodenum (D3) and distal second part (D2) are then separated from the pancreatic head and uncinate process. The mobile, devascularised distal duodenum is then excised, again with linear stapler. For benign lesions close to the papilla, the latter is cannulated with a 4F infant feeding tube via a duodenotomy to facilitate proximal transection with preservation of the ampullary complex. PD is undertaken if the lesion involved to papilla. The proximal, blind end of jejunum is delivered through a window in the transverse mesocolon to permit a sutured side-to-side isoperistaltic duodenojejunostomy, which is performed with 3-0 or 4-0 polydioxanone (PDS®II, Ethicon, Edinburgh, United Kingdom) sutures in a single continuous layer. In cases assessed at risk of malnutrition or anticipated delayed gastric emptying, as in patients with preoperative gastric outlet obstruction, a fine bore nasojejunal feeding tube is placed across the anastomosis for postoperative feeding. A cholecystectomy is performed if the gallbladder is still in situ. A peritoneal drain is placed selectively if there is of a perceived risk of postoperative pancreatic fistulation following dissection on or close to the pancreas.
Postoperative care is initially in a surgical high dependency unit and has, in recent years, proceeded according to enhanced recovery after surgery (ERAS) principles[11]. Somatostatin analogues are not used routinely. The peritoneal drain, if used, is removed on the third postoperative day provided fluid amylase does not exceed three times serum amylase and there is no evidence of enteric content.
All patients undergoing PPDD within a large teaching hospital were identified using a prospectively maintained database and crosschecked against a hospital database of pathology specimens. Electronic healthcare records were reviewed for relevant information.
Between 2003 and 2016, 24 patients were explored with the intention of performing PPDD. In 3 patients, malignant involvement of the superior mesenteric artery precluded resection and palliative gastroenterostomy was undertaken. Two patients had intraoperative findings that necessitated PD, as the malignant process was close to the papilla and adequate resection margins could not have been obtained without a PD. Thus, 19 patients proceeded to PPDD. Median Charlson co-morbidity index was 4 (range 0-6). Patient characteristics and modes of presentation and are shown in Table 1. Either of two surgeons (DNL, AJB) oversaw each operation.
| No. | Age | Sex | Presentation | Comorbidities | CCI | Radiological assessment | Endoscopic assessment | Nutritional support | Year |
| 1 | 67 | M | Asymptomatic (incidental on OGD) | Coeliac disease | 4 | CT | OGD, EUS | 2011 | |
| 2 | 56 | F | Weight loss, anaemia, vomiting | Malnutrition | 3 | CT, Ba study | OGD | NJ | 2004 |
| 3 | 66 | F | Vomiting | Asthma, depression | 4 | CT | - | 2015 | |
| 4 | 51 | M | Epigastric pain, weight loss, vomiting | Nil | 3 | CT | OGD | 2016 | |
| 5 | 68 | M | Epigastric pain, vomiting | Hiatus hernia | 4 | CT | - | 2015 | |
| 6 | 77 | M | Epigastric pain, weight loss, vomiting, anaemia | Bronchiectasis, GORD | 6 | CT | OGD, enteroscopy | 2016 | |
| 7 | 73 | F | Anaemia | Metachronous colonic cancer | 10 | CT | - | 2016 | |
| 8 | 61 | M | Asymptomatic (incidental on ultrasound) | GORD | 4 | CT | OGD, EUS | 2013 | |
| 9 | 48 | F | Epigastric pain, weight loss, vomiting | Nil | 2 | CT | OGD, EUS | 2014 | |
| 10 | 65 | M | Asymptomatic (incidental on aneurysm screening) | Nil | 4 | CT | OGD, EUS | 2016 | |
| 11 | 83 | F | Epigastric pain, weight loss, vomiting | Glaucoma, hypothyroidism | 6 | CT | - | 2016 | |
| 12 | 76 | F | Dyspepsia/reflux | NASH, cirrhosis, colectomy for cancer | 4 | CT | OGD | 2008 | |
| 13 | 76 | M | Epigastric pain, back pain | Functional asplenia | 3 | CT | OGD, EUS | 2006 | |
| 14 | 76 | M | HTN, Stroke, MI | 5 | - | OGD | 2007 | ||
| 15 | 64 | F | Dyspepsia/reflux | Hiatus hernia | 2 | CT | OGD, EUS | 2004 | |
| 16 | 68 | F | Epigastric pain, vomiting, early satiety | Stricture post EMR | 1 | CT, Wat Sol St | OGD, EUS | NJ | 2016 |
| 17 | 36 | M | Recurrent pancreatitis | Nil | 0 | CT | OGD | 2003 | |
| 18 | 80 | F | Melaena | Glaucoma | 4 | CT | OGD | 2004 | |
| 19 | 39 | F | Epigastric pain, weight loss, vomiting | Nil | 0 | CT, Wat Sol St | OGD | 2016 |
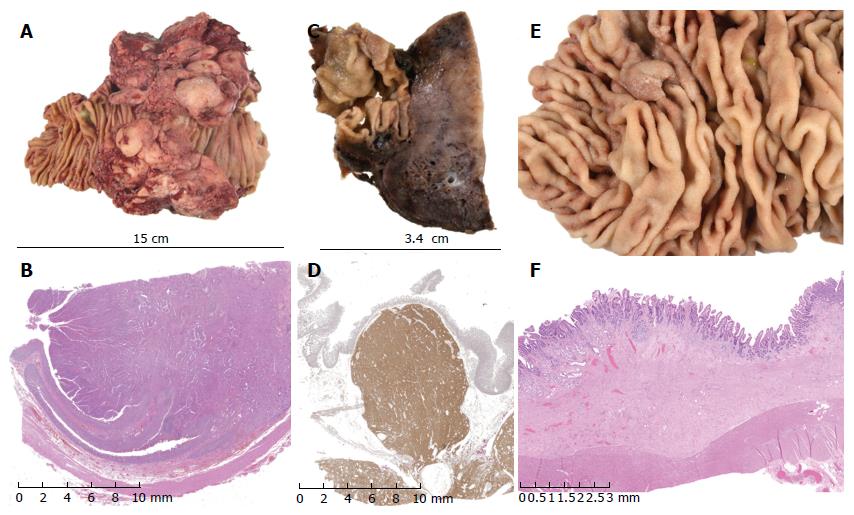
All patients survived 30 d and to discharge home. Two of nineteen patients required blood products and median postoperative length of stay was 8 d (range 4-21) The Mann-Whitney test was employed to compare length of stay (LOS) following PPDD vs PD undertaken in the same centre, using a continuous series of PD, February to August 2015 (n = 26). PPDD was associated with a significantly shorter LOS than PD [8 (6-14) d vs 11 (10-16) d, median (IQR), P = 0.026] No procedure-related deaths were observed in this series and only 1 patient went on to die of related disease within the follow up period. This patient developed distant recurrence (transcoelomic spread to rectouterine pouch). Neoplastic conditions accounted for 17 (90%) of operations (Table 2). Median (IQR) follow-up was 36 (11-114) mo. Representative images of pathological specimens are shown in Figure 5.
| No. | Diagnosis | Total lymph nodes | Nodes +ve | Proximal margin (mm) | Blood transfusion (units) | Length of stay (d) | FU (mo) | Complications | Outcome |
| 1 | Adenocarcinoma Mod diff pT4 Nx Mx G2 V0 R0 | 0 | - | 15 | 0 | 4 | 72 | - | Alive, disease free2 |
| 2 | Adenocarcinoma Mod diff pT3 N0 Mx G2 V1 R0 | 4 | 0 | 55 | NR | 14 | 155 | - | Alive, disease free |
| 3 | Adenocarcinoma Mod diff T4 N1 Mx V0 G2 R0 | 11 | 1 | 30 | 0 | 13 | 181 | Recurrence | Died (distant metastases)2 |
| 4 | Adenocarcinoma Poorly diff T4 N2 Mx V1 G3 R0 | 29 | 16 | 62 | 0 | 5 | 13 | - | Alive, disease free2 |
| 5 | Adenocarcinoma Mod diff pT3 N0 M0 V1 G2 R0 | 7 | 0 | 30 | 0 | 14 | 15 | Incisional hernia at 1 yr | Alive, disease free |
| 6 | Adenocarcinoma Mod diff pT3 N0 Mx V1 G2 R0 | 19 | 0 | 25 | 0 | 6 | 7 | - | Alive, disease free |
| 7 | Adenocarcinoma of colon | 10 | 1 | 2 | 7 | 100 | - | Alive, disease free2 | |
| 8 | GIST (low grade malignant potential) | 0 | 0 | 0 | 4 | 48 | - | Alive, disease free | |
| 9 | GIST (low grade malignant potential) | 0 | 0 | 0 | 10 | 36 | Anast. stricture; GJ at 2 yr | Alive, disease free | |
| 10 | GIST (low grade malignant potential) | 1 | 0 | 0 | 8 | 10 | - | Alive, disease free | |
| 11 | GIST (low grade malignant potential) | 6 | 0 | 0 | 6 | 5 | - | Alive, disease free | |
| 12 | Villous adenoma (high grade dysplasia) | NR | - | 8 | 19 | 21 | Ascitic leak (cirrhotic) | Died of unrelated causes | |
| 13 | Tubulovillous adenoma (high grade dysplasia) | NR | - | NR | 21 | 126 | OPSI | Alive, disease free | |
| 14 | Tubular adenoma (high grade dysplasia) | NR | - | 0 | 8 | 1021 | - | Died of unrelated causes | |
| 15 | Tubular adenoma (low grade dysplasia) | NR | - | 0 | 18 | 146 | Postoperative pneumonia | Alive, disease free | |
| 16 | Multiple tubular adenomas (low grade dysplasia) | 1 | 0 | 0 | 7 | 8 | - | Alive, disease free | |
| 17 | Lipoma | NR | - | NR | 10 | 168 | - | Alive, disease free | |
| 18 | Bleeding duodenal diverticulum | NR | - | NR | 13 | 144 | - | Alive, disease free | |
| 19 | Superior mesenteric artery syndrome | NR | - | 0 | 5 | 12 | Poor pain control, N&V | Alive, disease free |
In our experience, PPDD provides a valuable surgical treatment for a range of infra-papillary pathologies, which were in the most part neoplastic, including duodenal adenocarcinomas (n = 6, 32%), adenomas (n = 5, 26%) and gastrointestinal stromal tumours (n = 4, 21%).
PPDD avoids the potential complications associated with a pancreaticoenteric anastomosis. Although the infrequency of PPDD, along with differences in underlying disease, prevented meaningful comparison of morbidity and mortality between PD and PPDD, a significantly shorter median length of stay was observed following PPDD than PD.
Neither cross-sectional imaging nor forward-viewing endoscopies provide a detailed description of the relationship of the lesion to the papilla. For this reason, patients had consented to PD and selected cases underwent side-viewing on-table endoscopy and duodenotomy/papillary cannulation. The authors would advocate that PPDD should only be undertaken where expertise and facilities support progression to PD.
Eight of the nineteen PPDD undertaken in this 14-year period were performed in the last two years. It is likely that this reflects changes in referral patterns to the centre and an increase in the population catchment area, and better awareness of the option of pancreas preservation may have contributed to this increase. This raises the possibility that the technique of PPDD is underused and improved surgical awareness may prevent some patients undergoing unnecessary pancreatic resection with an associated longer hospital stay and likely increased morbidity and mortality.
To the knowledge of the authors, this series brings to 83 the total number of patients undergoing PPDD that have been reported in published literature, which comprises 4 other series and 10 reports of individual cases[3,8,9,12-22]. These are summarized in Table 3. Represented pathologies include 27 adenocarcinomas of the duodenum (33%), 20 gastrointestinal stromal tumours (24%), 12 adenomas (14%) and 5 trauma (6%) as well as lipoma and liposarcoma, locally invading colon cancer, metastases from seminoma and lung cancer, Crohn’s disease, plasmacytoma and lymphoma. Technical variation includes different longitudinal extent of resection and different anastomotic technique with end-to-end, end-to-side and side-to-side all represented. Three deaths within 30 d of PPDD have been reported; 2 due to cholecystitis and one due to anastomotic leak; giving a periprocedural mortality of 3.7%. Of the 27 patients undergoing PPDD for adenocarcinoma, 10 deaths were recorded and of the 17 patients alive at the time of publication of the individual reports, 7 had survived more than 36 mo. Procedural morbidity included cholangitis/ cholecystitis, anastomotic bleeding, delayed gastric emptying and, unexpectedly, pancreatic fistulae. Overall, morbidity was reported in 32 patients (39%).
| First Author | Year | No. of cases | Histology | Anastomosis | Complications | Outcome |
| Kerremans et al[8] | 1979 | 1 | 1 adenocarcinoma | - | Jejunocutaneous fistula | Death at 20 mo |
| Kawano et al[9] | 1995 | 1 | 1 GIST | 1 end-to-side | - | NR |
| Maher et al[12] | 1996 | 24 | 11 adenocarcinomas | 10 end-to-end | 1 death (anastomotic leak) | Adenocarcinoma; |
| 1 GIST | 8 end-to-side | 2 pancreatic fistulae | Median survival 18.5 mo | |||
| 2 adenomas | 3 side-to-end | 2 DGE | GIST; NR | |||
| 1 lymphoma | 3 side-to-side | 2 anastomotic bleeds | ||||
| 1 liposarcoma | ||||||
| 2 Crohn’s disease | ||||||
| 5 trauma | ||||||
| 1 peptic ulceration | ||||||
| Sohn et al[13] | 1998 | 2 | 2 adenocarcinomas | NR | 2 cholangitis | NR |
| Suzuki et al[14] | 1999 | 1 | 1 GIST | 1 end-to-side | DGE | Alive/ well 2 yr postop |
| Orda et al[15] | 2000 | 1 | 1 GIST | end-to-end | - | Alive/ well 13 yr postop |
| Ammori[16] | 2002 | 1 | 1 benign stricture | side-to-side | Intra-abdominal bleeding | NR |
| Eisenberger et al[17] | 2004 | 1 | 1 GIST | NR | - | Alive/ well 1 yr postop |
| Spalding et al[18] | 2007 | 14 | 5 adenocarcinomas | 14 end-to-end | 1 death (cholecystitis) | Adenocarcinoma; 1 death at 3 mo, |
| 4 GIST | 1 anastomotic stricture (reoperated) | Median survival 56 mo. | ||||
| 1 adenoma | 1 DGE | |||||
| 1 lipoma | 1 anastomotic bleed (reoperated) | GIST; 1 death at 3 mo, | ||||
| 1 metastatic seminoma | Median survival 120 mo | |||||
| 1 ulcer | ||||||
| 1 plasmacytoma | ||||||
| Cavaniglia et al[19] | 2012 | 1 | 1 GIST | 1 end-to-end | - | NR |
| Stauffer et al[3] | 2013 | 1 | 5 adenomas | 7 side-to-side | 1 DGE | NR |
| 2 adenocarcinomas | 2 end-to-side | 1 pancreatic fistula | ||||
| 1 lymphangiolipoma | gastrojejunostomy | |||||
| 1 GIST | ||||||
| 1 NET | ||||||
| Waisberg et al[20] | 2013 | 1 | 1 carcinoid | NR | NR | Death at 6 mo |
| Shimizu et al[21] | 2015 | 1 | 1 adenoma | 1 end-to-side | NR | NR |
| García-Molina et al[22] | 2015 | 8 | 1 adenocarcinoma | 1 death | Adenocarcinoma; 1 death at 12 mo | |
| 5 GIST | ||||||
| 1 metastasis from lung | GIST; 5 Alive/ well at 4-6 yr | |||||
| 1 colon cancer | ||||||
| Current series | 2017 | 19 | 6 adenocarcinomas | 19 side-to-side | 1 recurrent adenocarcinoma | Adenocarcinoma: 1 death at 18 mo |
| 5 adenomas | 1 anastomotic Stricture | Alive/well 1 < 1 yr, 2 > 1 yr, 1 > 6 yr, 1 > 12 yr | ||||
| 4 GIST | 1 incisional hernia | GIST: Alive/ well 2 < 1 yr, 2 > 3 yr | ||||
| 1 lipoma | ||||||
| 1 colon cancer | ||||||
| 1 bleeding diverticulum | ||||||
| 1 extrinsic compression |
Laparoscopic[3,16] and laparoscopic-assisted[23] approaches to distal duodenal resection have also been described and may offer patients the expected benefits of minimally invasive surgery. However, an open approach may be better to achieve adequate assessment and margins for lesions close to the papilla.
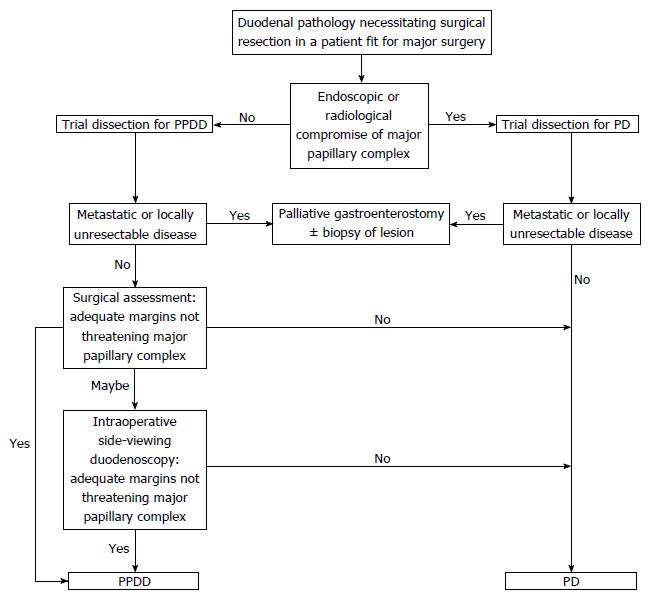
Concern may exist regarding the oncological effectiveness of PPDD. We suggest that no evidence exists to show benefit of including a pancreatic resection in the treatment of a distal duodenal cancer. This study shows that an adequate lymphadenectomy may be achieved with PPDD (Table 2). Consistent R0 margin status and adequate histopathological proximal resection margins have been achieved by conversion to PD if intraoperative doubt exists regarding the macroscopic relationship of the disease to surrounding structures. The only pattern of recurrence observed in this series was distant spread to pelvic peritoneum (after resection of T4 lesion with serosal involvement) and the authors propose that there would have been no oncological benefit from the addition of pancreatic head resection. An algorithm describing pre- and intraoperative decision making is presented (Figure 6).
PPDD is a valuable technique for the treatment of a wide range of infra-papillary duodenal lesions and an expanding body of published literature exists to support its use. It should be undertaken where expertise and facilities permit conversion to PD if necessitated by intraoperative findings.
Neoplastic lesions of the duodenum are treated conventionally by pancreaticoduodenectomy. Lesions distal to the major papilla may be suitable for a pancreas-preserving distal duodenectomy (PPDD), potentially reducing morbidity and mortality. Limited awareness of this technique may deprive patients of the opportunity to avoid pancreas-specific complications following treatment for infrapapillary diseases.
Early series suggested poor outcomes after PPDD for duodenal adenocarcinoma. Adenocarcinoma may thus be considered a contentious indication for PPDD.
The authors widen the range of conditions treated with this surgery, provide detail on lymph node harvests, demonstrate a shorter length of stay than after pancreaticoduodenectomy, and we present relatively good outcomes after PPDD for adenocarcinoma.
This work supports the consideration of PPDD in the resection of any infrapapillary lesion but demonstrates that, in a minority of patients, intraoperative findings may mandate proceeding to a formal pancreaticoduodenectomy.
The authors consider a circumferential full thickness resection of an infrapapillary portion of the duodenal tube without macroscopic resection of pancreas to constitute a pancreas-preserving distal duodenectomy; this is typically after full Kocherisation and with a primary duodenojejunal anastomosis.
It's a well-written manuscript. The authors described surgical technique and its results of pancreas preserving distal duodenectomy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Andrianello S, Kawabata Y, Schafer M, Takahashi H S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Perey B. Neoplasms of the duodenum. In: Scott H, Sawyers JL, editor Surgery of the Stomach, Duodenum and Small Intestine. Boston: Blackwell Scientific Publications, 1987: 571-584. . [Cited in This Article: ] |
| 2. | Jefferson G. Carcinoma of suprapapillary duodenum casually associated with pre-existing simple ulcer. Br J Surg. 1916;4:209. [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Stauffer JA, Raimondo M, Woodward TA, Goldberg RF, Bowers SP, Asbun HJ. Laparoscopic partial sleeve duodenectomy (PSD) for nonampullary duodenal neoplasms: avoiding a whipple by separating the duodenum from the pancreatic head. Pancreas. 2013;42:461-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 5. | Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide in-hospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann Surg. 2016;264:1082-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 6. | Chung RS, Church JM, vanStolk R. Pancreas-sparing duodenectomy: indications, surgical technique, and results. Surgery. 1995;117:254-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Al-Sarireh B, Ghaneh P, Gardner-Thorpe J, Raraty M, Hartley M, Sutton R, Neoptolemos JP. Complications and follow-up after pancreas-preserving total duodenectomy for duodenal polyps. Br J Surg. 2008;95:1506-1511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kerremans RP, Lerut J, Penninckx FM. Primary malignant duodenal tumors. Ann Surg. 1979;190:179-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Kawano N, Ryu M, Kinoshita T, Konishi M, Iwasaki M, Furuse J, Yoshino M, Hasebe T. Segmental resection of the duodenum for treating leiomyosarcoma associated with von Recklinghausen’s disease: a case report. Jpn J Clin Oncol. 1995;25:109-112. [PubMed] [Cited in This Article: ] |
| 10. | Cattell RB, Braasch JW. A technique for the exposure of the third and fourth portions of the duodenum. Surg Gynecol Obstet. 1960;111:378-379. [PubMed] [Cited in This Article: ] |
| 11. | Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 12. | Maher MM, Yeo CJ, Lillemoe KD, Roberts JR, Cameron JL. Pancreas-sparing duodenectomy for infra-ampullary duodenal pathology. Am J Surg. 1996;171:62-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Sohn TA, Lillemoe KD, Cameron JL, Pitt HA, Kaufman HS, Hruban RH, Yeo CJ. Adenocarcinoma of the duodenum: factors influencing long-term survival. J Gastrointest Surg. 1998;2:79-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Suzuki H, Yasui A. Pancreas-sparing duodenectomy for a huge leiomyosarcoma in the third portion of the duodenum. J Hepatobiliary Pancreat Surg. 1999;6:414-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Orda R, Sayfan J, Wasserman I. Surgical treatment of leiomyosarcoma of the distal duodenum. Dig Surg. 2000;17:410-412. [PubMed] [Cited in This Article: ] |
| 16. | Ammori BJ. Laparoscopic pancreas-preserving distal duodenectomy for duodenal stricture related to nonsteroidal antiinflammatory drugs (NSAIDs). Surg Endosc. 2002;16:1362-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Eisenberger CF, Knoefel WT, Peiper M, Yekebas EF, Hosch SB, Busch C, Izbicki JR. Pancreas-sparing duodenectomy in duodenal pathology: indications and results. Hepatogastroenterology. 2004;51:727-731. [PubMed] [Cited in This Article: ] |
| 18. | Spalding DR, Isla AM, Thompson JN, Williamson RC. Pancreas-sparing distal duodenectomy for infrapapillary neoplasms. Ann R Coll Surg Engl. 2007;89:130-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Cavaniglia D, Petrucciani N, Lorenzon L, Caterino S, Cavallini M. Partial duodenectomy with end-to-end anastomosis for duodenal gastrointestinal stromal tumor. Am Surg. 2012;78:E273-E275. [PubMed] [Cited in This Article: ] |
| 20. | Waisberg J, Joppert-Netto G, Vasconcellos C, Sartini GH, Miranda LS, Franco MI. Carcinoid tumor of the duodenum: a rare tumor at an unusual site. Case series from a single institution. Arq Gastroenterol. 2013;50:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Shimizu K, Hashimoto D, Abe S, Chikamoto A, Baba H. Pancreas-preserving partial duodenectomy of the distal region for large duodenal adenoma: report of a case. Surg Today. 2015;45:390-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | García-Molina FJ, Mateo-Vallejo F, Franco-Osorio Jde D, Esteban-Ramos JL, Rivero-Henández I. Surgical approach for tumours of the third and fourth part of the duodenum. Distal pancreas-sparing duodenectomy. Int J Surg. 2015;18:143-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Abe N, Hashimoto Y, Kawaguchi S, Shimoyama H, Kojima Y, Yoshimoto E, Kondo E, Ohki A, Takeuchi H, Nagao G. Successful treatment of large adenoma extending close to the papilla in the duodenum by laparoscopy-assisted pancreas-sparing duodenectomy. Asian J Endosc Surg. 2016;9:52-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |