Published online Apr 14, 2017. doi: 10.3748/wjg.v23.i14.2592
Peer-review started: November 17, 2016
First decision: December 19, 2016
Revised: January 3, 2017
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: April 14, 2017
To investigate the significance of endothelial progenitor cells (EPCs) in predicting severe acute pancreatitis (SAP).
We recruited 71 patients with acute pancreatitis (AP) and excluded 11 of them; finally, cases of mild acute pancreatitis (MAP) (n = 30) and SAP (n = 30), and healthy volunteers (n = 20) were internalized to investigate levels of EPCs, C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), fibrinogen (FIB) and white blood cells (WBC) in peripheral blood.
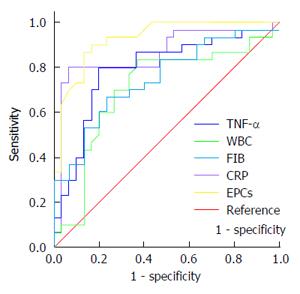
The levels of TNF-α, WBC, FIB and CRP were higher both in SAP and MAP cases than in healthy volunteers (P < 0.05, all). Interestingly, the level of EPCs was higher in SAP than MAP (1.63% ± 1.47% vs 6.61% ± 4.28%, P < 0.01), but there was no significant difference between the MAP cases and healthy volunteers (1.63% ± 1.47% vs 0.55% ± 0.54%, P > 0.05). Receiver operating characteristics curve (ROC) showed that EPCs, TNF-α, CRP and FIB were significantly associated with SAP, especially EPCs and CRP were optimal predictive markers of SAP. When the cut-off point for EPCs and CRP were 2.26% and 5.94 mg/dL, the sensitivities were 90.0% and 73.3%, and the specificities were 83.3% and 96.7%. Although, CRP had the highest specificity, and EPCs had the highest sensitivity and highest area under the curve value (0.93).
Data suggest that EPCs may be a new biological marker in predicting SAP.
Core tip: Endothelial progenitor cells (EPCs) may be used as a novel biological marker to predict the severity of acute pancreatitis (AP) considering the relation between endothelial cells and EPCs. We compared five markers, and concluded that EPCs had the highest area under the curve value (0.93) and Youden index (0.8), sensitivity (90.0%) and specificity (83.3%). EPCs may represent a new biological marker for predicting severe AP at the early stage.
- Citation: Ha XQ, Song YJ, Zhao HB, Ta WW, Gao HW, Feng QS, Dong JZ, Deng ZY, Fan HY, Peng JH, Yang ZH, Zhao Y. Endothelial progenitor cells in peripheral blood may serve as a biological marker to predict severe acute pancreatitis. World J Gastroenterol 2017; 23(14): 2592-2600
- URL: https://www.wjgnet.com/1007-9327/full/v23/i14/2592.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i14.2592
Acute pancreatitis (AP) is a frequent disease. Mild acute pancreatitis (MAP) is easy to treat and the cure rate is high. Although severe acute pancreatitis (SAP) accounts for only 15%-30% of AP cases, it has a high rate of multiple complications and a fatality rate of 5% to 70%[1].
The treatment strategy for SAP is different than that for MAP. The major treatment for MAP is conservative, while SAP requires enhanced monitoring and comprehensive care that includes enteral/abenteric nutrition support, antibiotics, or endoscopic sphincterotomy. Lack of accurate or timely evaluation of AP will lead to excessive medical treatments and a higher fatality rate. Therefore, correct appraisal of the severity of AP is key to clinical decision-making.
It is difficult to evaluate the severity of AP at the early stage. Over the past decade, only 19% of AP cases were graded accurately and only 67% of SAP cases received special therapy in the intensive care unit[2]. AP has a complex etiology, and disease progression does not always match clinical manifestations.
With the development of diagnostic tools - such as the Acute Physiology And Chronic Health Evaluation-II (APACHE-II) scoring system, Ranson criteria, the Balthazar scoring system, and the gold standard, contrast-enhanced computed tomography (CECT)[3,4] - the ability to accurately predict the severity and clinical outcome of AP patients can be up to 80%[5]. However, these methods are inconvenient to use and have limited clinical value[6-8].
The Ranson score focuses primarily on biochemical disturbances and must be completed more than 48 h after admission. The APACHE-II score focuses on physiologic variables. APACHE-II and Balthazar scoring can be done within the first 24 h, but the scores are based on a high number of variables, and the methods are not easily mastered. CECT also has several limitations; for example, iodinated contrast medium is contraindicated in some patients and carries the risk of nephrotoxicity. In addition, it usually requires patient transport to another hospital site.
Serum biochemical detection is objective, exact, economical, and enables real-time monitoring. Levels of tumor necrosis factor-alpha (TNF-α), white blood cell (WBC) count, C-reactive protein (CRP), and fibrinogen (FIB) all can predict SAP, yet endothelial progenitor cells (EPCs) may be a new biological marker.
Serum amylase (S-Amy) and urinary glandular amylase (U-Amy) play an important role in the final diagnosis of AP. However, the level of S-Amy in SAP may be lower than that in MAP due to extensive necrosis and calcification of the pancreas, which leads to consumption of S-Amy[9]. Therefore, it cannot be used to predict SAP exactly.
However, data show that other biomarkers, including CRP[10] and TNF-α[11], are significantly related to the severity and prognosis of AP. As a non-specific acute phase protein, CRP induces endothelial cell dysfunction, impairs vessel walls, and promotes inflammatory reactions. In addition, a certain level of CRP may impair the number and function of EPCs by depressing the expression of endothelial nitric oxide synthase mRNA[12].
In AP, TNF-α promotes a cascade of inflammatory factors, such as IL-6 and IL-1. These are produced by neutrophils and macrophages that infiltrate pancreatic tissue. IL-1 promotes aggregation of WBCs and apoptosis of pancreatic acinar cells[13].
Leukocyte-endothelial interaction and microcirculation disorder may be central to the start of AP progression[14]. In addition, both TNF-α and EPCs can promote apoptosis of pancreatic acinar cells by inducing the release of caspase-3 protease, thereby affecting the prognosis of AP[15,16]. At a certain level, TNF-α also induces premature aging of high proliferation EPCs by modulating the p38 mitogen-activated protein kinase pathway[17].
Various inflammatory mediators are produced and result in damage to cells and tissues. Highly coagulated blood also leads to microcirculation disorders and disseminated intravascular coagulation[18]. Activation of the coagulation and fibrinolytic systems cause other serious outcomes.
Damage to endothelial cells (ECs) is a key factor in systemic inflammatory mediator reactions and secondary organ injury; EPCs sustain ECs. During the embryonic period, EPCs differentiate from the outer layer of the blood-island[19]. Postnatal EPCs derive mainly from umbilical cord blood, bone marrow and peripheral blood[20].
Today, CD34+CD133+VEGFR+ cells, which are involved in neovascularization associated with angiogenic and vasculogenic mechanisms[21,22], are widely considered as EPCs[23]. As such, EPCs can also be used to predict progression or prognosis of cardiovascular diseases and tumors[24-26]. In AP, activated proteases, neutrophils and inflammatory mediums cause widespread damage to ECs, eventually leading to dysfunction of the endothelial barrier that activates coagulation and causes capillary leaks.
As stated above, damaged ECs are a critical factor in systemic inflammatory mediator reactions and secondary organ injury[27]. Previous studies report that impaired or apoptotic ECs are repaired through hyperplasia and lateral movement of peripheral mature ECs. However, in 1997, Asahara et al[28] first discovered that CD34+ hematopoietic stem cells were capable of differentiating into ECs and incorporating into sites of neovascularization in vitro.
The apoptotic bodies of ECs damaged in AP were shown to mobilize EPCs into peripheral blood from bone marrow, and to promote the proliferation and differentiation of EPCs[29]. These progressions were also shown to be mediated by inflammatory cells, e.g., WBCs and macrophages, and inflammatory factors such as TNF-α, CRP and interleukin-8 (IL-8).
The purpose of this study was to investigate the significance of EPCs in predicting SAP.
From September 2010 to October 2011, a total of 71 AP patients (38 women and 33 men; aged 22-80 years, median age of 50 years) were recruited within 24 h from the time of admission. The diagnosis of AP was made according to at least two of the following three criteria: (1) abdominal pain characteristic of AP; (2) S-Amy and/or lipase ≥ 3 times the upper limit of normal; and (3) characteristic findings of AP on a computed tomography (CT) scan. Informed consent was obtained from the patients and ethics approval was obtained from the Institutional Research Ethics Committee.
Reports show that in tumor patients, EPCs mobilize from bone marrow into peripheral blood; besides, age and chemotherapy also affect the number of EPCs. So, exclusion criteria included any of the following: age > 80 years, a diagnosis of cancer or hematological proliferative disease under treatment, current steroid or chemotherapy for any reason, normal findings on amylase and lipase testing, failure to find changes associated with pancreatitis on CT examination, and unavailable complete blood counts or medical records. Eleven patients with AP were excluded according to these criteria.
Patients with pancreatitis were classified as the SAP group (7 women, 13 men; median age of 57 ± 16 years) if they had organ failure, a Ranson score ≥ 3, an APACHE-II score ≥ 8, a class D or E Bathazar score, or a CT severity index ≥ 4. The remainder were classified as the MAP group (11 women, 9 men; median age of 47 ± 20 years)[9].
After MAP and SAP had been diagnosed according to Chinese criteria[9], all patients received conventional treatments. Early prediction of SAP (according to EPCs, TNF-α, WBC, CRP, FIB and other criteria) was made within 24 h after admission. The control group consisted of 20 healthy volunteers (9 women and 11 men; median age of 47 years), and all of the AP patients were volunteers. In addition to the informed consent and ethics approval cited above, this study was also approved by ethics committee of Lanzhou Military Command General Hospital of the People’s Liberation Army. Again, written informed consent was obtained from every subject.
We obtained blood samples from healthy volunteers and AP patients within 24 h after admission. Blood samples for cytofluorimetric analysis were processed within 12 h, whereas plasma samples were stored at -20 °C until used for other analyses.
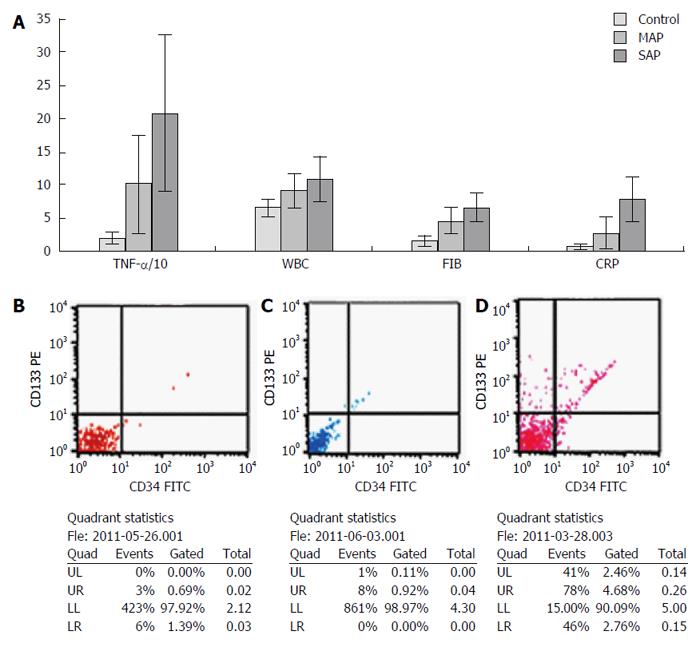
A total of 200 microliters of peripheral blood collected in ethylene diaminetetraacetic acid (EDTA)-containing tubes was incubated for 30 min at 4 °C with 5 μL of FITC-anti-CD34 and PE-anti-CD133. After red cell lysis, the samples were centrifuged and the pellets resuspended in 1 mL PBS buffer. Cells (1 × 105) were acquired by flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA, United States) and the percent of CD34+/CD133+ cells was analyzed using CellQuest software (BD Bioscience, San Jose, CA, United States).
TNF-α was detected by enzyme-linked immunosorbent assay (ELISA) kit (R&D, Minneapolis, MN, United States). CRP was investigated by LX20 automatic biochemical analyzer (Beckman Coulter, Brea, CA, United States). WBC was analyzed by blood cell analyzer. FIB was measured by ACL 9000 automatic coagulation/fibrinolysis analyzer (Instrumentation Laboratory, Milan, Italy). To determine the diagnostic value of EPCs, TNF-α, WBC, FIB and CRP, we compared the area under the curve (AUC) and selected optimal cut-off points for distinguishing SAP from MAP. We also calculated sensitivity, specificity and the Youden index (YI) of each marker (Table 1).
| Cut-off value | Sensitivity | Specificity | YI | AUC | |
| EPCs, % | 2.26 | 90.0% | 83.3% | 0.73 | 0.926 |
| TNF-α, pg/mL | 103.12 | 80.0% | 80.0% | 0.60 | 0.790 |
| WBCs, 109/L | 8.98 | 83.3% | 63.3% | 0.47 | 0.704 |
| FIB, g/L | 5.85 | 66.7% | 76.7% | 0.43 | 0.749 |
| CRP, mg/dL | 5.94 | 73.3% | 96.7% | 0.70 | 0.859 |
We used the Statistical Package for Social Sciences (SPSS) for Windows (Version 17.0; IBM SPSS, Armonk, NY, United States). Data are shown as mean ± SD. We compared subjects using multivariate analysis of variance (ANOVA). Correlations among the five markers were analyzed using Spearman’s rank correlation. We constructed receiver operating characteristic (ROC) curves taking SAP as the positive group and MAP as the negative group to predict SAP (Figure 1). The AUC was used to evaluate the diagnostic value of the five biomarkers.
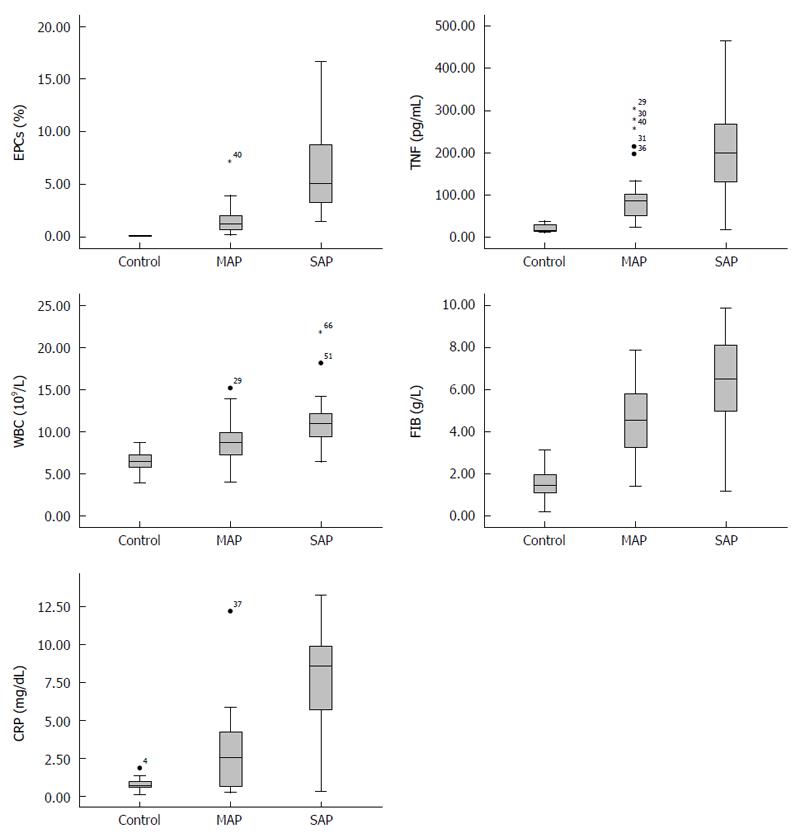
The box plot shows that the distribution of data (i.e., EPCs, TNF-α, WBC, FIB and CRP) in each group was asymmetrical (Figure 2). Furthermore, there were different levels of the characteristics among the three groups. Though, the level of EPCs in the control and MAP groups was similar, there was a significant difference between the MAP and SAP groups.
ANOVA showed that there was no significant difference in age or sex among the three groups. In the SAP, MAP and control groups, the serum levels of TNF-α, WBC, FIB and CRP decreased sequentially; differences were significant (P < 0.05, all). The level of EPCs was higher in the SAP group compared with the MAP group (P < 0.01), but there was no significant difference between the MAP and control groups (P = 0.21) (Tables 2 and 3, Figure 3).
| Patient characteristic | Control group | MAP group | SAP group |
| Number | 20 | 30 | 30 |
| Average years of age | 47.65 ± 15.14 | 48.17 ± 16.85 | 54.97 ± 15.35 |
| Sex (male/female) | 10/10 | 14/16 | 17/13 |
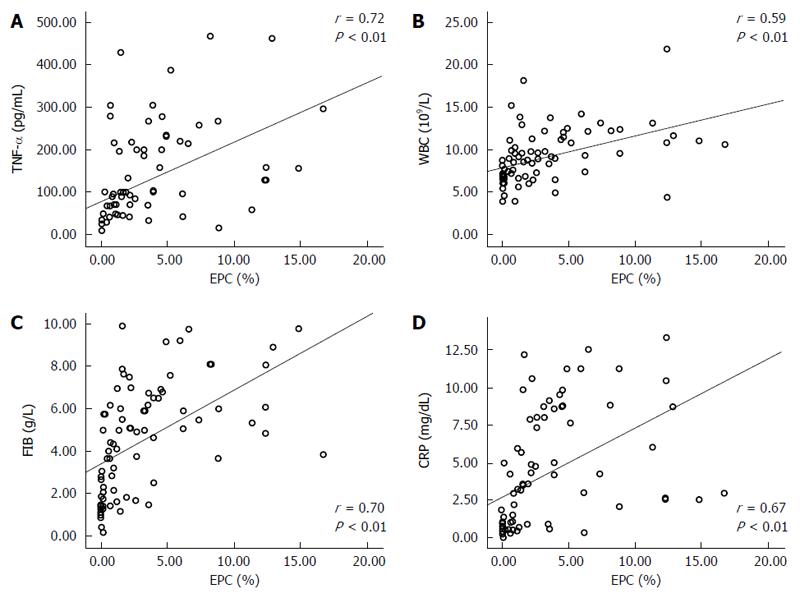
Correlations between the five biomarkers were positive (P < 0.01, all). EPCs had the closest correlation with TNF-α (r = 0.721, P = 0.00) (Table 4, Figure 4).
The optimal cut-off values of EPCs, TNF-α, FIB and CRP were 2.26%, 103.12 pg/mL, 5.85 g/L and 5.94 mg/dL, respectively. A comparison of AUCs showed AUC-EPCs (0.93) > AUC-CRP (0.86) > AUC-TNF-α (0.79) > AUC-FIB (0.75) (P < 0.01, all). Although AUC-WBC was 0.704 (AUC > 0.70), WBC 8.98 × 109 could not be used to predict SAP, perhaps due to distortions from drugs.
According to AUC or YI, EPC may be an optimal marker to predict SAP, followed by CRP. Besides the highest AUC value (0.93) and YI (0.73), EPCs also had the highest sensitivity (90%), while CRP had the highest specificity (96.7%). In serial tests, the YI of combinations including EPCs was higher than that of other combinations without EPCs. EPCs combined with CRP had the highest specificity (99.4%). Combining more markers did not improve diagnostic value according to YI.
Systemic inflammatory response syndrome and multiple organ dysfunction syndrome induced by various inflammatory mediators are lethal factors in AP[30]. Inflammation and imbalance of coagulation are two keys to these pathologic processes. Therefore, inflammatory and coagulation factors may serve as biological markers to predict the severity and prognosis of AP.
EPCs have a close relation with the endothelial system, and may be antigen-presenting cells[31]. That means EPCs may contribute to the processes of AP, and may be a potential marker to predict the severity and prognosis of AP at the early stage. This investigation supports that hypothesis.
Data indicate that the normal level of EPCs in peripheral blood range from 0% to 0.05% of circulating mononuclear cells[32,33]. We found that the mean level of EPCs in peripheral blood of the control, MAP and SAP patients were 0.55% ± 0.54%, 1.63% ± 1.47% and 6.61% ± 4.28%, respectively. The difference between the MAP and SAP groups was significant (P < 0.01). However, the level of EPCs in the control and MAP groups was similar. Furthermore, the control group level of EPCs (mean of 0.55% ± 0.54%, range of 0% to 0.16%) was higher than reported. This may be attributed to national, altitude and other factors. EPCs also had positive correlations with the other four markers in AP patients and controls (P < 0.01, all).
According to AUC value and YI, EPCs and CRP appeared to be optimal biomarkers for predicting SAP. Although CRP had the highest specificity (96.7%), EPCs had the highest sensitivity (90.0%) and highest AUC value (0.93) compared with the other five markers. CRP is produced by the liver after the stimulation of IL-6 and other hormones so that the peak of CRP appears 24-48 h later than IL-6; as well, the different level of CRP between MAP and SAP groups appears 2 d later, after clinical symptoms occur[34]. In contrast, EPCs are instantly mobilized, suggesting that EPCs might be superior to CRP in predicting SAP at the early stage.
CRP impairs the repairing effect of EPCs and leads to the dysfunction of ECs, finally resulting in progression of AP. This investigation showed that CRP has a positive correlation with EPCs that may be attributable to the peripheral blood level of CRP. CRP is accurate, cost-effective and popular. Therefore, it could be used as a significant independent biological marker[35,36].
The World Congress of Gastroenterology also suggests that CRP might be an independent risk factor for SAP. If CRP is > 150 mg/L in 72 h, it suggests SAP and the occurrence of complications. The Congress’s Report[37] included that the sensitivity, specificity, positive predictive value (PV+) and negative predictive value (PV-) of CRP were 86%, 87%, 75% and 93%, respectively. This investigation indicates that CRP > 5.94 mg/dL (59.4 mg/L) within 24 h after admission suggests SAP. Furthermore, at this cut-off value, the sensitivity, specificity, PV+ and PV- of CRP were 73.3%, 96.7%, 95.7% and 78.4%, respectively.
CRP is produced later in the progress of AP, with the peak sustained in only 24 h. That the optimal cut-off level is lower than reported, may be attributed to different patient admission times.
TNF-α rose rapidly at the early stage of AP, and it had a negative correlation with the rate of decay and the severity of AP. This investigation indicated that TNF-α, with a significant AUC value (AUC-TNF-α > 0.7), can be used as a marker to predict SAP at the early stage.
Since coagulation function disorder also occurs in the early stage of SAP, markers of coagulation function can also be used to predict the severity of AP. FIB is the most important coagulation factor, with the highest normal serum level of 2-4 g/L. Reports suggest that progressive change indicates poor prognosis. This investigation found that FIB > 5.85 g/L may predict SAP with a respective sensitivity and specificity of 66.7% and 76.7%.
According to the AUC value and YI, both TNF-α and FIB seemed to have lower diagnostic value than EPCs or CRP. Furthermore, WBC could be easily modified by anti-inflammatory drugs, such as aspirin, making it an unlikely biomarker to identify SAP or MAP.
In this investigation, we first proposed that EPCs may be used as a novel biological marker to predict the severity of AP considering the relation between ECs and EPCs. We compared five markers, and concluded that EPCs had the highest AUC value (0.93) and YI (0.8), sensitivity (90.0%) and specificity (83.3%). According to the YI, combination of CRP with EPCs would improve diagnostic value. Data suggest that EPCs may be a new biological marker in predicting SAP at the early stage.
Acute pancreatitis (AP) is a frequent disease. Mild acute pancreatitis is easy to treat and the cure rate is high. Although severe acute pancreatitis (SAP) accounts for only 15%-30% of AP cases, it has a high rate of multiple complications and a fatality rate of 5% to 70%. Lack of accurate or timely evaluation of AP will lead to excessive medical treatments and a higher fatality rate. Therefore, correct appraisal of the severity of AP is key to clinical decision-making. Data show that biomarkers, including C-reactive protein (CRP) and tumor necrosis factor-alpha (TNF-α) are significantly related to the severity and prognosis of AP. As a non-specific acute phase protein, CRP induces endothelial cell dysfunction, impairs vessel walls and promotes inflammatory reactions. In addition, a certain level of CRP may impair the number and function of endothelial progenitor cells (EPCs) by depressing the expression of endothelial nitric oxide synthase. The purpose of this study was to investigate the significance of EPCs in predicting SAP.
EPCs have a close relation with the endothelial system, and may be antigen-presenting cells. That means that EPCs may contribute to the processes of AP, and may be a potential marker to predict the severity and prognosis of AP at the early stage.
In this investigation, the authors first proposed that EPCs may be used as a novel biological marker to predict the severity of AP, considering the relation between ECs and EPCs. The authors compared five markers, and concluded that EPCs had the highest AUC value (0.93) and Youden index (YI) (0.8), as well as the highest sensitivity (90.0%) and the second highest specificity (83.3%) from among five markers evaluated. According to the YI, combination of CRP with EPCs will improve diagnostic value. Furthermore, this investigation showed that EPCs had positive correlations with the four other markers in AP patients.
This study suggests that EPCs may be used as a new biological marker in predicting SAP at the early stage.
EPCs may be used as a novel biological marker to predict the severity of AP considering the relation between ECs and EPCs. The authors compared five markers, and concluded that EPCs had the highest AUC value (0.93) and YI (0.8), sensitivity (90.0%) and specificity (83.3%). EPCs may be a new biological marker in predicting SAP at the early stage.
This article relooks at whether EPCs may be used as a novel biological marker to predict the severity of AP, considering the relation between ECs and EPCs. Compared with five markers, the authors concluded that EPCs had the highest AUC value (0.93) and YI (0.8), sensitivity (90.0%) and specificity (83.3%). So, EPCs may be useful as a new biological marker in predicting SAP at the early stage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liao KF S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Bruennler T, Hamer OW, Lang S, Gruene S, Wrede CE, Zorger N, Herold T, Siebig S, Rockmann F, Salzberger B. Outcome in a large unselected series of patients with acute pancreatitis. Hepatogastroenterology. 2009;56:871-876. [PubMed] [Cited in This Article: ] |
| 2. | Toh SK, Phillips S, Johnson CD. A prospective audit against national standards of the presentation and management of acute pancreatitis in the South of England. Gut. 2000;46:239-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Balthazar EJ, Ranson JH, Naidich DP, Megibow AJ, Caccavale R, Cooper MM. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767-772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 330] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Triantopoulou C, Lytras D, Maniatis P, Chrysovergis D, Manes K, Siafas I, Papailiou J, Dervenis C. Computed tomography versus Acute Physiology and Chronic Health Evaluation II score in predicting severity of acute pancreatitis: a prospective, comparative study with statistical evaluation. Pancreas. 2007;35:238-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Chakraborty S, Kaur S, Muddana V, Sharma N, Wittel UA, Papachristou GI, Whitcomb D, Brand RE, Batra SK. Elevated serum neutrophil gelatinase-associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am J Gastroenterol. 2010;105:2050-2059. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Johnson CD, Toh SK, Campbell MJ. Combination of APACHE-II score and an obesity score (APACHE-O) for the prediction of severe acute pancreatitis. Pancreatology. 2004;4:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Tang W, Zhang XM, Xiao B, Zeng NL, Pan HS, Feng ZS, Xu XX. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol. 2011;80:637-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Wang XP, Xu GM, Yuan YZ, Li ZS. China Guideline for the diagnosis and treatment of acute pancreatitis (Draft). Zhonghua Neike Zazhi. 2007;43:236-238. [Cited in This Article: ] |
| 10. | Werner J, Hartwig W, Uhl W, Müller C, Büchler MW. Useful markers for predicting severity and monitoring progression of acute pancreatitis. Pancreatology. 2003;3:115-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Gulcubuk A, Altunatmaz K, Sonmez K, Haktanir-Yatkin D, Uzun H, Gurel A, Aydin S. Effects of curcumin on tumour necrosis factor-alpha and interleukin-6 in the late phase of experimental acute pancreatitis. J Vet Med A Physiol Pathol Clin Med. 2006;53:49-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:2476-2482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Bhatia M. Inflammatory response on the pancreatic acinar cell injury. Scand J Surg. 2005;94:97-102. [PubMed] [Cited in This Article: ] |
| 14. | Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Knobbe CB, Trampe-Kieslich A, Reifenberger G. Genetic alteration and expression of the phosphoinositol-3-kinase/Akt pathway genes PIK3CA and PIKE in human glioblastomas. Neuropathol Appl Neurobiol. 2005;31:486-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Herbert BS, Rajashekhar G, Ingram DA, Yoder MC, Clauss M, Rehman J. Premature senescence of highly proliferative endothelial progenitor cells is induced by tumor necrosis factor-alpha via the p38 mitogen-activated protein kinase pathway. FASEB J. 2009;23:1358-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Bao XM, Wu CF, Lu GP. Atorvastatin inhibits homocysteine-induced oxidative stress and apoptosis in endothelial progenitor cells involving Nox4 and p38MAPK. Atherosclerosis. 2010;210:114-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Werner J, Rivera J, Fernandez-del Castillo C, Lewandrowski K, Adrie C, Rattner DW, Warshaw AL. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997;121:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ribatti D. The discovery of endothelial progenitor cells. An historical review. Leuk Res. 2007;31:439-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Murohara T. Cord blood-derived early outgrowth endothelial progenitor cells. Microvasc Res. 2010;79:174-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 361] [Cited by in F6Publishing: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 22. | Döme B, Hendrix MJ, Paku S, Tóvári J, Tímár J. Alternative vascularization mechanisms in cancer: Pathology and therapeutic implications. Am J Pathol. 2007;170:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 23. | Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952-958. [PubMed] [Cited in This Article: ] |
| 24. | Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D’Amario D, Rebuzzi AG, Crea F. From bone marrow to the arterial wall: the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Sieghart W, Fellner S, Reiberger T, Ulbrich G, Ferlitsch A, Wacheck V, Peck-Radosavljevic M. Differential role of circulating endothelial progenitor cells in cirrhotic patients with or without hepatocellular carcinoma. Dig Liver Dis. 2009;41:902-906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Zhang HR, Chen FL, Xu CP, Ping YF, Wang QL, Liang ZQ, Wang JM, Bian XW. Incorporation of endothelial progenitor cells into the neovasculature of malignant glioma xenograft. J Neurooncol. 2009;93:165-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1147] [Cited by in F6Publishing: 1239] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 28. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6624] [Cited by in F6Publishing: 6212] [Article Influence: 230.1] [Reference Citation Analysis (1)] |
| 29. | Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761-2766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 30. | Cuzzocrea S, Nocentini G, Di Paola R, Agostini M, Mazzon E, Ronchetti S, Crisafulli C, Esposito E, Caputi AP, Riccardi C. Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation. J Immunol. 2006;177:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Asakage M, Tsuno NH, Kitayama J, Kawai K, Okaji Y, Yazawa K, Kaisaki S, Osada T, Watanabe T, Takahashi K. Early-outgrowth of endothelial progenitor cells can function as antigen-presenting cells. Cancer Immunol Immunother. 2006;55:708-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Kalka C, Masuda H, Takahashi T, Gordon R, Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 349] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Rafii S, Heissig B, Hattori K. Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Ther. 2002;9:631-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Mayer JM, Raraty M, Slavin J, Kemppainen E, Fitzpatrick J, Hietaranta A, Puolakkainen P, Beger HG, Neoptolemos JP. Serum amyloid A is a better early predictor of severity than C-reactive protein in acute pancreatitis. Br J Surg. 2002;89:163-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scand J Gastroenterol. 2010;45:959-970. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Hjalmarsson C, Stenflo J, Borgström A. Activated protein C-protein C inhibitor complex, activation peptide of carboxypeptidase B and C-reactive protein as predictors of severe acute pancreatitis. Pancreatology. 2009;9:700-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Pongprasobchai S, Jianjaroonwong V, Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S, Pausawasdi N, Srikureja W, Chainuvati S, Prachayakul V. Erythrocyte sedimentation rate and C-reactive protein for the prediction of severity of acute pancreatitis. Pancreas. 2010;39:1226-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |