Published online Jan 7, 2017. doi: 10.3748/wjg.v23.i1.1
Peer-review started: August 24, 2016
First decision: September 12, 2016
Revised: September 27, 2016
Accepted: October 30, 2016
Article in press: October 31, 2016
Published online: January 7, 2017
Processing time: 135 Days and 12.8 Hours
Measurement of portal pressure is pivotal in the evaluation of patients with liver cirrhosis. The measurement of the hepatic venous pressure gradient represents the reference method by which portal pressure is estimated. However, it is an invasive procedure that requires significant hospital resources, including experienced staff, and is associated with considerable cost. Non-invasive methods that can be reliably used to estimate the presence and the degree of portal hypertension are urgently needed in clinical practice. Biochemical and morphological parameters have been proposed for this purpose, but have shown disappointing results overall. Splanchnic Doppler ultrasonography and the analysis of microbubble contrast agent kinetics with contrast-enhanced ultrasonography have shown better accuracy for the evaluation of patients with portal hypertension. A key advancement in the non-invasive evaluation of portal hypertension has been the introduction in clinical practice of methods able to measure stiffness in the liver, as well as stiffness/congestion in the spleen. According to the data published to date, it appears to be possible to rule out clinically significant portal hypertension in patients with cirrhosis (i.e., hepatic venous pressure gradient ≥ 10 mmHg) with a level of clinically-acceptable accuracy by combining measurements of liver stiffness and spleen stiffness along with Doppler ultrasound evaluation. It is probable that the combination of these methods may also allow for the identification of patients with the most serious degree of portal hypertension, and ongoing research is helping to ensure progress in this field.
Core tip: This Editorial analyzes the newest and promising methods for estimating portal pressure non-invasively in cirrhotic patients with portal hypertension. Measurements of liver and spleen stiffness, combined with Doppler ultrasound evaluation, allow for the identification of patients without clinically-significant portal hypertension and are also promising for estimation of the degree of portal pressure in patients with portal hypertension.
- Citation: Bolognesi M, Di Pascoli M, Sacerdoti D. Clinical role of non-invasive assessment of portal hypertension. World J Gastroenterol 2017; 23(1): 1-10
- URL: https://www.wjgnet.com/1007-9327/full/v23/i1/1.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i1.1
Measurement of portal pressure is pivotal in the evaluation of patients with liver cirrhosis. Indeed, portal hypertension is a complication of cirrhosis that affects prognosis and the natural history (disease stage). Portal hypertension is the main etiology underlying the opening of collateral circulation and the onset of hyperdynamic circulatory syndrome, which can result in esophageal varices (EV), gastrointestinal bleeding, ascites, hepatorenal syndrome, spontaneous bacterial peritonitis and/or hepatic encephalopathy[1]. Therefore, upper gastrointestinal endoscopy and measurement of portal pressure are recommended for patients with suspected liver cirrhosis and portal hypertension[1]. Moreover, the measurement of portal pressure represents the only valid method currently available for evaluation of effectiveness of portal hypertension therapies (pharmacological, surgical, interventional radiology)[2].
Increase in portal pressure has been shown to be clinically significant (clinically significant portal hypertension, CSPH) when it corresponds to a porto-hepatic gradient of ≥ 10 mmHg[2,3]. CSPH is considered the threshold beyond which complications like EV and ascites may develop[2,4]. However, in the evaluation of portal hypertension, it is not sufficient to merely determine the presence or absence of CSPH. Indeed, the degree of portal hypertension defines different levels of risk, with progressively worse prognostic significance.
Risk of EV, ascites and decompensation after surgery for hepatocellular carcinoma is associated with portal pressure > 10 mmHg, while risk of bleeding of EV is associated with a portal pressure of ≥ 12 mmHg[5]. Portal pressure > 16 mmHg is reported as correlated with survival, first clinical decompensation in patients with varices, and higher risk of esophageal rebleeding and mortality in patients with decompensated cirrhosis; higher than 20 mmHg is correlated with failure to control bleeding in patients with active bleeding from varices and to mortality, > 22 mmHg is correlated with mortality in patients with alcoholic cirrhosis and acute alcoholic hepatitis, and > 30 mmHg is correlated with spontaneous bacterial peritonitis[3,5,6]. In contrast, improvement of portal hypertension is associated with improved prognosis. In particular, a reduction in portal pressure to a level of < 12 mmHg, or to at least of 20% of the baseline values, is necessary to obtain clinical efficacy of portal hypertension therapy[2,7,8]. It is clear, therefore, that when portal hypertension is suspected in patients with liver cirrhosis, it is not only necessary to know whether CSPH is present but also to quantify the level of portal hypertension and, further, to evaluate the change in portal pressure over time.
The actual reference method for the measurement of portal pressure is measurement of the hepatic venous pressure gradient (HVPG), an indirect estimate of portal pressure obtained by use of catheterization of the hepatic veins. It allows measurement of the level of sinusoidal pressure by calculating the difference between the pressure in a hepatic vein that has been inserted with the occluding catheter and free pressure[5,7]. In the cirrhosis condition, portal hypertension is mainly due to sinusoidal and post-sinusoidal hypertension, with the sinusoidal pressure corresponding to the pressure in the portal vein. Introduction of balloon catheters to this measurement approach has resulted in marked improvement in reliability of the measurement[9].
HVPG is an indirect method, which is only able to correctly evaluate portal pressure in patients with increased portal pressure at the sinusoidal level (i.e., cirrhosis), and it has no value in patients with pre-sinusoidal and pre-hepatic portal hypertension. Yet, detection of its normality can sometimes help in differential diagnosis of those forms of pre-hepatic portal hypertension.
With this limitation, HVPG, if executed according to the guidelines, is a safe and reproducible technique, and has emerged as the reference method for measurement of the pressure gradient between the portal vein and the inferior vena cava in cirrhosis (sinusoidal portal hypertension)[2]. Unfortunately, the method is invasive and relies on the commitment of significant hospital resources, equating to a considerable cost and requiring experienced staff. As such, it is routinely performed in only a few centers, particularly those specializing in the treatment of portal hypertension[10].
Non-invasive methods that can be used reliably to determine the presence and estimate the degree of portal hypertension have been in great demand for at least 30 years. Despite substantial efforts to generate such a method, up until a few years ago only disappointing and unsatisfactory results were obtained.
Since the primary cause of portal hypertension is the mechanical increase in intrahepatic resistance due to fibrosis and distortion of liver architecture, it is reasonable to assume that non-invasive parameters of liver fibrosis may indicate the presence of portal hypertension.
A number of the biochemical and morphological parameters that have been proposed for evaluation of the degree of liver fibrosis have been analyzed for their potential in use for the evaluation of portal hypertension and/or the presence of EV[11]. Even if there is a broad correlation between these indices and portal pressure or the presence of EV, confirming the role of liver fibrosis in the genesis of this condition, it is a fact that the low coefficients of the correlations do not support clinical use of these parameters for this purpose. Various indices have also been proposed[3,12,13]. The platelet count/spleen diameter ratio (Plt/Spl) was reported to be independently associated with the presence of EV, as shown in a multivariate analysis. A Plt/Spl cut-off value of 909 had 100% negative predictive value for diagnosis of EV[14,15]. Another study determined that this parameter is also related to the presence of portal hypertension[16]. A model combining albumin, aspartate aminotransferase (AST) and the international normalized ratio (INR) had an area under the receiver operating characteristic curve (AUROC) of 0.952 for prediction of CSPH in a group of patients with compensated cirrhosis[17].
In a study by Sebastiani et al[18], a combination of the Lok index (an index derived by AST and alanine aminotransferase (ALT) levels, platelet counts and prothrombin time (PT)-INR; using a cut-off of 1.5) and the Forns’ index (an index derived by age, platelet counts, gamma-glutamyl transferase (GGT) and cholesterol; using a cut-off of 8.8) had an AUROC of 0.80 (95%CI: 0.76-0.84) and a high negative predictive value (> 90%) for excluding clinically-relevant EV[18].
Overall, the results for the proposed parameters and indices have not been satisfactory[3]. Serum markers may be useful as a first-line tool to identify cirrhotic patients in whom the risk of clinically-relevant EV is trivial[17]. However, the possibility of replacing upper endoscopy with simple serum non-invasive markers is still not practical for the vast majority of patients[3,11,13,17,18]. Accordingly, the biochemical/morphological tests may be of help to diagnose patients with suspected CSPH, but not to estimate the degree of portal pressure. These tests do not allow for clinical decisions on their own, nor can they be used alone in a clinical context; although, they may be sufficient in use as a first-level test[19] (laboratory tests require no clinical skillfulness, distinctive from Doppler ultrasound and measurement of tissue stiffness), but their use would not exempt a clinician from undertaking further analysis with more accurate tests.
The introduction of ultrasound and Doppler techniques generated great expectations in the 1990s for non-invasive assessment of portal hypertension. Doppler ultrasound evaluation was a major step forward in the clinical evaluation of patients with portal hypertension. Indeed, many parameters indicating the presence of portal hypertension could be identified non-invasively, including the presence of collateral vessels, spleen enlargement, ascites, change in the portal vein parameters (e.g. increase in diameter, disappearance of caliper variation during respiration, decrease in blood flow velocity, increase in the congestion index), increase in hepatic and splenic arterial resistance indices, and decrease in the damping index of hepatic veins[5,12,20-22]. Scores obtained by the combinations of measuring portal vein blood velocity, portal vein diameter, the hepatic artery resistance index and splenic artery resistance index[4,12,23-26] were proposed and demonstrated to be useful in the clinical monitoring of patients with cirrhosis and portal hypertension.
Some of these parameters, such as the presence of collateral circulation in patients with cirrhosis, can be considered as having a specificity of 100% for the diagnosis of CSPH[2]; although, all of these parameters have low diagnostic sensitivity for identifying the condition.
In patients with known cirrhosis, Doppler ultrasound has > 80% specificity for diagnosis of CSPH, but sensitivity does not exceed 40%-70%, particularly in compensated patients[5]. Therefore, while the presence of one or more Doppler ultrasound signs can establish the presence of CSPH, their absence cannot exclude it. Moreover, Doppler ultrasound is not useful for evaluating the effect of pharmacological therapy on portal hypertension, as vasoactive drugs used in the therapy of portal hypertension modify Doppler parameters (i.e., vascular blood velocity, resistance indices) per se, in a manner independent of the final modification of portal pressure.
On the contrary, Doppler parameters may have major utility in the evaluation of the effect of surgical therapy and of liver transplantation on portal hypertension. Indeed, in these conditions, normalization of portal hemodynamics and of splenic Doppler resistance indices has been proposed as confirmatory for having achieved a good resolution of portal hypertension after surgery[27].
Thus, although indispensable in the evaluation and monitoring of patients with cirrhosis and portal hypertension, Doppler ultrasonography cannot be used on its own as a screening method to exclude CSPH, nor as a method for monitoring portal pressure over time. Doppler ultrasound, however, does detect signs, such as portal-collateral circulation, ascites and portal vein thrombosis, that, if present, allow for a certain diagnosis of CSPH.
Color Doppler ultrasonography is a useful non-invasive modality for assessing gastric, duodenal and rectal varices[28-31]. Contrast-enhanced ultrasonography analysis of transit time of microbubble contrast agent through the liver has demonstrated that a decrease in the transit time between the hepatic vein and the hepatic artery or the portal vein (a sign of porto-hepatic shunting) is related to the degree of portal hypertension in cirrhosis[12,21,32]. Moreover, a relation has been reported for the presence of portal hypertension and a number of other parameters derived by the analysis of time-intensity curves of contrast agent in the various liver structures; these parameters include regional hepatic perfusion[33], portal vein/hepatic artery strength ratio, area under the portal vein/hepatic artery time-intensity curve ratio, and portal vein/hepatic artery wash-in perfusion slope ratio[34]. Unfortunately, most of the correlations reported between these parameters and portal pressure are weak, indicating that they cannot predict the presence of CSPH in single patients with sufficient accuracy.
Recently, a new non-invasive approach to quantify portal pressure has been proposed that is based upon subharmonic emission from ultrasound contrast agent[35,36]. The changes of subharmonic signal amplitude are reported as correlating with portal pressure changes[36]; moreover, Eisenbrey et al[37] demonstrated that subharmonic-aided pressure estimation (SHAPE) was in good overall agreement with HVPG (r = 0.82). This method seems promising and deserves further study.
Another important advancement in the non-invasive assessment of portal hypertension has been the introduction of non-invasive measurement of liver stiffness (LS) by transient elastography (TE). Originally proposed and designed as a non-invasive approach for detecting the presence of fibrosis in the liver, after initial doubts, the method has gradually imposed itself as a routine method used in the clinical evaluation of patients with chronic liver disease. TE has proven sensitive for estimating the absence of liver fibrosis or the presence of high-degree liver fibrosis, yet patients with moderate fibrosis remain more difficult to assess[26]. TE has also been shown to be related to the degree of portal pressure[10,38,39]. Such a correlation is somewhat expected because liver fibrosis is the first and main determinant both of tissue stiffness and of intrahepatic resistance to portal blood flow[3]. LS can increase independently of fibrosis due to food ingestion, inflammation, cholestasis and liver congestion[3]. Even with the limitations cited above, a number of studies have demonstrated that the related method allows not only for estimation of liver fibrosis but also determination of CSPH presence[13,40,41]. In patients with chronic liver disease, LS can predict CSPH (HVPG ≥ 10 mmHg) with a very high accuracy, having an AUROC of 0.945 (95%CI: 0.904-0.987); when the cut-off value was set at 21 kPa, this procedure accurately predicted CSPH in 92% of the patients for whom LS was successful[40]. Lemoine et al[41] confirmed that LS can predict CSPH, but highlighted that the cut-off is higher, with a better performance, in alcoholic patients; in particular, the AUROC for diagnosis of CSPH was reported as 0.76 ± 0.07 in patients with hepatitis C virus (HCV) infection (best cut-off at 20.5 kPa) and 0.94 ± 0.03 (best cut-off at 34.9 kPa) in alcoholic patients[41].
These results justify the proposal to use this method in clinical practice for identifying patients with CSPH[5]. Therefore, TE can be used as a screening method for CSPH in patients with compensated liver cirrhosis[2,5].
Vizzutti et al[39] showed that the correlation between LS and portal pressure in cirrhosis is very good up to 10-12 mmHg, while it is substantially lacking for higher values. This finding has been explained by the fact that - while in the early stages of the disease the main factor determining portal hypertension is liver fibrosis, therefore it is well related to portal pressure - once CSPH is established, the progression of portal hypertension depends not only on liver fibrosis but also on other factors, especially those related to the hyperdynamic circulation, the splanchnic vasodilatation and the resistance in portosystemic collaterals[42-44]. Unfortunately, these factors are not estimated by LS[10,16,39].
According to the collective data, TE can be very useful for ruling out or ruling in CSPH[5]; however, the technique is not accurate enough to replace HVPG in quantifying the exact severity of portal hypertension[5]. Furthermore, TE is unlikely to be useful in monitoring hemodynamic response to drug therapy, the effect of which is mediated primarily by decreases in splanchnic blood flow and partially by modifications in hepatic and collateral resistance, and not by improvements in hepatic fibrosis and LS[38].
LS has been demonstrated to be as effective as HVPG for predicting clinical decompensation and portal hypertension-related complications in patients with chronic liver disease[45]. The usefulness of LS in predicting portal hypertensive complications was confirmed by Kitson et al[46].
In recent years, additional techniques have been proposed for the evaluation of LS, each of which appear to overcome some of the limitations presented by traditional TE[47,48]; these include acoustic radiation force impulse imaging (ARFI) and shear-wave velocity estimation. In particular, the real-time shear-wave elastography (SWE) allows for real-time viewing of the area under investigation, contrary to TE which is done blindly, as well as integration of the assessment of TE with traditional ultrasound and Doppler[1,12,21,47,49-51]. In this context, it may be possible to integrate the measurement of LS with Doppler ultrasound parameters and, therefore, improve the accuracy of portal hypertension evaluation. The reported technical success rate of SWE is significantly better than that of TE[52]. Choi et al[53] also proposed that non-invasive measurement of LS by SWE may be useful for monitoring efficacy of the medical therapy of portal hypertension.
A very noteworthy advancement in this field is the application of non-invasive evaluation of parenchymal stiffness (via TE, ARFI and SWE) in the spleen[16,54,55]. An interesting study[16] showed that in patients with HCV-related cirrhosis, there is a very good correlation between HVPG and spleen stiffness (SS) (r2 = 0.78), with a correlation that is maintained even when portal pressure is > 10 mmHg, which contrasts with LS. This study suggests that SS increases in close parallel with the progression of portal hypertension from the early to the late stages of cirrhosis[16].
Similarly to LS, SS measurement has also been reported as useful for predicting of clinical complications in compensated cirrhosis[56]. In patients with HCV-related cirrhosis, a SS and model for end-stage liver disease (MELD) predictive model represented an accurate predictor of clinical decompensation, with accuracy at least equivalent to that of HVPG[56]. A value for SS of < 54 kPa ruled out the risk of complications in the subsequent 2 years[56]. SS has been shown to decrease after orthotopic liver transplantation, when portal hypertension is resolved[57]. This is a behavior similar to splenic resistance indices[27].
Although not all of the subsequent studies yielded such reassuring results[51,52], this study highlighted that spleen parameters probably reflect the levels of portal hypertension more accurately, due to the peculiar modifications that occur in the spleen during portal hypertension as a result of congestion and hyperplasia[58]. In cirrhosis, splenomegaly is not only due to passive congestion but also to tissue hyperplasia, and is characterized by a combination of angiogenesis, fibrogenesis, and enlargement and hyperactivation of the splenic lymphoid compartment[16,58]. This condition of hyperplasia, with increased flow, participates in the hyperdynamic circulatory syndrome of portal hypertension[58].
Stiffness and hemodynamics of the spleen are probably sensitive sensors of portal pressure and of portal vein resistance. Therefore, it seems that the next route to follow will be the combination of SS with the Doppler splenic resistance indices, and possibly platelet count and spleen size. Indeed, individually, these parameters have shown better accuracy in the prediction of portal hypertension. SS is probably related to splenic congestion due to portal hypertension in an organ with a rigid capsule. The platelet count/spleen diameter ratio is probably the simplest index for determining the presence of portal hypertension and EV[14-16,59,60]. Doppler splenic resistance indices are related to portal blood flow resistance and to HVPG[22,24].
As evidence for the central role of splenic hemodynamics in portal hypertension, a few studies have shown the usefulness of combining the value of LS with splenic parameters to improve the identification of patients with portal hypertension. Among these parameters are the LS-spleen diameter to platelet ratio score (LSPS)[13,16,59] and the portal hypertension risk score, the latter of which combines LS, sex and spleen diameter/platelet count ratio[61]. This portal hypertension risk score had the highest AUROC value (0.935), as compared with LS alone or LSPS, for identifying patients with CSPH.
A limitation of this method is the significant number of patients for whom the measurement of LS and/or SS could not be completed or yielded unreliable results. Reportedly, valid measurement of LS is not obtained in approximately 20% of patients[16,19,47]. TE cannot be performed in patients with ascites, and the failure rate of TE is generally higher in obese patients[47]. Aminotransferase flares, food intake, extrahepatic cholestasis, steatosis, increased central venous pressure and the use of beta-blockers can influence the accuracy of LS assessment by TE[36,62].
Moreover, LS and SS measurement are considered reliable for estimating portal hypertension only when the coefficient of variation among the successful measurements in a single patient is low[51,52]. In the study by Elkrief et al[52], the designation of excellent accuracy (i.e., patients with variation coefficient of TE measurement < 10%) was achieved in < 50% of the patients. Procopet et al[51] proposed that SWE measurement of LS can be considered “highly reliable” only when measurements have a coefficient of variation < 10% and a depth of measurement < 5.6 cm; when these criteria are fulfilled, the rate of patients considered well-classified for the presence or absence of CSPH is close to 100%.
Other methods have been proposed for non-invasive assessment of LS related to portal hypertension, namely magnetic resonance elastography, quantitative magnetic resonance imaging and computed tomography (CT). Although very interesting, at present these methods cannot be recommended as routine for measuring LS and SS. Magnetic resonance elastography can decompose tissue viscoelastic parameters into different components, including stiffness, elasticity and viscosity, allowing for better differentiation of fibrosis from congestion[63]. CT has the hypothetical capacity to assess portal pressure by using computational fluid dynamic modeling[64], and has already been proposed for use in evaluation of the fractional flow coronary reserve[65]. While magnetic resonance techniques are very promising[66,67], they are too expensive and the use of CT also seems impractical due to the high cost and the time-consuming nature of the computational fluid dynamic modeling.
A number of studies have shown that LS and SS would also be able to identify, with acceptable accuracy, patients with EV at risk of bleeding[16,59-61,68]. The findings of these studies, however, have been contradicted by other research groups[13,52,69]. The identification of patients with risk of bleeding from EV may be better with the measurement of SS[16,70,71], and particularly as related to LS[16,60].
Considering that the measurement of LS and SS can be considered a good method to identify patients with CSPH, and that EV develops only in the presence of CSPH, it may be reasonable to propose the measurement of LS and SS as a screening method for identifying chronic liver disease patients with HVPG < 10 mmHg (these patients should not have EV). Also, it is important to note that CSPH is a necessary, but not sufficient, condition for development of EV[19]. Therefore, measurement of LS and SS can exclude the need for a screening esophagogastroduodenoscopy, but cannot identify who among the patients with CSPH is at risk of esophageal bleeding[1,59,72].
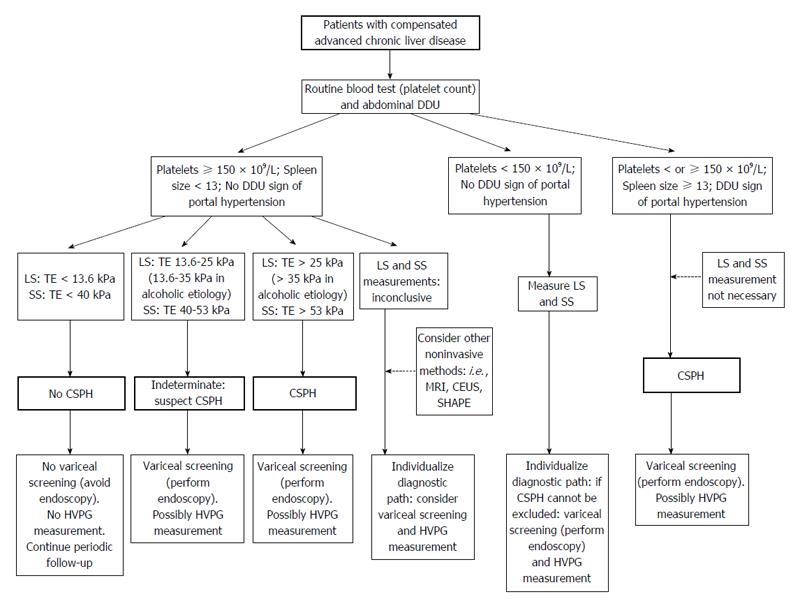
According to the Baveno VI criteria, TE and platelet count may be used to discriminate such patients, without the need for screening varices[2]. On the other hand, imaging analyses have shown that collateral circulation is sufficient for ruling-in CSPH in patients with compensated advanced chronic liver diseases of all etiologies[2].
A hypothetical algorithm of non-invasive methods for screening and evaluation of CSPH and to discriminate patients with or without a need for screening varices is presented in Figure 1.
According to the data published to date in the publicly available literature, it appears possible to rule-out CSPH with a clinically-acceptable accuracy through the combination of LS and SS measurements[10,16,50,59,73] along with Doppler ultrasound evaluation. It is probable that the combination of these methods may also allow for the identification of patients with the most serious degree of portal hypertension. Indeed, progress is being made in this field.
To conclude, however, advancement in the non-invasive evaluation of portal hypertension has included the introduction of methods to clinical practice that are able to measure stiffness in the liver and stiffness/congestion in the spleen. These methods, combined with Doppler ultrasound evaluation, allow for the identification of patients without CSPH. They are also promising for their ability to estimate the degree of portal pressure in patients with CSPH.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Fernandez-Rodriguez CM, Fierbinteanu-Braticevici C, La Mura V, Makisalo H, Maruyama H, Sato T, Tantau M S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Castéra L, García-Tsao G. When the spleen gets tough, the varices get going. Gastroenterology. 2013;144:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2293] [Article Influence: 229.3] [Reference Citation Analysis (3)] |
| 3. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 4. | Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811-16819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Procopeţ B, Tantau M, Bureau C. Are there any alternative methods to hepatic venous pressure gradient in portal hypertension assessment? J Gastrointestin Liver Dis. 2013;22:73-78. [PubMed] |
| 7. | Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 523] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 8. | Merkel C, Bolognesi M, Berzigotti A, Amodio P, Cavasin L, Casarotto IM, Zoli M, Gatta A. Clinical significance of worsening portal hypertension during long-term medical treatment in patients with cirrhosis who had been classified as early good-responders on haemodynamic criteria. J Hepatol. 2010;52:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Maleux G, Willems E, Fieuws S, Heye S, Vaninbroukx J, Laleman W, Cassiman D, Verslype C, Nevens F. Prospective study comparing different indirect methods to measure portal pressure. J Vasc Interv Radiol. 2011;22:1553-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Jeong SW. Liver stiffness measurement: is it a non-invasive substitution for HVPG? Clin Mol Hepatol. 2013;19:367-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Deng H, Qi X, Guo X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Colecchia A, Marasco G, Taddia M, Montrone L, Eusebi LH, Mandolesi D, Schiumerini R, Di Biase AR, Festi D. Liver and spleen stiffness and other noninvasive methods to assess portal hypertension in cirrhotic patients: a review of the literature. Eur J Gastroenterol Hepatol. 2015;27:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Cho EJ, Kim MY, Lee JH, Lee IY, Lim YL, Choi DH, Kim YJ, Yoon JH, Baik SK. Diagnostic and Prognostic Values of Noninvasive Predictors of Portal Hypertension in Patients with Alcoholic Cirrhosis. PLoS One. 2015;10:e0133935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] |
| 15. | Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, Sohaey R, Verhey P, Peck-Radosavljevic M, Mansi C. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 17. | Berzigotti A, Gilabert R, Abraldes JG, Nicolau C, Bru C, Bosch J, García-Pagan JC. Noninvasive prediction of clinically significant portal hypertension and esophageal varices in patients with compensated liver cirrhosis. Am J Gastroenterol. 2008;103:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Sebastiani G, Tempesta D, Fattovich G, Castera L, Halfon P, Bourliere M, Noventa F, Angeli P, Saggioro A, Alberti A. Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol. 2010;53:630-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Augustin S, Millán L, González A, Martell M, Gelabert A, Segarra A, Serres X, Esteban R, Genescà J. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 20. | Bolognesi M, Sacerdoti D, Merkel C, Gerunda G, Maffei-Faccioli A, Angeli P, Jemmolo RM, Bombonato G, Gatta A. Splenic Doppler impedance indices: influence of different portal hemodynamic conditions. Hepatology. 1996;23:1035-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Kim MY, Jeong WK, Baik SK. Invasive and non-invasive diagnosis of cirrhosis and portal hypertension. World J Gastroenterol. 2014;20:4300-4315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Lee CM, Jeong WK, Lim S, Kim Y, Kim J, Kim TY, Sohn JH. Diagnosis of Clinically Significant Portal Hypertension in Patients with Cirrhosis: Splenic Arterial Resistive Index versus Liver Stiffness Measurement. Ultrasound Med Biol. 2016;42:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Iwao T, Toyonaga A, Oho K, Tayama C, Masumoto H, Sakai T, Sato M, Tanikawa K. Value of Doppler ultrasound parameters of portal vein and hepatic artery in the diagnosis of cirrhosis and portal hypertension. Am J Gastroenterol. 1997;92:1012-1017. [PubMed] |
| 24. | Bolognesi M, Sacerdoti D, Merkel C, Bombonato G, Gatta A. Noninvasive grading of the severity of portal hypertension in cirrhotic patients by echo-color-Doppler. Ultrasound Med Biol. 2001;27:901-907. [PubMed] |
| 25. | Piscaglia F, Donati G, Serra C, Muratori R, Solmi L, Gaiani S, Gramantieri L, Bolondi L. Value of splanchnic Doppler ultrasound in the diagnosis of portal hypertension. Ultrasound Med Biol. 2001;27:893-899. [PubMed] |
| 26. | Zardi EM, Di Matteo FM, Pacella CM, Sanyal AJ. Invasive and non-invasive techniques for detecting portal hypertension and predicting variceal bleeding in cirrhosis: a review. Ann Med. 2014;46:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Bolognesi M, Sacerdoti D, Bombonato G, Merkel C, Sartori G, Merenda R, Nava V, Angeli P, Feltracco P, Gatta A. Change in portal flow after liver transplantation: effect on hepatic arterial resistance indices and role of spleen size. Hepatology. 2002;35:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Komatsuda T, Ishida H, Konno K, Hamashima Y, Ohnami Y, Naganuma H, Asanuma Y, Masamune O. Color Doppler findings of gastrointestinal varices. Abdom Imaging. 1998;23:45-50. [PubMed] |
| 29. | Sato T, Yamazaki K, Toyota J, Karino Y, Ohmura T, Suga T. Color Doppler findings of gastric varices compared with findings on computed tomography. J Gastroenterol. 2002;37:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Sato T, Yamazaki K, Toyota J, Karino Y, Ohmura T, Akaike J. Diagnosis of rectal varices via color Doppler ultrasonography. Am J Gastroenterol. 2007;102:2253-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Sato T, Yamazaki K, Akaike J. Diagnosis of gastric varices and evaluation of the effectiveness of treatment using transabdominal color Doppler ultrasonography. J Ultrasound Med. 2009;28:1125-1131. [PubMed] |
| 32. | Maruyama H, Shiha G, Yokosuka O, Kumar A, Sharma BC, Ibrahim A, Saraswat V, Lesmana CR, Omata M. Non-invasive assessment of portal hypertension and liver fibrosis using contrast-enhanced ultrasonography. Hepatol Int. 2016;10:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Berzigotti A, Nicolau C, Bellot P, Abraldes JG, Gilabert R, García-Pagan JC, Bosch J. Evaluation of regional hepatic perfusion (RHP) by contrast-enhanced ultrasound in patients with cirrhosis. J Hepatol. 2011;55:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Qu EZ, Zhang YC, Li ZY, Liu Y, Wang JR. Contrast-enhanced sonography for quantitative assessment of portal hypertension in patients with liver cirrhosis. J Ultrasound Med. 2014;33:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Dave JK, Halldorsdottir VG, Eisenbrey JR, Raichlen JS, Liu JB, McDonald ME, Dickie K, Wang S, Leung C, Forsberg F. Noninvasive LV pressure estimation using subharmonic emissions from microbubbles. JACC Cardiovasc Imaging. 2012;5:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Dave JK, Halldorsdottir VG, Eisenbrey JR, Merton DA, Liu JB, Zhou JH, Wang HK, Park S, Dianis S, Chalek CL. Investigating the efficacy of subharmonic aided pressure estimation for portal vein pressures and portal hypertension monitoring. Ultrasound Med Biol. 2012;38:1784-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Eisenbrey JR, Dave JK, Halldorsdottir VG, Merton DA, Miller C, Gonzalez JM, Machado P, Park S, Dianis S, Chalek CL. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;268:581-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 39. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 527] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 40. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 41. | Lemoine M, Katsahian S, Ziol M, Nahon P, Ganne-Carrie N, Kazemi F, Grando-Lemaire V, Trinchet JC, Beaugrand M. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol-related cirrhosis. Aliment Pharmacol Ther. 2008;28:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 151] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 42. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (3)] |
| 43. | Sacerdoti D, Pesce P, Di Pascoli M, Brocco S, Cecchetto L, Bolognesi M. Arachidonic acid metabolites and endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 2015;120:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Di Pascoli M, Zampieri F, Verardo A, Pesce P, Turato C, Angeli P, Sacerdoti D, Bolognesi M. Inhibition of epoxyeicosatrienoic acid production in rats with cirrhosis has beneficial effects on portal hypertension by reducing splanchnic vasodilation. Hepatology. 2016;64:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, Bureau C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 46. | Kitson MT, Roberts SK, Colman JC, Paul E, Button P, Kemp W. Liver stiffness and the prediction of clinically significant portal hypertension and portal hypertensive complications. Scand J Gastroenterol. 2015;50:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: current status and future perspectives. Eur J Radiol. 2014;83:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Kim TY, Jeong WK, Sohn JH, Kim J, Kim MY, Kim Y. Evaluation of portal hypertension by real-time shear wave elastography in cirrhotic patients. Liver Int. 2015;35:2416-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Şirli R, Sporea I, Popescu A, Dănilă M. Ultrasound-based elastography for the diagnosis of portal hypertension in cirrhotics. World J Gastroenterol. 2015;21:11542-11551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC, Bosch J. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol. 2015;62:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 52. | Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A, Durand F, Valla D, Lebrec D. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 53. | Choi SY, Jeong WK, Kim Y, Kim J, Kim TY, Sohn JH. Shear-wave elastography: a noninvasive tool for monitoring changing hepatic venous pressure gradients in patients with cirrhosis. Radiology. 2014;273:917-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 54. | Attia D, Schoenemeier B, Rodt T, Negm AA, Lenzen H, Lankisch TO, Manns M, Gebel M, Potthoff A. Evaluation of Liver and Spleen Stiffness with Acoustic Radiation Force Impulse Quantification Elastography for Diagnosing Clinically Significant Portal Hypertension. Ultraschall Med. 2015;36:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 56. | Colecchia A, Colli A, Casazza G, Mandolesi D, Schiumerini R, Reggiani LB, Marasco G, Taddia M, Lisotti A, Mazzella G. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014;60:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Chin JL, Chan G, Ryan JD, McCormick PA. Spleen stiffness can non-invasively assess resolution of portal hypertension after liver transplantation. Liver Int. 2015;35:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis. 2002;34:144-150. [PubMed] |
| 59. | Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Kim DY. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 60. | Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, Pinzani M, Bosch J. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144:102-111.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 62. | Petta S, Maida M, Macaluso FS, Di Marco V, Cammà C, Cabibi D, Craxì A. The severity of steatosis influences liver stiffness measurement in patients with nonalcoholic fatty liver disease. Hepatology. 2015;62:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 63. | Ronot M, Lambert S, Elkrief L, Doblas S, Rautou PE, Castera L, Vilgrain V, Sinkus R, Van Beers BE, Garteiser P. Assessment of portal hypertension and high-risk oesophageal varices with liver and spleen three-dimensional multifrequency MR elastography in liver cirrhosis. Eur Radiol. 2014;24:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 64. | Qi X, Li Z, Huang J, Zhu Y, Liu H, Zhou F, Liu C, Xiao C, Dong J, Zhao Y. Virtual portal pressure gradient from anatomic CT angiography. Gut. 2015;64:1004-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 835] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 66. | Guo J, Büning C, Schott E, Kröncke T, Braun J, Sack I, Althoff C. In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Invest Radiol. 2015;50:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Palaniyappan N, Cox E, Bradley C, Scott R, Austin A, O’Neill R, Ramjas G, Travis S, White H, Singh R. Non-invasive assessment of portal hypertension using quantitative magnetic resonance imaging. J Hepatol. 2016;65:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 68. | Kazemi F, Kettaneh A, N’kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, Beaugrand M. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol. 2006;45:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 268] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 69. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 273] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 70. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92-101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 71. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Doi A, Matsueda K, Yamamoto H. Prediction of oesophageal variceal bleeding by measuring spleen stiffness in patients with liver cirrhosis. Gut. 2016;65:354-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, Zheng MH. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013;33:62-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 73. | Bureau C, Di Martino V, Calès P. A major new step in non-invasive evaluation of portal hypertension: elastography. Liver Int. 2013;33:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |