Copyright
©The Author(s) 2016.
World J Gastroenterol. Feb 28, 2016; 22(8): 2415-2423
Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2415
Published online Feb 28, 2016. doi: 10.3748/wjg.v22.i8.2415
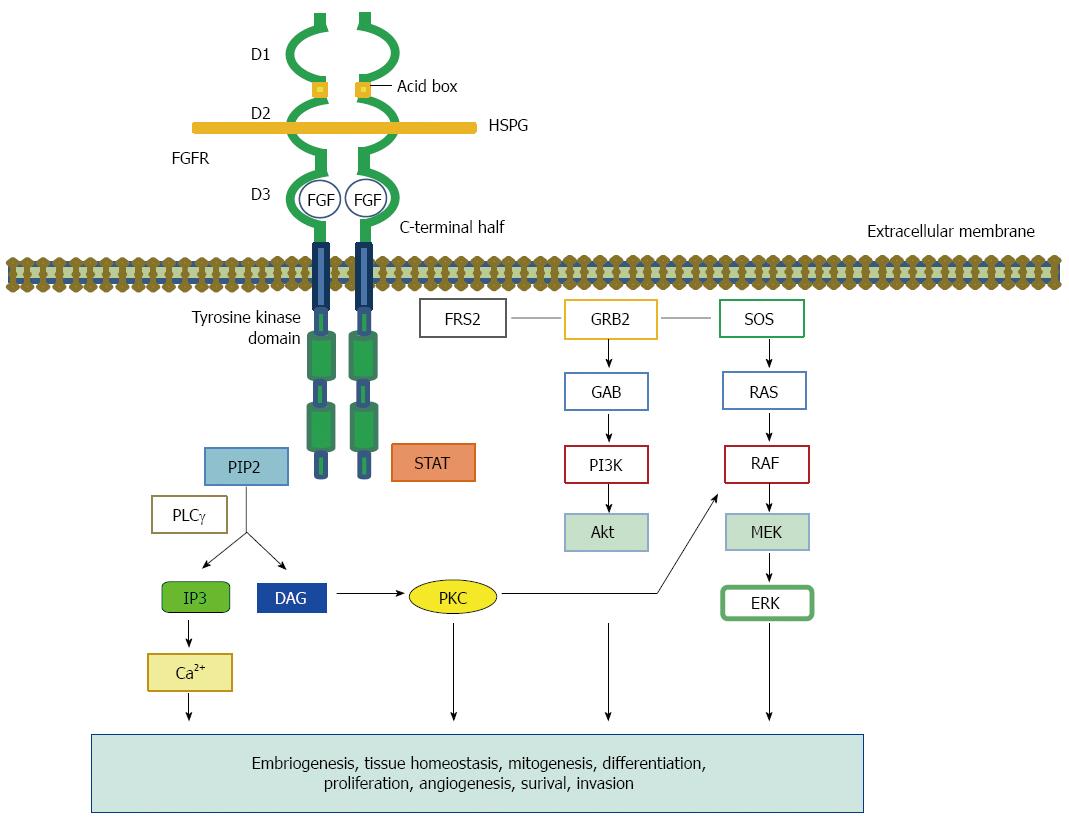
Figure 1 The fiblobrast growth factor structure and signaling network.
The Fibroblast growth factor (FGF)-FGFR complex consists of two receptor molecules, two FGFs, and a heparan sulfate proteoglycan (HSPG). The HSPG stabilizes and sequesters the FGFs. The FGFR comprises three extracellular Ig-like domains (D1-3), a transmembrane helix, and an intracellular split tyrosine kinase domain. D1 and D3 are separated by an acidic box. Ligand specificity of FGFR is regulated by alternative splicing within the C-terminal half of the third Ig loop (D3) in the ligand binding domain. Ligand binding and FGFR dimerization trigger the kinase domain phosphorylation, which leads to the docking of adapter residues and the activation of downstream pathways. Ligand-stimulated FGFRs phosphorylate the FGFR-associated cytosolic docking protein, FRS2. FRS2 activates SOS and GRB2, which in turn activate the Ras-Raf-MAPK pathway. A different complex involves GAB1, which activates the PI3K-Akt pathway. PLCγ hydrolyzes PIP2 to IP3 and DAG. PIP3 produces calcium. DAG also activates PKC, which results in the activation of the MAPK pathway by phosphorylating Raf. HSPG: Heparan sulfate proteoglycan; DAG: Diacylglycerol; FRS2α: FGFR substrate 2α; GRB2: Growth factor receptor-bound 2; IP3: Inositol triphosphate; PI3K: Phosphoinositide 3-kinase; PIP: 2-phosphatidylinositol-4,5-biphosphate; PKC: Protein kinase C; SOS: Son of sevenless; GAB1: GRB2-associated binding protein 1; PLCγ: Phospholipase Cγ.
- Citation: Yashiro M, Matsuoka T. Fibroblast growth factor receptor signaling as therapeutic targets in gastric cancer. World J Gastroenterol 2016; 22(8): 2415-2423
- URL: https://www.wjgnet.com/1007-9327/full/v22/i8/2415.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i8.2415