Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10592
Peer-review started: July 20, 2016
First decision: September 20, 2016
Revised: October 17, 2016
Accepted: November 23, 2016
Article in press: November 28, 2016
Published online: December 28, 2016
Processing time: 160 Days and 23.7 Hours
To clarify risk based upon segment length, diagnostic histological findings, patient age and year of surveillance, duration of surveillance and gender.
Patients registered with the United Kingdom Barrett’s Oesophagus Registry from 9 United Kingdom centers were included. The outcome measures were (1) development of all grades of dysplasia; (2) development of high-grade of dysplasia or adenocarcinoma; and (3) development of adenocarcinoma. Prevalent cases and subjects with < 1 year of follow-up were excluded. The covariates examined were segment length, previous biopsy findings, age at surveillance, duration of surveillance, year of surveillance and gender.
One thousand and one hundred thirty six patients were included (total 6474 patient-years). Fifty-four patients developed adenocarcinoma (0.83% per annum), 70 developed high-grade dysplasia/adenocarcinoma (1.1% per annum) and 190 developed any grade of dysplasia (3.5% per annum). High grade dysplasia and adenocarcinoma increased with age and duration of surveillance. The risk of low-grade dysplasia development was not dependent on age at surveillance. Segment length and previous biopsy findings were also significant factors for development of dysplasia and adenocarcinoma.
The risk of development of low-grade dysplasia is independent of age at surveillance, but high-grade dysplasia and adenocarcinoma were more commonly found at older age. Segment length and previous biopsy findings are also markers of risk. This study did not demonstrate stabilisation of the metaplastic segment with prolonged surveillance.
Core tip: Current surveillance guidelines for Barrett’s oesophagus base the enrolment into surveillance and surveillance interval on segment length, presence or absence of intestinal metaplasia and dysplasia. This study demonstrates the importance of age as an important risk factor for high-grade dysplasia and adenocarcinoma development and that stabilisation of the epithelium does not reliably occur at long-term follow-up.
- Citation: Gatenby P, Bhattacharjee S, Wall C, Caygill C, Watson A. Risk stratification for malignant progression in Barrett’s esophagus: Gender, age, duration and year of surveillance. World J Gastroenterol 2016; 22(48): 10592-10600
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10592.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10592
The incidence of adenocarcinoma in Barrett’s esophagus has been estimated to be around 0.5% per annum[1-3] and 0.32% in patients without dysplasia at index endoscopy[4]. This would suggest a lifetime risk of around 1:8 to 1:14 of developing adenocarcinoma and 1:5 to 1:6 of developing either high-grade dysplasia or adenocarcinoma[5]. A meta-analysis has shown a trend for a decrease in incidence of adenocarcinoma to be observed with time (which did not reach statistical significance, P = 0.117)[3] and in recent years large population-based cohort studies[6-8] have demonstrated lower adenocarcinoma incidence rates (0.22%, 0.43% and 0.12% per annum respectively). We have observed that the age at diagnosis of patients with Barrett’s esophagus is falling[9] and that the life expectancy of those diagnosed with Barrett’s esophagus is increasing[5]. The Danish pathology registry has demonstrated in their cohort that the adenocarcinoma incidence in Barrett’s increases with older age[8].
The currently accepted principal risk factors for dysplasia and cancer development in Barrett’s oesophagus are presence/absence of intestinal metaplasia, dysplasia and segment length[10-12]. These are the factors on which guidance for surveillance intervals are determined.
These observations prompt examination of what the time trends in cancer incidence in Barrett’s esophagus are: Are the annual incidences of dysplasia and cancer changing in the population with Barrett’s esophagus undergoing surveillance? Does an individual’s risk change over time dependent on the patient’s age at the time of surveillance? Does the Barrett’s segment stabilize with prolonged follow-up such that patients might be reassured and discharged from further follow-up?
This study seeks to examine whether there is a demonstrable change in incidence of dysplasia and adenocarcinoma over time in patients undergoing surveillance of Barrett’s esophagus who are registered with the United Kingdom Barrett’s Oesophagus Registry and which are the most important demographic, histological and endoscopic features with regard to dysplasia and cancer risk.
One thousand one hundred and thirty six patients who had been registered with the United Kingdom Barrett’s Oesophagus Registry from 9 centers who did not have prevalent adenocarcinoma (diagnosed at index endoscopy or within one year of the index endoscopy) and who had a minimum of one year of follow-up were included in the study cohort. The three outcome measures were (1) development of any grade of dysplasia; (2) development of high-grade dysplasia or adenocarcinoma; and (3) development of adenocarcinoma. Follow-up time commenced at the diagnostic biopsy and was censored at the first biopsy reported as demonstrating the histological outcome or, when this was not attained, the final surveillance endoscopy and biopsy. The influence of 7 factors was then considered to provide further clarity as to an individual’s risk of development of dysplasia and cancer. These were: (1) date (year) at which surveillance biopsies were undertaken; (2) age of the patient at which surveillance endoscopy and biopsy were undertaken; (3) length of time during which the patient had been undergoing surveillance; (4) patient gender; (5) segment length; (6) histological findings at the most recent (previous) endoscopy; and (7) histological findings at first and second endoscopies (in keeping with national guidelines on enrolment into surveillance programmes[10-12]).
Classification of histological results were: columnar-lined oesophagus without intestinal metaplasia, columnar-lined oesophagus with intestinal metaplasia, indefinite changes for dysplasia, low-grade dysplasia, high-grade dysplasia and adenocarcinoma.
The associations of dysplasia/adenocarcinoma risk with age at surveillance, year of surveillance and duration of surveillance were examined.
The associations between these factors and risk of development of dysplasia or cancer were examined using binary logistic regression. The model was modified to exclude factors which did not reach statistical significance with removal of factors which showed no association with dysplasia or cancer risk. Due to the co-linearity of age, surveillance duration and year of surveillance, only the most closely-associated of these three variables was included in the final analyses.
Segment length data were available for 92.4% of cases and the analyses were repeated with the exclusion of segment length. Segment length was examined as a continuous variable and when separated into three similarly sized and clinically relevant groups: short segment (< 3 cm), 3-5 cm and > 5 cm.
Approval for studies of this kind conducted by UKBOR was given by the London Multi-Centre Research Ethics Committee on 14th March 2002 number MREC/02/2/5.
Incidence calculations were undertaken using a patient-years at risk method and expressed as an annual percentage (cases per one hundred patient-years follow-up) and 95%CI were evaluated using an exact Poisson distribution. Logistic regression to ascertain the magnitude of the effect of the covariates was undertaken. P values < 0.05 were taken to be statistically significant.
The 1136 patients who fulfilled the criteria for inclusion in the study cohort comprised 783 males and 353 females diagnosed between 1974 and 2009. The mean age at diagnosis was 59.8 years, mean follow-up was 5.70 years and total follow-up was 6474 patient-years. The mean age at surveillance was 62.3 years, with a small trend for this to rise in males (60 in the 1990s, 61 in 2000-2004 and 62 from 2005 onwards), but no change in females (mean age at surveillance 65). During the follow-up period 54 patients developed adenocarcinoma, 70 developed either high-grade dysplasia or adenocarcinoma and 190 developed changes of any grade of dysplasia or cancer. The overall annual incidence of development of adenocarcinoma was 0.83% per annum, of high-grade dysplasia or adenocarcinoma, 1.1% per annum and of all grades of dysplasia and adenocarcinoma, 3.5% per annum.
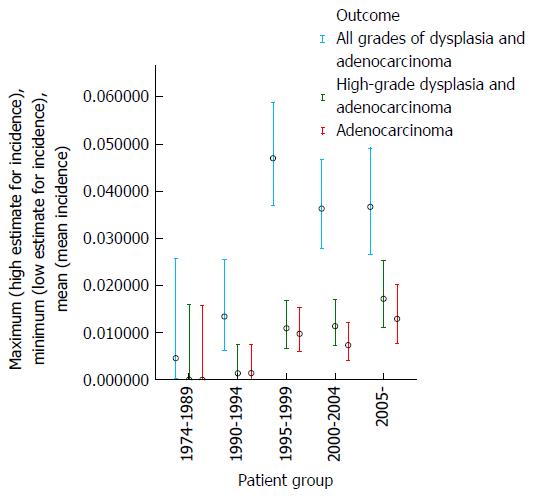
During the first 15 years of the cohort from 1974-1989 only a small number of patients (76) were diagnosed and underwent surveillance, hence these patients have been grouped together; thereafter, dysplasia and adenocarcinoma incidence were examined over consecutive 5 year periods (Table 1).
| Calendar years | Total patient-years follow-up | Adenocarcinoma | High-grade dysplasia and adenocarcinoma | All grades of dysplasia and adenocarcinoma | |||
| Number of cases | Annual incidence | Number of cases | Annual incidence | Number of cases | Annual incidence | ||
| 1974-1989 | 237 | 0 | 0.00% | 0 | 0.00% | 1 | 0.46% |
| 1990-1994 | 753 | 1 | 0.13% | 1 | 0.13% | 9 | 1.33% |
| 1995-1999 | 1950 | 19 | 0.97% | 21 | 1.09% | 76 | 4.70% |
| 2000-2004 | 2058 | 15 | 0.73% | 23 | 1.12% | 60 | 3.63% |
| 2005- | 1477 | 19 | 1.29% | 25 | 1.17% | 44 | 3.66% |
| Total | 6474 | 54 | 0.83% | 70 | 1.09% | 190 | 3.54% |
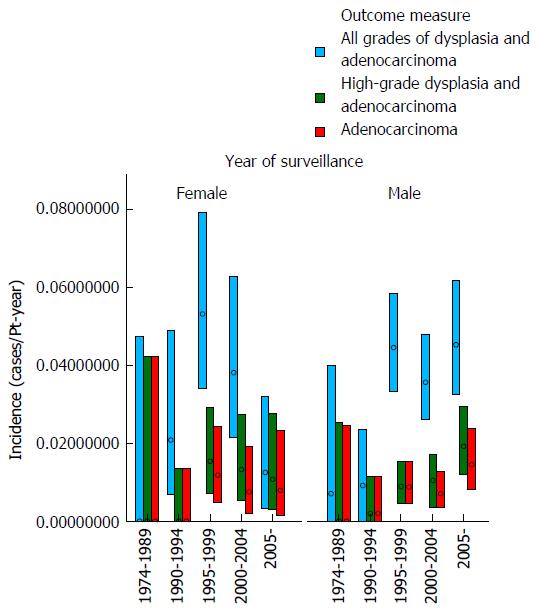
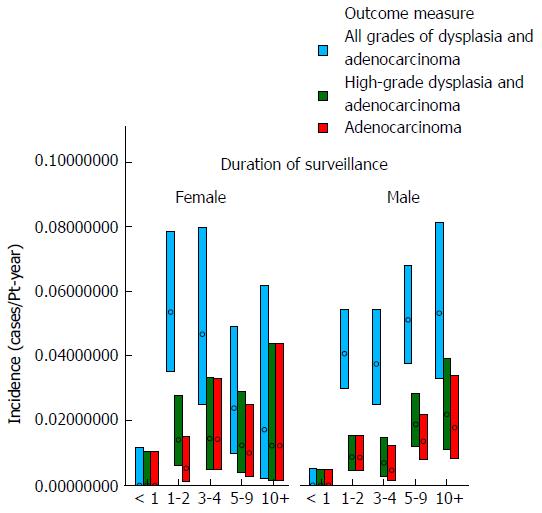
These data demonstrate that there was no clear trend for the incidence of either dysplasia or adenocarcinoma to fall appreciably during the study period (as examined by year of surveillance). The mean age at surveillance remained constant throughout the cohort; however there was an increased proportion of males in the latter portion of the cohort. When males and females were examined separately, there was no demonstrable change in the incidence of either adenocarcinoma or high-grade dysplasia for females with year of surveillance and a trend for a slight increase in adenocarcinoma incidence with later year of surveillance for males. The adenocarcinoma and high-grade dysplasia incidences were similar in males and females, but slightly lower in females. These results are depicted graphically in Figures 1 and 2.
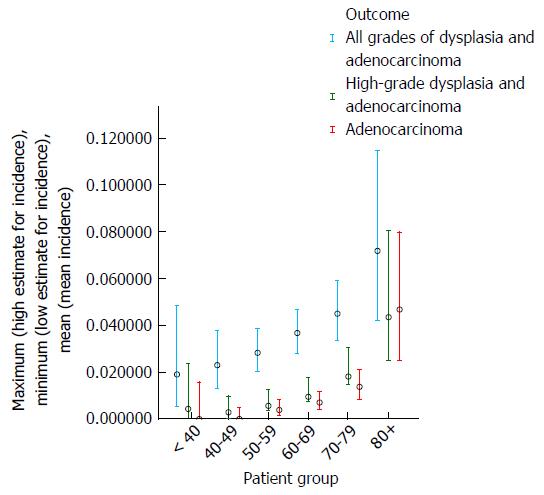
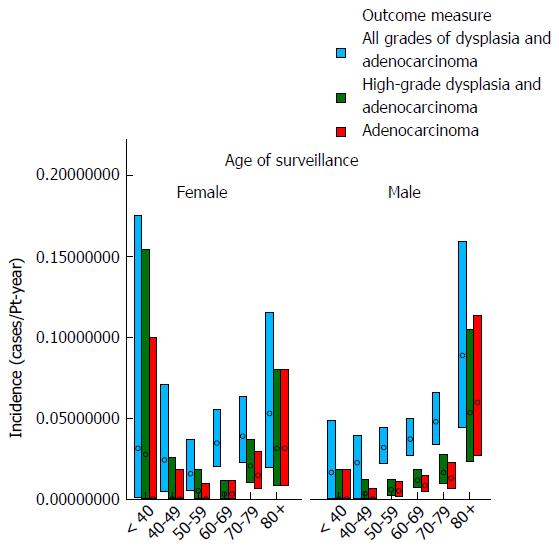
Table 2 and Figures 3 and 4 show the effect of age at surveillance on the detection of dysplasia and adenocarcinoma. This demonstrates a trend for an increasing risk of dysplasia detection as age at surveillance increases. When males and females were analysed separately, there was a trend for higher rates of dysplasia and adenocarcinoma incidence in males compared to females for specific decade of age at surveillance, but overall similar rates due to females being older than males at surveillance (40% of surveillance of females within the cohort occurring at age > 70, compared to 25% for males).
| Age at surveillance | Total patient-years follow-up | Adenocarcinoma | High-grade dysplasia and adenocarcinoma | All grades of dysplasia and adenocarcinoma | |||
| Number of cases | Annual incidence | Number of cases | Annual incidence | Number of cases | Annual incidence | ||
| < 40 | 236 | 0 | 0.00% | 1 | 0.42% | 4 | 1.89% |
| 40-49 | 740 | 0 | 0.00% | 2 | 0.27% | 15 | 2.30% |
| 50-59 | 1602 | 6 | 0.37% | 9 | 0.56% | 39 | 2.82% |
| 60-69 | 2154 | 15 | 0.70% | 20 | 0.94% | 63 | 3.66% |
| 70-79 | 1464 | 20 | 1.37% | 26 | 1.81% | 52 | 4.49% |
| 80+ | 278 | 13 | 4.67% | 12 | 4.34% | 17 | 7.18% |
| Total | 6474 | 54 | 0.83% | 70 | 1.09% | 190 | 3.54% |
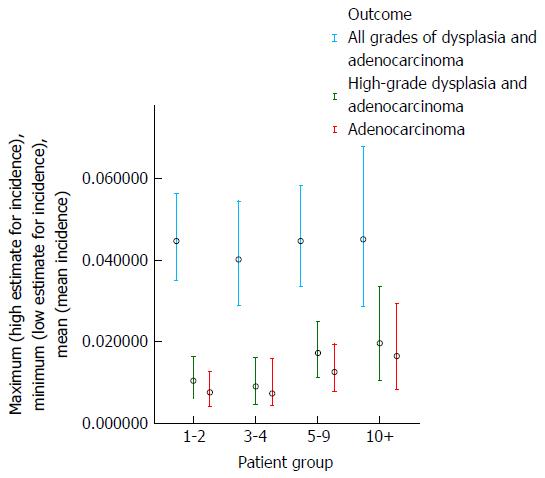
The results of the analysis which examined whether the risk of development of dysplasia and adenocarcinoma remains constant during the course of an individual patient’s surveillance are shown in Table 3 and Figures 5 and 6. These demonstrate that there is a trend for increasing risk of development of high-grade dysplasia and adenocarcinoma with increasing duration of surveillance, but not of development of low-grade dysplasia. When males and females were analyzed separately, there did not appear to be a significant effect of gender on the results.
| Duration of surveillance (yr) | Total patient-years follow-up | Adenocarcinoma | High-grade dysplasia and adenocarcinoma | All grades of dysplasia and adenocarcinoma | |||
| Number of cases | Annual incidence | Number of cases | Annual incidence | Number of cases | Annual incidence | ||
| 1-2 | 1848 | 14 | 0.76% | 19 | 1.04% | 72 | 4.47% |
| 3-4 | 1235 | 9 | 0.73% | 11 | 0.90% | 41 | 4.02% |
| 5-9 | 1587 | 20 | 1.26% | 27 | 1.72% | 54 | 4.47% |
| 10+ | 668 | 11 | 1.65% | 13 | 1.96% | 23 | 4.52% |
Initially all 7 variables were included in the logistic regression analyses. Gender was excluded as there was no association with the outcome measures and year at surveillance and duration of surveillance were also excluded as the association between the outcome variables was strongest with age at surveillance. For the outcome variables of high-grade dysplasia and adenocarcinoma: the final model showed that the statistically significantly associated factors were age at surveillance, segment length and previous histological biopsy grading. For the development of all grades of dysplasia and cancer: segment length and the findings at the first two biopsies reached statistical significance. These results are shown in Table 4.
| Factor | Development of all grades of dysplasia and cancer | Development of high-grade dysplasia or cancer | Development of cancer |
| Age | P = 0.187 | P = 0.400 | P = 0.034 |
| Segment length | P = 0.043 | P = 0.121 | P = 0.129 |
| Most recent biopsy results | P = 0.058 | P = 0.047 | |
| First two biopsy results | P < 0.001 |
These results demonstrate that there was no clear evidence of any change in overall adenocarcinoma or dysplasia incidence throughout the cohort period nor for the risk of development of all grades of dysplasia to change with increasing duration of follow-up. The risk of development of high-grade dysplasia and adenocarcinoma tended to increase with increased duration of surveillance, but more strikingly: there was a relationship between older age at surveillance and higher rate of detection of high-grade dysplasia and adenocarcinoma.
These observations demonstrate that the metaplastic segment does not remain stable with prolonged follow-up: the rate of development of all grades of dysplasia remained around 3.5% per annum and there was a trend for increased risk of high-grade dysplasia and adenocarcinoma development at increased duration of follow-up in common with the Dutch cohort[7].
From the logistic regression, the most significant time-dependent factor is age at surveillance (with increasing risks of development of high-grade dysplasia or cancer at older age).
Subsequently, this analysis has not demonstrated that after a prolonged period of stability there are patients who might be safely discharged from surveillance.
This observation would suggest that younger patients could undergo less intensive surveillance than older patients (with respect to development of high-grade dysplasia or adenocarcinoma), however younger patients potentially have longer periods of exposure to the risk of developing these changes and conceivably an increased lifetime risk[13]. These observations parallel those on esophageal cancer incidence in the United Kingdom population[14]. We have also observed that the average age at diagnosis of Barrett’s esophagus is falling[9], which, whilst this may subsequently result in a reduction in the annual incidence of adenocarcinoma for the surveillance cohort overall, it will not result in any risk reduction for an individual patient[5].
Overall, females and males had similar risk of development of dysplasia and cancer in this cohort, however it is likely that this is in part due to the larger proportion of females undergoing surveillance at older age compared to males and that the risk for females when age-matched is lower than in males. Similar results were shown in the Dutch national cohort[7]. With higher life expectancy for females, this is an important consideration and this study does not provide data to support the suggestion that females might be safely discharged from surveillance. The Dutch cohort also confirmed the high cancer risk in older females[7]. Different proportions of older patients undergoing surveillance may explain some of the variability seen in the incidence of high-grade dysplasia and adenocarcinoma in published studies.
The risk for any individual patient will depend on a number of factors, some of which are modifiable and others which cannot be modified[15]. Caucasian ethnic origin[16], male gender[6,8,17], older age at diagnosis[8,18,19], longer Barrett’s segment length[3,17,20,21] and the presence of dysplasia at diagnosis[6,8,20,22] have each been reported to be markers of higher malignant risk determined at the time of diagnosis. Longer duration of surveillance was also associated with increased cancer risk, but without separately examining the age at which surveillance was undertaken[20]. The role of intestinal metaplasia remains controversial[1,6,23-26]. Obesity[17,27,28], optimal reflux control[29-35], diet (poor intake of fruit, vegetables and anti-oxidants)[17,36,37], smoking[17,28,38,39] and medication use (particularly aspirin[40], non-steroidal anti-inflammatory drugs[41-43] and statins[40,44-46]) will influence an individual’s risk at the time of diagnosis and subsequently. These modifiable factors should be addressed by public health programs and medical care. Additionally, absence of Helicobacter Pylori infection has been associated with higher prevalence of Barrett’s esophagus and dysplasia than in subjects who have had Helicobacter Pylori infection[47]. It seems likely that much of the fate of the metaplastic segment is determined prior to the time of diagnosis. Once diagnosed, reflux control, smoking, medication use, diet and control of obesity may be managed, but unless the potentially malignant tissue is either completely resected or ablated, cancer risk in this tissue remains.
Encouraging data on the role of endoscopic ablation of the metaplastic segment have now been published, however long-term follow-up with respect to cancer development is awaited[48-50].
Unfortunately, the incidence of Barrett’s esophagus[51-55] and esophageal adenocarcinoma[56-58] are increasing and without improved strategies to reduce the risk of cancer development within the population at risk and the size of this population, the number of cases of esophageal adenocarcinoma is likely to continue to rise[13].
The results of this study demonstrate that age at surveillance is an important factor for high-grade dysplasia and adenocarcinoma development and should be incorporated (with segment length and previous biopsy findings) into risk assessment in Barrett’s oesophagus surveillance.
We would like to thank the registrants for enabling the work to take place (see Supplementary Table).
The age at diagnosis of patients with Barrett’s esophagus is falling and that the life expectancy of those diagnosed with Barrett’s esophagus is increasing. The Danish pathology registry has demonstrated in their cohort that the adenocarcinoma incidence in Barrett’s increases with older age.
The currently accepted principal risk factors for dysplasia and cancer development in Barrett’s oesophagus are presence/absence of intestinal metaplasia, dysplasia and segment length. These are the factors on which guidance for surveillance intervals are determined.
Seek to clarify risk based upon segment length, diagnostic histological findings, patient age and year of surveillance, duration of surveillance and gender.
This study demonstrates the importance of age as an important risk factor for high-grade dysplasia and adenocarcinoma development and that stabilisation of the epithelium does not reliably occur at long-term follow-up.
Interesting and practical paper, the relation between Barrett's esophagus and adenocarcinoma was very important.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ono T, Stasek M S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Yousef F, Cardwell C, Cantwell MM, Galway K, Johnston BT, Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 2. | Sikkema M, de Jonge PJ, Steyerberg EW, Kuipers EJ. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235-244; quiz e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26:1465-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Desai TK, Krishnan K, Samala N, Singh J, Cluley J, Perla S, Howden CW. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: a meta-analysis. Gut. 2012;61:970-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Gatenby P, Caygill C, Wall C, Bhatacharjee S, Ramus J, Watson A, Winslet M. Lifetime risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. World J Gastroenterol. 2014;20:9611-9617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 513] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | de Jonge PJ, van Blankenstein M, Looman CW, Casparie MK, Meijer GA, Kuipers EJ. Risk of malignant progression in patients with Barrett’s oesophagus: a Dutch nationwide cohort study. Gut. 2010;59:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 8. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 976] [Article Influence: 69.7] [Reference Citation Analysis (1)] |
| 9. | Wall CM, Charlett A, Caygill CP, Gatenby PA, Ramus JR, Winslet MC, Watson A. Are newly diagnosed columnar-lined oesophagus patients getting younger? Eur J Gastroenterol Hepatol. 2009;21:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 871] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 11. | Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology. 2011;140:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 801] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 12. | Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 786] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 13. | Gatenby PA, Hainsworth A, Caygill C, Watson A, Winslet M. Projections for oesophageal cancer incidence in England to 2033. Eur J Cancer Prev. 2011;20:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Office for National Statistics: Cancer Registrations in England, 2010. In: Office for National Statistics. Newport, Wales, UK: UK Statistics Authority, 2012. . |
| 15. | Gatenby P, Soon Y. Barrett’s oesophagus: Evidence from the current meta-analyses. World J Gastrointest Pathophysiol. 2014;5:178-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Khoury JE, Chisholm S, Jamal MM, Palacio C, Pudhota S, Vega KJ. African Americans with Barrett’s esophagus are less likely to have dysplasia at biopsy. Dig Dis Sci. 2012;57:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Pohl H, Wrobel K, Bojarski C, Voderholzer W, Sonnenberg A, Rösch T, Baumgart DC. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | den Hoed CM, van Blankenstein M, Dees J, Kuipers EJ. The minimal incubation period from the onset of Barrett’s oesophagus to symptomatic adenocarcinoma. Br J Cancer. 2011;105:200-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Gatenby PA, Caygill CP, Ramus JR, Charlett A, Watson A. Barrett’s columnar-lined oesophagus: demographic and lifestyle associations and adenocarcinoma risk. Dig Dis Sci. 2008;53:1175-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Sikkema M, Looman CW, Steyerberg EW, Kerkhof M, Kastelein F, van Dekken H, van Vuuren AJ, Bode WA, van der Valk H, Ouwendijk RJ. Predictors for neoplastic progression in patients with Barrett’s Esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106:1231-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Gatenby PA, Caygill CP, Ramus JR, Charlett A, Fitzgerald RC, Watson A. Short segment columnar-lined oesophagus: an underestimated cancer risk? A large cohort study of the relationship between Barrett’s columnar-lined oesophagus segment length and adenocarcinoma risk. Eur J Gastroenterol Hepatol. 2007;19:969-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 22. | Gatenby P, Ramus J, Caygill C, Shepherd N, Winslet M, Watson A. Routinely diagnosed low-grade dysplasia in Barrett’s oesophagus: a population-based study of natural history. Histopathology. 2009;54:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kelty C, Gough M, Van Wyck Q, Stephenson T, Ackroyd R: Barrett’s oesophagus: Intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;21:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Gatenby PA, Ramus JR, Caygill CP, Shepherd NA, Watson A. Relevance of the detection of intestinal metaplasia in non-dysplastic columnar-lined oesophagus. Scand J Gastroenterol. 2008;43:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Dias Pereira A, Chaves P. Columnar-lined oesophagus without intestinal metaplasia: results from a cohort with a mean follow-up of 7 years. Aliment Pharmacol Ther. 2012;36:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Westerhoff M, Hovan L, Lee C, Hart J. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:1232-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Duggan C, Onstad L, Hardikar S, Blount PL, Reid BJ, Vaughan TL. Association between markers of obesity and progression from Barrett’s esophagus to esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PLoS One. 2013;8:e52192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Braghetto I, Korn O, Valladares H, Debandi A, Díaz JC, Brunet L. Laparoscopic surgical treatment for patients with short- and long-segment Barrett’s esophagus: which technique in which patient? Int Surg. 2011;96:95-103. [PubMed] |
| 30. | Zaninotto G, Parente P, Salvador R, Farinati F, Tieppo C, Passuello N, Zanatta L, Fassan M, Cavallin F, Costantini M. Long-term follow-up of Barrett’s epithelium: medical versus antireflux surgical therapy. J Gastrointest Surg. 2012;16:7-14; discussion 14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Li YM, Li L, Yu CH, Liu YS, Xu CF. A systematic review and meta-analysis of the treatment for Barrett’s esophagus. Dig Dis Sci. 2008;53:2837-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Corey KE, Schmitz SM, Shaheen NJ. Does a surgical antireflux procedure decrease the incidence of esophageal adenocarcinoma in Barrett’s esophagus? A meta-analysis. Am J Gastroenterol. 2003;98:2390-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Rees JR, Lao-Sirieix P, Wong A, Fitzgerald RC: Treatment for Barrett’s oesophagus. Cochrane Database Syst Rev. 2010;CD004060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Gatenby PA, Ramus JR, Caygill CP, Charlett A, Winslet MC, Watson A. Treatment modality and risk of development of dysplasia and adenocarcinoma in columnar-lined esophagus. Dis Esophagus. 2009;22:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Kastelein F, Spaander MC, Steyerberg EW, Biermann K, Valkhoff VE, Kuipers EJ, Bruno MJ. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Lukić M, Segec A, Segec I, Pinotić L, Pinotić K, Atalić B, Solić K, Vcev A. The impact of the vitamins A, C and E in the prevention of gastroesophageal reflux disease, Barrett’s oesophagus and oesophageal adenocarcinoma. Coll Antropol. 2012;36:867-872. [PubMed] |
| 37. | Ibiebele TI, Hughes MC, Nagle CM, Bain CJ, Whiteman DC, Webb PM. Dietary antioxidants and risk of Barrett’s esophagus and adenocarcinoma of the esophagus in an Australian population. Int J Cancer. 2013;133:214-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Coleman HG, Bhat S, Johnston BT, McManus D, Gavin AT, Murray LJ. Tobacco smoking increases the risk of high-grade dysplasia and cancer among patients with Barrett’s esophagus. Gastroenterology. 2012;142:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Ramus JR, Gatenby PA, Caygill CP, Watson A, Winslet MC. The relationship between smoking and severe dysplastic disease in patients with Barrett’s columnar-lined oesophagus. Eur J Cancer Prev. 2012;21:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett’s oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol. 2012;24:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Wang F, Lv ZS, Fu YK. Nonsteroidal anti-inflammatory drugs and esophageal inflammation - Barrett’s esophagus - adenocarcinoma sequence: a meta-analysis. Dis Esophagus. 2011;24:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Liao LM, Vaughan TL, Corley DA, Cook MB, Casson AG, Kamangar F, Abnet CC, Risch HA, Giffen C, Freedman ND. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442-452.e5; quiz e22-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Thrift AP, Pandeya N, Smith KJ, Green AC, Webb PM, Whiteman DC. The use of nonsteroidal anti-inflammatory drugs and the risk of Barrett’s oesophagus. Aliment Pharmacol Ther. 2011;34:1235-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Kantor ED, Onstad L, Blount PL, Reid BJ, Vaughan TL. Use of statin medications and risk of esophageal adenocarcinoma in persons with Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2012;21:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Alexandre L, Clark AB, Cheong E, Lewis MP, Hart AR. Systematic review: potential preventive effects of statins against oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2012;36:301-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 47. | Thrift AP, Pandeya N, Smith KJ, Green AC, Hayward NK, Webb PM, Whiteman DC. Helicobacter pylori infection and the risks of Barrett’s oesophagus: a population-based case-control study. Int J Cancer. 2012;130:2407-2416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Phoa KN, van Vilsteren FG, Weusten BL, Bisschops R, Schoon EJ, Ragunath K, Fullarton G, Di Pietro M, Ravi N, Visser M. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 437] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 49. | Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 968] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 50. | Fleischer DE, Overholt BF, Sharma VK, Reymunde A, Kimmey MB, Chuttani R, Chang KJ, Muthasamy R, Lightdale CJ, Santiago N. Endoscopic radiofrequency ablation for Barrett’s esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, Quesenberry C, Buffler P. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994-2006. Gut. 2009;58:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 52. | Caygill CP, Reed PI, Johnston BJ, Hill MJ, Ali MH, Levi S. A single centre’s 20 years’ experience of columnar-lined (Barrett’s) oesophagus diagnosis. Eur J Gastroenterol Hepatol. 1999;11:1355-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Coleman HG, Bhat S, Murray LJ, McManus D, Gavin AT, Johnston BT. Increasing incidence of Barrett’s oesophagus: a population-based study. Eur J Epidemiol. 2011;26:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 55. | Alexandropoulou K, van Vlymen J, Reid F, Poullis A, Kang JY. Temporal trends of Barrett’s oesophagus and gastro-oesophageal reflux and related oesophageal cancer over a 10-year period in England and Wales and associated proton pump inhibitor and H2RA prescriptions: a GPRD study. Eur J Gastroenterol Hepatol. 2013;25:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103:2694-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 57. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, van Sandick JW, Cats A, Verheij M, Coebergh JW. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |