Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9984
Peer-review started: September 12, 2016
First decision: October 11, 2016
Revised: October 18, 2016
Accepted: November 15, 2016
Article in press: November 16, 2016
Published online: December 7, 2016
To investigate the clinical significance of routinely used glycemic parameters in a cohort of colorectal cancer (CRC) patients.
Pre-treatment fasting blood glucose, insulin, HbA1c and homeostasis model of risk assessment (HOMA-IR) were retrospectively evaluated in a case-control study of 224 CRC and 112 control subjects matched for sex, obesity and diabetes frequency and blood lipid profile. Furthermore, the prognostic value of routinely used glycemic parameters towards progression-free (PFS) and overall survival (OS) was prospectively evaluated.
Fasting blood glucose, insulin, HOMA-IR and HbA1c (all P < 0.0001) levels were higher in non-diabetic CRC patients compared with obesity-matched controls. All parameters were associated with increased CRC risk at ROC analysis, but no relationship with clinical-pathological variables or survival outcomes was observed for glycemia, insulinemia or HOMA-IR. Conversely, advanced CRC stage (P = 0.018) was an independent predictor of increased HbA1c levels, which were also higher in patients who had disease progression compared with those who did not (P = 0.05). Elevated HbA1c levels showed a negative prognostic value both in terms of PFS (HR = 1.24) and OS (HR = 1.36) after adjustment for major confounders, which was further confirmed in a subgroup analysis performed after exclusion of diabetic patients.
HbA1c might have a negative prognostic value in CRC, thus suggesting that glycemic metabolic markers should be carefully monitored in these patients, independently of overt diabetes.
Core tip: The clinical significance of routinely used pre-treatment fasting blood glucose, insulin, HbA1c and homeostasis model of risk assessment was investigated in a cohort of colorectal cancer (CRC) patients. Despite all four metabolic markers were elevated in non-diabetic CRC patients, only elevated HbA1c levels were significantly associated with advanced CRC stage and disease progression, showing a negative prognostic value both in terms of progression-free (HR = 1.24) and overall (HR = 1.36) survival after adjustment for major confounders. These results suggest that glycemic metabolic markers, mainly HbA1c, should be carefully monitored in CRC patients as they could provide important risk stratification information.
- Citation: Ferroni P, Formica V, Della-Morte D, Lucchetti J, Spila A, D'Alessandro R, Riondino S, Guadagni F, Roselli M. Prognostic value of glycated hemoglobin in colorectal cancer. World J Gastroenterol 2016; 22(45): 9984-9993
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9984.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9984
Type 2 diabetes (T2D) and colorectal cancer (CRC) are among the major causes of morbidity and mortality worldwide, and their increasing prevalence represents a significant public health burden[1-3]. Several epidemiological studies and meta-analyses of published trials suggested that there is a significant association between T2D and increased risk of CRC[4-6], which is further suggested by the findings that combined treatment of metformin and 5-fluorouracil had a better anticancer effect than 5-fluorouracil alone, possibly by reverting epithelial-mesenchymal transition phenotype in cancer cells[7].
Among the mechanisms advocated to explain this association, hyperinsulinemia may play a pivotal role, acting as a potent cell mitogen capable of promoting colon cancer growth[8], an hypothesis that is corroborated by indirect findings of a harmful effect of insulin use for CRC risk[9,10]. Other proposed explanations for the increased risk include obesity-related insulin resistance (IR)[11] and cytokine production[12], which may directly influence CRC growth not only through promotion of angiogenesis[13,14], but also by inducing host inflammatory response[13,15] or decreasing natural killer (NK) lymphocyte cytotoxic activity and, thus, the immunological defense against cancer[16].
Independently of the pathogenetic mechanism(s) advocated for the relationship between T2D and CRC, elevated levels of fasting glucose, fasting insulin, glycated hemoglobin (HbA1c) and IR evaluated by homeostasis model of risk assessment (HOMA-IR) have all been associated with increased CRC risk[17-30], but the results were often conflicting, with some studies suggesting a superiority of HbA1c[20,26] and others placing emphasis on fasting blood glucose, insulin or on the composite index HOMA-IR[31].
Furthermore, whilst most of the studies have focused on the association between T2D or metabolic markers of impaired glucose metabolism and CRC risk, little is known on the impact of a deregulation of the glucoseinsulin axis on CRC progression and survival. The few available data come from selected diabetic populations[32], or obese individuals[30], while additional indirect evidences can be derived, once again, from T2D patients with CRC treated with metformin, suggesting that it could moderately improve survival outcomes[33-37].
Based on the above, we sought to investigate the potential prognostic value of routinely used glycemic parameters in non-diabetic CRC patients. To this purpose, we first designed a case-control study aimed at evaluating the behavior of glucose metabolism indexes in a population of CRC patients, representative of a general practice cohort, compared with non-cancer controls matched for sex, obesity and T2D frequency and blood lipid profile, in order to minimize possible metabolic-related confounders. Thereafter, based on the hypothesis that an association between pre-treatment fasting blood glycemic indexes (blood glucose, insulin, HbA1c and HOMA-IR) and CRC-related survival outcomes might exist independently of T2D, the prognostic value of routinely used glycemic parameters towards progression-free and overall survival was prospectively evaluated.
Starting from January 2007, the PTV Bio.Ca.Re. (Policlinico Tor Vergata Biospecimen Cancer Repository) and the Interinstitutional Multidisciplinary Biobank of the IRCCS San Raffaele Pisana (SR-BioBIM, Rome, Italy) are actively involved in the recruitment of ambulatory patients with primary or metastatic cancer, who are prospectively followed under the appropriate Institutional ethics approvals, as part of a Clinical Database and Biobank project. Among these, a cohort of 224 CRC patients was eligible for the study. Inclusion criteria for the CRC patients from whom serum samples were stored in our Biobanks were: age above 18 years, an Eastern Cooperative Oncology Group performance status (ECOG-PS) ≤ 2 and adequate hematological, hepatic and renal functions. History of alcohol or drug abuse, concurrent infectious or inflammatory diseases were all considered as exclusion criteria for the current analysis.
CRC was staged according to the TNM classification. Surgery was performed in 104 patients with primary CRC and 8 with resectable synchronous metastasis. The remaining 112 patients had relapsing/metastatic disease and entered the study prior to the start of chemotherapy. Among the non-metastatic population, 21/224 (10%) and 83/224 (38%) patients received neoadjuvant and adjuvant therapies, respectively. First-line chemotherapy was instituted in all patients with metastatic disease. Anti-cancer regimens used were all 5-fluorouracil-based in combination either with irinotecan (n = 97) or with platinum compounds (n = 114). Bevacizumab or cetuximab were administered in 68 (57%) and 36 (30%) metastatic CRC patients, respectively. Supportive drugs included erythropoiesis-stimulating agents (n = 3, 1%), granulocyte colony stimulating factors (n = 7, 2%) or corticosteroids (n = 40, 12%). No patient was lost at follow-up. Clinical features of CRC patients are summarized in Table 1.
| IGT or T2D | P value | ||
| NO (n = 173) | YES (n = 51) | ||
| Age (yr) | |||
| mean ± SD (range) | 64 ± 10 (30-83) | 66 ± 8 (54-80) | 0.127 |
| Sex, n (%) | |||
| Males | 103 (60) | 30 (59) | |
| Females | 70 (40) | 21 (41) | 0.927 |
| Body mass index | |||
| mean ± SD (range) | 25.4 ± 4.1 (17.2-38.4) | 26.5 ± 4.2 (17.2-38.4) | 0.105 |
| HOMA index | |||
| Median (IQR) | 2.80 (1.83-4.79) | 6.48 (3.95-10.26) | < 0.0001 |
| HbA1c | |||
| mean ± SD (range) | 5.72 ± 0.36 (4.30-7.10) | 6.87 ± 1.25 (5.30-13.0) | < 0.0001 |
| Blood lipids (mg/dL) | |||
| Total cholesterol | 187 ± 42 | 189 ± 48 | 0.782 |
| HDL cholesterol | 47 ± 12 | 44 ± 13 | 0.284 |
| LDL cholesterol | 113 ± 38 | 117 ± 41 | 0.618 |
| Triglycerides | 136 ± 69 | 138 ± 52 | 0.838 |
| Site, n (%) | |||
| Colon | 126 (73) | 33 (65) | |
| Rectum | 47 (27) | 18 (35) | 0.293 |
| Histological diagnosis, n (%) | |||
| Adenocarcinoma | |||
| Mucinous | 30 (17) | 10 (20) | |
| Non-mucinous | 140 (81) | 40 (78) | |
| Others1 | 3 (2) | 1 (2) | 0.933 |
| ECOG performance status, n (%) | |||
| 0 | 150 (91) | 40 (82) | |
| 1 | 14 (8) | 9 (18) | |
| 2 | 1 (1) | 0 (0) | 0.129 |
| Stage, n (%) | |||
| I | 3 (2) | 1 (2) | |
| IIA | 26 (15) | 6 (12) | |
| IIB | 4 (2) | 1 (2) | |
| IIIA | 3 (2) | 1 (2) | |
| IIIB | 37 (21) | 8 (15) | |
| IIIC | 9 (5) | 5 (10) | |
| Metastatic | 91 (53) | 29 (57) | 0.875 |
As control group, 112 unrelated individuals (mean age 60 ± 13, ranging from 31 to 83 years), paired for T2D rate (22%), obesity (BMI: 25.7 ± 4.4; 18% obese, 33% overweight) and blood lipid parameters were recruited in a 2:1 ratio from otherwise healthy individuals enrolled in the SR-BioBIM.
The study was performed in accordance with the principles embodied in the Declaration of Helsinki. All patients gave written informed consent, previously approved by our Institutional Ethics Committees.
Fasting serum samples were obtained from each recruited subject, aliquoted and stored at -80 °C in the facilities of the PTV Bio.Ca.Re. or the SR-BioBIM. Samples from CRC patients were obtained at baseline prior to chemotherapy.
Routine chemistry studies, including fasting blood glucose (Hexokinase/Glucose-6-phosphate dehydrogenase-based methodology; Abbott Laboratories, Abbott Park, IL, United States), were performed on fresh samples within one hour from blood withdrawal on an ARCHITECT c8000 System (Abbott Laboratories). Fasting insulin levels were analyzed on serum samples using a fully automated Lumipulse G 600 II chemiluminescent enzyme immunoassay analyzer (Fujirebio Inc. Tokyo, Japan) according to the manufacturer’s instructions.
The HOMA index (a marker of insulin resistance) was retrospectively calculated for each participating subject from fasting blood glucose and insulin according to the formula: glucose (mg/dL) × insulin (μIU/mL)/405[38].
HbA1c levels were immediately measured on EDTA anticoagulated whole blood by the Tosoh G7 Automated HPLC Analyzer - HbA1c Variant Analysis Mode (Tosoh Bioscience, Rivoli, TO, Italy), certified by the NGSP (National Glycohemoglobin Standardization Program) and traceable to the Diabetes Control and Complications Trial.
All measurements were ascertained while blinded to the sample origin and to study endpoint.
Sample size of the study was based on the agreement to inclusion criteria and willingness to provide informed consent rather than on sample size calculations. However, estimation was later performed and showed that, given the observed proportions for patients and control groups for HbA1c values and using a type I error probability of 0.05, the recruited population yielded a statistical power greater than 95%.
Data are presented as percentages, mean ± SD, or median and interquartile range. Student’s unpaired t-test and ANOVA test were used for normally distributed variables. Appropriate non-parametric tests (Mann-Whitney U-test and Kruskal-Wallis ANOVA and median test) were employed for all the other variables. The cut-off values were generated from continuous data by receiver operating characteristic (ROC) curve analyses performed by MedCalc Statistical Software version 13.1.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
Progression free (PFS) and overall survival (OS) represented the study endpoints. PFS was calculated from the date of enrollment until relapse or progression of disease. OS was calculated from the date of enrollment until death from disease. If a patient had not progressed or died, PFS or OS were censored at the time of the last follow-up. Survival curves were calculated by the Kaplan-Meier method and the significance level was assessed according to the log-rank test using a computer software package (Statistica 8.0, StatSoft Inc., Tulsa, OK). Cox-proportional hazards analysis was performed by a free web-based application (http://statpages.org/) to evaluate the association between clinical-pathological variables and PFS. For administrative censoring, follow-up was ended the date of March 31st, 2016. All tests were two-tailed and only P values lower than 0.05 were regarded as statistically significant.
Of 224 prospectively recruited CRC patients, 51 (23%) had an established diagnosis of IGT (n = 15) or T2D (n = 36). In addition, 86 (38%) and 27 (12%) of the patients were overweight or obese, respectively. Fasting blood glycemic indexes (blood glucose, insulin, HbA1c) and HOMA-IR were retrospectively reviewed in the overall population, demonstrating that fasting insulin (P < 0.0001), HOMA-IR (P < 0.0001) and HbA1c (P < 0.001), but not blood glucose levels were higher in CRC patients compared to non-cancer controls matched for IGT/T2D or obesity (Table 2). Of interest, pre-treatment levels of all four metabolic markers, including blood glucose, were significantly higher in CRC patients compared with controls once diabetic or overweight/obese subjects were excluded from comparative analysis (Table 2).
| CRC | Controls | P value | |
| Overall population | n = 224 | n = 112 | |
| Fasting blood glucose | 106 ± 35 | 101 ± 39 | |
| (mg/dL) | (65-415) | (61-289) | 0.219 |
| Fasting insulin (μIU/mL) | 14.2 (8.7-23.3) | 9.0 (6.0-12.2) | < 0.0001 |
| HbA1c (%) | 5.98 ± 0.83 | 5.63 ± 1.10 | |
| (4.30-13.0) | (3.90-9.70) | 0.001 | |
| HOMA index | 3.5 (2.0-6.1) | 2.0 (1.3-3.3) | < 0.0001 |
| Non-diabetic | n = 173 | n = 87 | |
| Fasting blood glucose | 100 ± 25 | 87 ± 12 | |
| (mg/dL) | (65-254) | (61-123) | < 0.0001 |
| Fasting insulin (μIU/mL) | 12.5 (8.5-18.6) | 7.6 (5.1-10.9) | < 0.0001 |
| HbA1c (%) | 5.72 ± 0.36 | 5.14 ± 0.42 | |
| (4.30-7.10) | (3.90-6.10) | < 0.0001 | |
| HOMA index | 2.8 (1.8 -4.8) | 1.7 (1.0-2.5) | < 0.0001 |
| Non-diabetic normoweight | n = 90 | n = 51 | |
| Fasting blood glucose | 97 ± 19 | 84 ± 16 | |
| (mg/dL) | (65-175) | (61-112) | 0.0001 |
| Fasting insulin (μIU/mL) | 10.8 (8.1 17.5) | 6.5 (4.3-10.0) | < 0.0001 |
| HbA1c (%) | 5.69 ± 0.31 | 5.13 ± 0.40 | |
| (4.70-6.60) | (4.00-6.10) | 0.0005 | |
| HOMA index | 2.5 (1.8-4.0) | 1.4 (1.0-2.4) | < 0.0001 |
ROC curves were, thus, generated from continuous variables measured in non-diabetic individuals. As summarized in Table 3, the areas under the curve for fasting blood glucose, insulin, HOMA-IR and HbA1c were: 0.657, 0.728, 0.738 and 0.860, respectively. At the designated criterion values all three parameters were associated with an increased CRC risk, although HbA1c yielded the best performance, being associated with a sensitivity and specificity > 80% and a 4.5 positive likelihood ratio (+LR) of CRC risk (Table 3).
| Fasting blood glucose | Fasting insulin | HOMA-IR | HbA1c | |
| AUC (SE) | 0.657 (0.04) | 0.728 (0.03) | 0.738 (0.03) | 0.860 (0.03) |
| 95%CI | 0.596-0.714 | 0.670-0.781 | 0.680-0.791 | 0.812-0.900 |
| Criterion | 97 mg/dL | 12.5 μIU/mL | 1.7% | 5.4% |
| Sensitivity | 42% | 50% | 81% | 82% |
| Specificity | 85% | 84% | 55% | 82% |
| +LR (CI) | 2.79 (1.63-5.06) | 3.09 (1.87-5.41) | 1.81 (1.43-2.30) | 4.46 (2.96-7.06) |
| -LR (CI) | 0.69 (0.61-0.81) | 0.60 (0.52-0.72) | 0. 35 (0.24-0.50) | 0.22 (0.17-0.30) |
| P value1 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
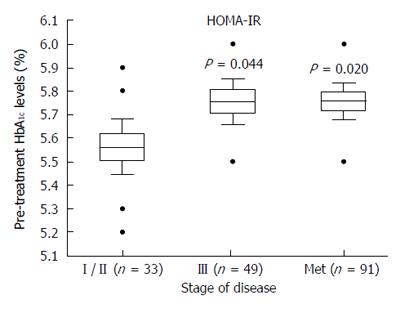
Despite the discriminative power of glycemic indexes at ROC analysis, there were no significant associations between pre-treatment fasting blood glucose or insulin and clinical-pathologic variables. On the other hand, mean pre-treatment HbA1c was higher in mucinous (5.9% ± 0.4%) compared with non-mucinous (5.7% ± 0.3%, P = 0.007) adenocarcinomas and showed a trend to increasing concentrations from early (5.6%) to both advanced (5.8%, P = 0.044) and metastatic (5.8%, P = 0.020) CRC stages in non-diabetic patients (Anova test: F = 3.9, P = 0.021) (Figure 1). Thus, multivariate regression analysis (including age, sex, obesity, HOMA-IR, ECOG-PS and tumor site, histology and stage) was performed to assess possible determinants of HbA1c in non-diabetic CRC patients. The final model by forward stepping showed that HOMA-IR (regression coefficient = 0.200, SE = 0.074, P = 0.008), mucinous features (regression coefficient = 0.196, SE = 0.072, P = 0.007) and advanced stage (regression coefficient = 0.177, SE = 0.074, P = 0.018) were all independent predictors of increased HbA1c.
Overall, the mean time of follow up was 24.8 mo. Among patients with primary non metastatic CRC, 81/104 (78%) remained clinically free of disease, while 23/104 (22%) had progressive disease. Among patients with metastatic CRC, 3/120 (3%) had stable disease, 28/120 (23%) had a complete/partial response during chemotherapy, while 89/120 (74%) had CRC progression. Pre-treatment HbA1c levels, but not fasting glycemia or insulinemia, were higher in patients who had CRC progression (5.8%) compared with patients in whom the disease did not progress (5.6%, P = 0.05).
To further evaluate its prognostic value, pre-treatment HbA1c levels were divided into quartiles according to value distribution (median and interquartile range) in the 224 CRC patients (Q1: ≤ 5.3%; Q2: > 5.3% and ≤ 5.7%; Q3: > 5.7% and ≤ 6.1%; Q4: > 6.1%). Overall, IGT/T2D or obesity were not associated to either PFS or OS at multivariate Cox proportional hazards survival analyses (data not shown). On the other hand, univariate Cox proportional hazards survival analyses showed that elevated pre-treatment HbA1c levels had a negative prognostic value in terms of PFS [HR = 1.30 (95%CI: 1.09-1.54)] or OS [HR= 1.43 (95%CI: 1.16-1.76)]. No association with survival outcomes was observed for fasting blood glucose, insulinemia or the composite index HOMA-IR. These results were largely unmodified after adjustment for obesity or other variables known to be associated with PFS or OS, including disease stage, ECOG-PS, and tumor site and histotype [HRs for HbA1c quartiles: PFS: 1.24 (95%CI: 1.01-1.53); OS: 1.36 (95%CI: 1.05-1.74)] (Table 4). Of interest, the negative prognostic value of HbA1c in terms of OS was further confirmed in a subgroup analysis performed after exclusion of IGT/T2D patients (Table 4).
| Variable | Progression-free survival | Overall survival | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Overall population (n = 224) | ||||
| Sex | 0.96 (0.68-1.36) | 0.809 | 0.82 (0.54-1.23) | 0.336 |
| Obesity1 | 0.95 (0.73-1.24) | 0.701 | 0.88 (0.63-1.22) | 0.448 |
| Site | 0.93 (0.62-1.41) | 0.745 | 0.81 (0.49-1.34) | 0.412 |
| Histological diagnosis | 0.90 (0.58-1.39) | 0.629 | 0.88 (0.53-1.45) | 0.604 |
| Stage of disease2 | 1.95 (1.11-3.42) | 0.021 | 1.17 (0.58-2.34) | 0.662 |
| ECOG-PS | 1.54 (0.96-2.47) | 0.075 | 1.84 (1.05-3.23) | 0.035 |
| Type of treatment3 | 1.37 (0.71-2.62) | 0.346 | 1.94 (0.80-4.74) | 0.145 |
| Fasting glucose4 | 0.99 (0.80-1.21) | 0.892 | 1.06 (0.83-1.34) | 0.657 |
| Fasting insulin5 | 0.90 (0.58-1.38) | 0.619 | 1.01 (0.62-1.62) | 0.981 |
| Fasting HbA1c6 | 1.24 (1.01-1.53) | 0.040 | 1.36 (1.05-1.74) | 0.018 |
| Non-diabetic patients (n = 173) | ||||
| Sex | 1.05 (0.69-1.59) | 0.824 | 0.79 (0.48-1.31) | 0.355 |
| Obesity1 | 1.11 (0.83-1.50) | 0.482 | 0.91 (0.62-1.34) | 0.640 |
| Site | 1.09 (0.67-1.76) | 0.740 | 0.98 (0.54-1.79) | 0.952 |
| Histological diagnosis | 0.91 (0.55-1.52) | 0.728 | 0.88 (0.49-1.61) | 0.687 |
| Stage of disease2 | 1.78 (0.93-3.40) | 0.083 | 0.87 (0.39-1.93) | 0.735 |
| ECOG-PS | 1.69 (0.98-2.92) | 0.060 | 1.98 (1.02-3.85) | 0.044 |
| Type of treatment3 | 1.46 (0.69-3.08) | 0.321 | 2.93 (1.01-8.56) | 0.050 |
| Fasting glucose3 | 0.94 (0.74-1.20) | 0.625 | 0.91 (0.62-1.22) | 0.531 |
| Fasting insulin5 | 1.01 (0.60-1.68) | 0.976 | 0.91 (0.51-1.64) | 0.755 |
| Fasting HbA1c6 | 1.25 (0.97-1.62) | 0.092 | 1.40 (1.03-1.92) | 0.034 |
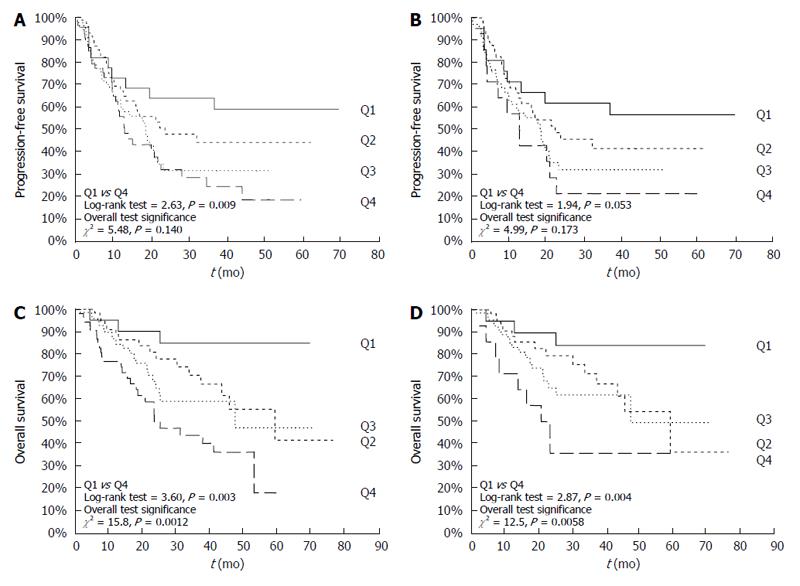
Figure 2A and C demonstrates the Kaplan-Meier PFS and OS curves for CRC patients stratified on the basis of pre-treatment HbA1c levels. As shown, patients with HbA1c in the highest quartile had a worse 5-year PFS and OS rate compared to patients with HbA1c levels in the lower quartile. Similar results were confirmed in non-diabetic CRC (Figure 2B and 2D).
Although the presence of a causal link between T2D and CRC risk has been proposed since long time, the role of a deregulation of glucose metabolic axis in CRC prognosis is far less explored, and mostly relying on indirect findings on glucose lowering drugs in T2D patients with cancer[33-37]. Furthermore, earlier studies have mainly used a “single marker approach” that does not take into account the complex synergistic interactions among the various metabolic features.
In the present study, we demonstrate the presence of increased pre-treatment fasting insulin and HbA1c levels in a population of CRC patients representative of a general practice cohort, as compared to control subjects matched for obesity and T2D frequency. Furthermore, we provide evidence of an elevation of glycemic metabolic markers in normoweight non-diabetic CRC compared with matched control subjects.
Contrary to what reported for other tumors[39,40], however, neither hyperglycemia nor hyperinsulinemia - and thus HOMA-IR - were associated to CRC clinical-pathological features, whereas pre-treatment HbA1c levels were associated with advanced disease stage, especially of the mucinous histotype, in non-diabetic CRC patients. Most importantly, elevated pre-treatment HbA1c levels acted as a negative prognostic factors for OS (HR = 1.43), independently of other well established prognostic factors (e.g., stage or ECOG-PS). Indeed, patients with HbA1c levels in the highest quartile (i.e., > 6.1%) had an approximately three-fold increased risk of dying from disease (5-year survival rate 24%) compared to patients with HbA1c levels in the lowest quartile (i.e., < 5.3%; 5-year survival rate 85%).
These results are in agreement and extend previous reports in cancer patients with T2D, in whom HbA1c levels were evaluated as a marker of glycemic control[41,42]. In particular, Siddiqui et al[41] reported that poorly controlled T2D - defined as a HbA1c level ≥ 7.5% - was independently associated with a more advanced CRC stage at time of diagnosis and poorer 5-year survival, thus suggesting that in CRC patients with T2D, poor glycemic control is associated with a clinically aggressive cancer phenotype. Furthermore, no differences were observed in stage at presentation and 5-year mortality from CRC between well-controlled T2D and non-diabetic controls, suggesting that meticulous glycemic control might lead to a reduction in CRC mortality[41]. These findings are only in apparent disagreement with data obtained in the present study. Indeed, Siddiqui et al[41] performed a comparative analysis between well-controlled T2D and non-diabetic CRC patients (both control groups in their study) without reporting the impact of increasing HbA1c on survival in either group, thus its possible prognostic significance. Furthermore, in our study the negative prognostic value of HbA1c on survival outcomes was assessed in a general practice cohort of CRC patients that included only a moderate rate (23%) of IGT/T2D patients. Accordingly, we did not categorized HbA1c on currently accepted cut-off value for metabolic control, but on quartiles calculated on the basis of the value distribution in the overall population. Of interest, CRC patients in the highest HbA1c quartile (> 6.1%) had the poorest survival. However, the small number of T2D patients enrolled did not confer enough power to perform a separate subgroup analysis. On the other hand, Boursi et al[42] reported no association between HbA1c levels within 6 mo prior to cancer diagnosis and overall survival for CRC. This study is barely comparable with the results obtained in ours, since it enrolled only T2D patients in whom HbA1c was evaluated as a marker of glycemic control.
More recently, Hope et al[43] reported the results of a meta-analysis investigating the relationship between HbA1c and cancer in people with or without T2D. Major finding was that the majority of studies that investigated HbA1c levels in relation to CRC risk identified positive associations. In particular, CRC cases were found to have higher HbA1c levels than control subjects; however, the possibility of reverse causality cannot be completely excluded due to many shortcomings in population selection (e.g., different ethnicity) and lack of correction for CRC-related confounding factors known to increase HbA1c levels such as iron deficiency anemia[43]. Thus, data to support the role of HbA1c either for the identification of people at risk of cancers or to provide some insight into the potential progression of the disease are still lacking.
On the other hand, and to best of our knowledge, this is the first report suggesting that HbA1c may have a prognostic significance for PFS of non-diabetic CRC, in whom HbA1c was capable of discriminating a subset of patients with a more favorable outcome, with a 3-year PFS rate of 56% in patients with HbA1c levels in the lower quartile (< 5.3%) compared with 21% of those in the upper quartile (> 6.1%). These results are scarcely comparable with the currently available evidences, as there are no data on the prognostic value of HbA1c in the PFS of non-diabetic CRC.
As reported above, HbA1c is a common measure of glucose metabolism, as it reflects average glycemia over the previous three months[44]. Accordingly, an elevated HbA1c indicates poor long-term glycemic control and, possibly, chronic hyperinsulinemia. The data here reported are in agreement with the current knowledge that glycemic control is important in determining CRC development and progression. Moreover, the finding of a prognostic role of HbA1c in non diabetic CRC patients could be explained by the common knowledge that, at the time of T2D diagnosis, patients usually have had hyperglycemia for more than a decade[45]. Thus, it is conceivable to hypothesize that colorectal carcinogenesis may recognize, among its causative mechanisms, a deregulated glucose metabolism and that CRC might become manifest long before T2D diagnosis.
There are, of course, some limitations that need to be acknowledged. The most obvious resides in the relatively small sample size that might have weakened the statistical power. Another limitation resides in the fact that, as evidenced by Hope et al[43], data on iron deficiency anemia were missing also in our study. However, the fact that erythropoiesis stimulating agents were administered to three patients only, indicates a satisfactory hemoglobin asset in the overall population. On the other hand, the strength of our analysis was represented by the use of samples collected and processed using standard operating procedures in the context of two large Biobanks. In addition, tests were run by a single laboratory under ongoing quality control protocols, hence minimizing the difference in sample analyses.
Based on the above, we may conclude that pre-treatment HbA1c levels might have a negative prognostic value in CRC patients and, as such, should be carefully monitored, as they could provide important information in risk stratification. These results, however, should be regarded with caution and additional studies are required to prospectively evaluate their clinical value. Future investigations specifically designed to address the role of HbA1c in the management of CRC, independently of T2D, may provide the rationale for lifestyle or glucose targeting dietary/pharmacologic interventions as a means of improving CRC outcomes.
The team expresses deep gratitude to all patients and their families for providing the opportunity to conduct the present research project. Authors also wish to thank the nursery staff of the Day Hospital of the Medical Oncology Unit, Tor Vergata Clinical Center who have contributed and supported the researchers to the overall success of the PTV Bio.Ca.Re. project.
Although metabolic markers of impaired glucose metabolism have been often associated with increased colorectal cancer (CRC) risk, little is known on the impact of a deregulation of the glucose-insulin axis on CRC progression and survival. The few available data come from selected diabetic populations, or obese individuals, while indirect evidences can be derived from type 2 diabetes (T2D) patients with CRC treated with metformin, suggesting that combined treatment of metformin and 5-fluorouracil had a better anticancer effect than 5-fluorouracil alone.
Future investigations specifically designed to address the role of HbA1c in the management of CRC, independently of T2D, may provide the rationale for lifestyle or glucose targeting dietary/pharmacologic interventions as a means of improving CRC outcomes.
This study provides evidences that pre-treatment HbA1c levels might have a negative prognostic value in CRC patients. Glycemic metabolic markers, mainly HbA1c, should be carefully monitored in CRC patients, independently of T2D, as they could provide important information in risk stratification.
Clinicians should be alert to the potential risk of impaired glycemic control and advise CRC patients about lifestyle intervention, weight loss, and exercise as a part of their therapeutic plan. In the context of a precision medicine approach, HbA1c guided incorporation of metformin might aid as adjunctive therapy in CRC management.
HbA1c is a glycation product resulting from hemoglobin’s exposure to plasma glucose. As such, it provides and estimate of average blood glucose levels over the previous three months, as this is the lifespan of red blood cells. It is used in the diagnosis of T2D and as a marker of glycemic control.
The study is interesting and the observational results are intriguing.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B,B
Grade C (Good): C,C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Martinez-Zorzano VS, M’Koma A, Ziogas DE S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2650] [Cited by in F6Publishing: 2577] [Article Influence: 198.2] [Reference Citation Analysis (0)] |
| 2. | Beagley J, Guariguata L, Weil C, Motala AA. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2014;103:150-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 328] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 3. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20838] [Article Influence: 2315.3] [Reference Citation Analysis (2)] |
| 4. | Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 714] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 5. | Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911-121; quiz 1922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Giouleme O, Diamantidis MD, Katsaros MG. Is diabetes a causal agent for colorectal cancer? Pathophysiological and molecular mechanisms. World J Gastroenterol. 2011;17:444-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Zhang HH, Guo XL. Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol. 2016;78:13-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164-179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 552] [Cited by in F6Publishing: 520] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 9. | Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044-1050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 10. | Yin S, Bai H, Jing D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients: a systemic review and meta-analysis. Diagn Pathol. 2014;9:91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Vazzana N, Riondino S, Toto V, Guadagni F, Roselli M, Davi G, Ferroni P. Obesity-driven inflammation and colorectal cancer. Curr Med Chem. 2012;19:5837-5853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20:5177-5190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 128] [Cited by in F6Publishing: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo. 2007;21:147-161. [PubMed] [Cited in This Article: ] |
| 14. | Ferroni P, Palmirotta R, Spila A, Martini F, Formica V, Portarena I, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic value of carcinoembryonic antigen and vascular endothelial growth factor tumor tissue content in colorectal cancer. Oncology. 2006;71:176-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Roselli M, Guadagni F, Martini F, Spila A, Mariotti S, D’Alessandro R, Aloe S, Gazzaniga PP, Basili S, Cosimelli M. Association between serum carcinoembryonic antigen and endothelial cell adhesion molecules in colorectal cancer. Oncology. 2003;65:132-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Piątkiewicz P, Miłek T, Bernat-Karpińska M, Ohams M, Czech A, Ciostek P. The dysfunction of NK cells in patients with type 2 diabetes and colon cancer. Arch Immunol Ther Exp (Warsz). 2013;61:245-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, Rinaldi S, Zeleniuch-Jacquotte A, Shore RE, Riboli E. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 454] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 19. | Palmqvist R, Stattin P, Rinaldi S, Biessy C, Stenling R, Riboli E, Hallmans G, Kaaks R. Plasma insulin, IGF-binding proteins-1 and -2 and risk of colorectal cancer: a prospective study in northern Sweden. Int J Cancer. 2003;107:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Ma J, Giovannucci E, Pollak M, Leavitt A, Tao Y, Gaziano JM, Stampfer MJ. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 243] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 21. | Lin J, Ridker PM, Pradhan A, Lee IM, Manson JE, Cook NR, Buring JE, Zhang SM. Hemoglobin A1c concentrations and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:3010-3012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia. 2006;49:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-peptide, insulin-like growth factor-I, insulin-like growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int J Cancer. 2007;120:2007-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Stattin P, Björ O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 25. | Rinaldi S, Rohrmann S, Jenab M, Biessy C, Sieri S, Palli D, Tumino R, Mattiello A, Vineis P, Nieters A. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3108-3115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Stocks T, Rapp K, Bjørge T, Manjer J, Ulmer H, Selmer R, Lukanova A, Johansen D, Concin H, Tretli S. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS Med. 2009;6:e1000201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Hsu YC, Chiu HM, Liou JM, Chang CC, Lin JT, Liu HH, Wu MS. Glycated hemoglobin A1c is superior to fasting plasma glucose as an independent risk factor for colorectal neoplasia. Cancer Causes Control. 2012;23:321-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Dankner R, Shanik MH, Keinan-Boker L, Cohen C, Chetrit A. Effect of elevated basal insulin on cancer incidence and mortality in cancer incident patients: the Israel GOH 29-year follow-up study. Diabetes Care. 2012;35:1538-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Ollberding NJ, Cheng I, Wilkens LR, Henderson BE, Pollak MN, Kolonel LN, Le Marchand L. Genetic variants, prediagnostic circulating levels of insulin-like growth factors, insulin, and glucose and the risk of colorectal cancer: the Multiethnic Cohort study. Cancer Epidemiol Biomarkers Prev. 2012;21:810-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Parekh N, Lin Y, Vadiveloo M, Hayes RB, Lu-Yao GL. Metabolic dysregulation of the insulin-glucose axis and risk of obesity-related cancers in the Framingham heart study-offspring cohort (1971-2008). Cancer Epidemiol Biomarkers Prev. 2013;22:1825-1836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Xu J, Ye Y, Wu H, Duerksen-Hughes P, Zhang H, Li P, Huang J, Yang J, Wu Y, Xia D. Association between markers of glucose metabolism and risk of colorectal cancer. BMJ Open. 2016;6:e011430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 32. | Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 33. | He XK, Su TT, Si JM, Sun LM. Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore). 2016;95:e2749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014;7:867-885. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Mei ZB, Zhang ZJ, Liu CY, Liu Y, Cui A, Liang ZL, Wang GH, Cui L. Survival benefits of metformin for colorectal cancer patients with diabetes: a systematic review and meta-analysis. PLoS One. 2014;9:e91818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Yin M, Zhou J, Gorak EJ, Quddus F. Metformin is associated with survival benefit in cancer patients with concurrent type 2 diabetes: a systematic review and meta-analysis. Oncologist. 2013;18:1248-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, Crowe FL, Farmer AJ, Harrison S, Hirst JA. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593-2603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1665] [Cited by in F6Publishing: 1721] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 39. | Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30:164-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Ferroni P, Riondino S, Laudisi A, Portarena I, Formica V, Alessandroni J, D’Alessandro R, Orlandi A, Costarelli L, Cavaliere F. Pretreatment Insulin Levels as a Prognostic Factor for Breast Cancer Progression. Oncologist. 2016;21:1041-1049. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer: a case-control study. Dig Dis Sci. 2008;53:2486-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Boursi B, Giantonio BJ, Lewis JD, Haynes K, Mamtani R, Yang YX. Serum glucose and hemoglobin A1C levels at cancer diagnosis and disease outcome. Eur J Cancer. 2016;59:90-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Hope C, Robertshaw A, Cheung KL, Idris I, English E. Relationship between HbA1c and cancer in people with or without diabetes: a systematic review. Diabet Med. 2016;33:1013-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978;200:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 677] [Cited by in F6Publishing: 645] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815-819. [PubMed] [Cited in This Article: ] |