Copyright
©The Author(s) 2016.
World J Gastroenterol. Sep 21, 2016; 22(35): 7892-7907
Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.7892
Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.7892
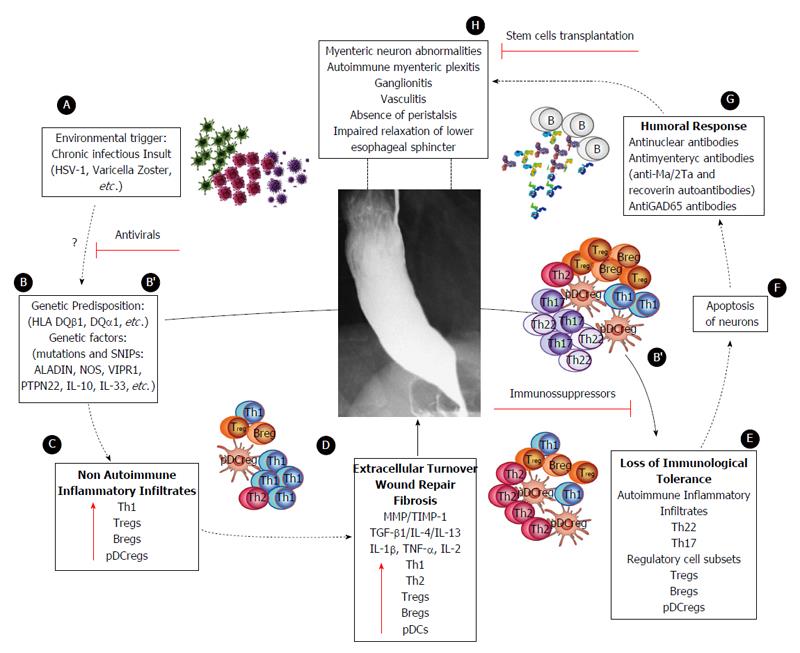
Figure 5 Proposed model of achalasia pathophysiology (modified from Furuzawa-Carballeda et al[6]).
A: An initial active or latent infectious insult, likely involving a neurotropic virus such as the herpes family of viruses or varicella zoster, which have predilection for squamous epithelium and neurons and may cause ganglion cell damage which would be limited to the esophagus; B: Some individuals with genetic predisposition will progress with an aggressive inflammatory response; C: At a very early stage of the disease, the inflammatory infiltrates may be predominantly composed of Th1, Th2 and regulatory cell subsets [T regulatory cells (Tregs), B regulatory cells (Bregs) and plasmacytoid dendritic regulatory cells (pDCregs)]; D: Repair of tissue after injury would require orchestrated coordination of several cell types and biosynthetic processes and would be coordinated by an interacting group of pro- and anti-inflammatory cytokines, fibrous extracellular matrix (ECM) proteins to replace lost or damaged tissue, and products of metabolism such as oxygen radicals. The most prominent pro-fibrogenic cytokines are TGFβ, IL-4 and IL-13. ECM also mediates cellular crosstalk, and does so in two ways. Newly deposited ECM is then rebuilt over time to emulate normal tissue. Matrix proteinases and their inhibitors (TIMPs) also are important, during wound repair, tissue remodeling and fibrosis. B’; E: If steps A-D occur repeatedly, such as in a chronic infection condition, only those individuals with genetic predisposition to developing a long-lasting autoinflammatory response will progress to development of the disease (loss of peripheral tolerance). Thus, autoinflammatory infiltrates would be predominantly composed of Th22, Th17 and regulatory subpopulations; F: Degeneration and significant loss of nerve fibers, associated with autoinflammatory infiltrates of the myenteric plexus, provide evidence of an immune-mediated destruction of the inhibitory neurons, not only by necrosis but also apoptosis (Fas/FasL overexpression); G: Autoimmune etiology of achalasia is further supported by the presence of anti-myenteric autoantibodies in sera; H: Pathophysiologically, achalasia is caused by autoinflammation, degeneration of nerves in the esophagus, plexitis, abnormalities in microvasculature, ganglionitis, and finally by the loss of inhibitory ganglion in the myenteric plexus. Red lines: Potential therapeutic targets.
- Citation: Furuzawa-Carballeda J, Torres-Landa S, Valdovinos M&, Coss-Adame E, Martín del Campo LA, Torres-Villalobos G. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroenterol 2016; 22(35): 7892-7907
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/7892.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.7892