Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.7857
Peer-review started: July 6, 2016
First decision: July 29, 2016
Revised: August 16, 2016
Accepted: August 30, 2016
Article in press: August 30, 2016
Published online: September 21, 2016
Processing time: 71 Days and 17.8 Hours
The development of liver cirrhosis and portal hypertension (PH), one of its major complications, are structural and functional alterations of the liver, occurring in many patients with chronic liver diseases (CLD). Actually the progressive deposition of hepatic fibrosis has a key role in the prognosis of CLD patients. The subsequent development of PH leads to its major complications, such as ascites, hepatic encephalopathy, variceal bleeding and decompensation. Liver biopsy is still considered the reference standard for the assessment of hepatic fibrosis, whereas the measurement of hepatic vein pressure gradient is the standard to ascertain the presence of PH and upper endoscopy is the method of choice to detect the presence of oesophageal varices. However, several non-invasive tests, including elastographic techniques, are currently used to evaluate the severity of liver disease and predict its prognosis. More recently, the measurement of the spleen stiffness has become particularly attractive to assess, considering the relevant role accomplished by the spleen in splanchnic circulation in the course of liver cirrhosis and in the PH. Moreover, spleen stiffness as compared with liver stiffness better represents the dynamic changes occurring in the advanced stages of cirrhosis and shows higher diagnostic performance in detecting esophageal varices. The aim of this review is to provide an exhaustive overview of the actual role of spleen stiffness measurement as assessed by several elastographic techniques in evaluating both liver disease severity and the development of cirrhosis complications, such as PH and to highlight its potential and possible limitations.
Core tip: Spleen elastography is an attractive tool used as an alternative and/or complementary method to assess liver fibrosis, portal hypertension and complications related to cirrhosis. There are several elastography techniques to measure spleen stiffness, all characterized by non-invasiveness and repeatability. Current data from the literature show the higher accuracy of spleen stiffness as compared to liver stiffness, in predicting major complications of cirrhosis. Thus, despite some limitations, spleen stiffness seems to be a better prognostic predictor in patients with chronic liver disease.
- Citation: Giunta M, Conte D, Fraquelli M. Role of spleen elastography in patients with chronic liver diseases. World J Gastroenterol 2016; 22(35): 7857-7867
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/7857.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.7857
Elastographic techniques are among the most promising tools developed in the last decade for the non-invasive evaluation of patients with chronic liver disease. Liver stiffness (LS) measurement using transient elastography (Fibroscan®) is now a widely accepted and validated method to predict the severity and prognosis of liver disease[1-3]. However, there is mounting evidence that its diagnostic accuracy can be flawed by the presence of some confounders, such as liver cell inflammation and cholestasis[4-9] and its performances may be limited in patients with a high body mass index (BMI), narrow intercostal space, or ascites.
Besides this evidence, liver elastography measures hepatic fibrosis which only correlates with the fixed component of portal hypertension (PH) related to intrahepatic resistance but is unable to account for the dynamic component related to hyperdinamic splanchnic circulation and portal venous blood flow[10]. Given these considerations and the known relationship between advanced liver disease and spleen tissue changes, spleen elastography has been proposed and tested as an alternative and/or complementary method to LS for assessing liver fibrosis, predicting complications related to cirrhosis, detecting PH and predicting any presence of esophageal varices (EV).
The aim of this review is to provide, from the current literature data, an extensive overview of the different elastographic techniques used to measure spleen stiffness (SS), with the focus on their feasibility, reproducibility, limitations and diagnostic accuracy to better predict the prognosis of patients affected by chronic liver diseases.
All the elastographic techniques applied to assess SS were at first developed as non-invasive methods to assess LS for staging liver fibrosis and predicting the presence of PH.
Transient elastography: Transient elastography (TE) is the first elastographic technique: it became available 13 years ago with the development of FibroScan® (Echosens, Paris, France) used to assess LS. An ultrasound transducer probe is mounted on the axis of a vibrator. Vibrations of mild amplitude and low frequency are transmitted by the transducer, inducing an elastic shear wave that propagates through the underlying tissues. Pulse-echo ultrasound acquisitions are used to follow the propagation of the shear wave and to measure its velocity, which is directly related to the tissue stiffness of the elastic modulus: the stiffer the tissue, the faster the shear wave propagates.
A large amount of literature, consisting of both primary studies and meta-analyses using histology as the reference standard, has analyzed the role of TE in predicting the stage of fibrosis in patients with CLD caused by different etiologies, and has showed that TE accurately predicts hepatic fibrosis stage with very good accuracy especially for severe fibrosis/cirrhosis. TE cut-off values for the different fibrosis stages have been provided from many large-scale studies[11-14] and by meta-analysis. Results obtained by meta-analysis show cut-offs of TE for liver fibrosis in the range of 7.3-7.9 kPa for the diagnosis of METAVIR fibrosis stage F ≥ 2 and 13.0-15.6 kPa for the diagnosis of liver cirrhosis with AUROC between 0.84-0.87 and between 0.93-0.96 respectively[15-19].
The main disadvantages of TE are that it requires a dedicated device, the region of interest (ROI) cannot be chosen, it cannot be performed in patients with ascites and can be difficult to obtain in patients with obesity.
Currently TE is also the most common technique used to assess SS. In the absence of guidelines for the measurement of SS, all studies applied the same main rules approved for the measurement of LS (e.g., fasting period, success rate, IQR and the minimum number of valid measurements). To date, for the assessment of SS transient elastography is performed with the same probe used to perform LS, with the patient lying supine or prone. Besides the same technical limitations mentioned for LS which are valid also for SS assessment, in some patients the operator is unable to locate the splenic parenchyma because the spleen surface is smaller than the liver surface. In a previous study from our own group both the rate of indeterminate results and failures of SS measurements, decreased over time with operator experience. In particular, the rate of failures decreased from 8%-12%, in the first six months of the study, to 0%-2% in the subsequent 18-24 mo, whereas the rate of unreliable results was 16.6%. Also the inter-observer agreement of SS as expressed by ICC was optimal, being 0.89 in patients with chronic liver disease[20].
A relevant technical aspect is that SS measurements by TE are obtained using a probe validated only for the measurement of LS. Indeed, the acquisition parameters of the FibroScan® (Echosens, Paris, France) were optimized for the stiffness assessment of liver tissues, especially in terms of low-frequency excitation. To accurately assess the stiffness of organs harder than the liver, the acquisition parameters should be probably adapted. Normal spleen stiffness, also in healthy volunteers, is higher in terms of kPa than that of the liver, ranging from 9.4 to 65.2 kPa[20]. Therefore, the use of the Fibroscan® (Echosens, Paris, France) on the spleen of patients with cirrhosis might lead to overestimated stiffness values. Often in patients with cirrhosis and portal hypertension SS measurements reach the maximum value reported by machine, i.e., 75 kPa, in order to identify the correct range of SS values and to better stratify them both Calvaruso et al[21] and Stefanescu et al[22] asked Echosens to perform an analysis of their elastograms using software and calculation algorithms that allow for stiffness measurements up to 150 kPa. Using this modified spleen stiffness, the authors obtained interesting results in terms of prediction of both esophageal varices and liver decompensation[23]. However, those authors correctly pointed out that this modified SS measurement probably is not the real spleen stiffness value but only an estimation of it thus efforts should be made to develop a modified probe and software with frequency correction and depth optimization.
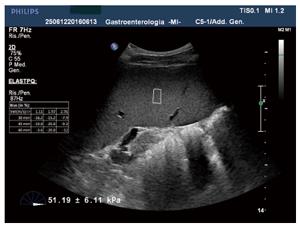
Point shear-wave elastography: This is an elastographic technique recently developed and integrated into several conventional ultrasound machines (e.g., Siemens, Philips, Hitachi, Esaote etc.). It allows the quantitative assessment of tissue stiffness, providing measurements of shear-wave velocity within a small region (fixed ROI size 0.5 cm × 1.5 cm), its localization being monitored by real-time B-mode ultrasound. Results may be expressed in units of shear-wave velocity (m/s) or converted into units of Young’s modulus (kPa). The advantage of point shear-wave elastography (pSWE) relates to the opportunity for the operator of positioning the region of interest where to perform measurements with an adequate ultrasound window. In addition, the exam can be performed also in presence of ascites, obesity or narrow intercostal spaces. Several recent reports and meta-analyses have demonstrated that the measurement of LS by pSWE is a valuable method of assessing liver fibrosis[24-27] while only a few studies have investigated spleen stiffness with this method[28-30]. The failure rate of acoustic radiation force impulse (ARFI) for liver stiffness determination is similar to that of TE (2.9%)[27,31] and also the reproducibility is good for LS determination whereas insufficient data is available to date regarding the rate of failure and unreliable measurements of SS by pSWE. Its feasibility seems to be similar to that for LS and inter-observer agreement seems to be acceptable but inferior to that on LS[30,32].
Figure 1 shows an example of spleen stiffness determination by pSWE.
Real-time two-dimensional SWE: Real-time two-dimensional SWE (RT 2D-SWE) has the peculiarity that the shear-wave velocities can be measured in a bi-dimensional area (a box of 2.5 cm × 3.5 cm) rather than in a single small point: this way the limitation of pSWE to accidentally investigate small regions of greater or lesser stiffness than average is overcome. Also this technique is incorporated in conventional ultrasound machines and its results are expressed either in m/sec or in kPa. Its failure rate is significantly lower than that of TE and preliminary results suggest that SWE may be better than TE at the diagnosis of clinically significant fibrosis and more reliable in patients with ascites[33-37]. SS measurement using 2D-SWE has been investigated in even fewer studies. A preliminary study by Cassinotto et al[38] showed excellent intra-observer (ICC 0.96) and inter-observer (ICC 0.87) agreements but the rate of failure was quite high (30%) probably due to the thinness and the more cranial position of the splenic parenchyma and to movements owed to the proximity of the left cardiac ventricle. Findings regarding the diagnostic accuracy of SS determination for the assessment of liver fibrosis are sporadic even if its diagnostic accuracy for liver cirrhosis seems good: a study by Grgurevic et al[39] found an AUROC of 0.82 using a cut-off value of 24 kPa.
Regarding ARFI techniques (pSWE and RT 2D-SWE) there are two relevant critical issues that should be taken into account. Firstly, the quality criteria for the correct interpretation of results (for SS and even for LS) remain to be adequately defined. Secondly, the elastographic techniques which are incorporated into conventional ultrasound devices enabling the choice of where to place the region of interest and whether to confirm or exclude each single measurement, can be more operator-dependent than TE[40].
Magnetic resonance elastography: Magnetic resonance elastography (MRE) is an imaging technique that provides full organ coverage[41] and low variability for stiffness measurement as evidenced by excellent inter-scan reproducibility[42-44] and inter-reader agreement[45]. A correlation between LS by MRE and hepatic fibrosis has been established[46] and MRE has shown to have considerably high reproducibility, feasibility and validity for assessing liver fibrosis[47-50].
SS measurement using MRE has been investigated in a few studies. Firstly, Talwalkar et al[51] suggested the feasibility of MRE in assessing SS as a non-invasive measure of portal pressure by assessing 38 patients with chronic liver disease. The authors found a strong linear relationship between LS and SS (r2 = 0.75, P < 0.001) and with a mean SS valued ≥ 10.5 kPa they noted a detection rate of 100% for EV. Similarly, Morisaka et al[52] found that SS had the highest correlation with EV as observed at endoscopy. For discriminating large EV vs no EV or small EV only SS showed a significant association (OR = 1.82) with an AUROC of 0.81. Moreover, the authors showed the reproducibility of SS measurements by MRE (Pearson’s correlation coefficient 0.898). A recent retrospective study confirmed that LS and SS measured by MRE are strongly associated with the presence of EV[53]. However, all these studies cannot confirm a direct correlation between SS and hepatic vein pressure gradient (HVPG) because the measurement of HVPG was not performed and the presence of PH was only assessed indirectly by the presence of EV at endoscopy. Only in animal models a strong correlation was reported (r > 0.80) between SS measured with MRE and HVPG[54].
A more recent study[55] showed that the performance of SS by MRE for the detection of liver cirrhosis was good (AUROC of 0.87 and 0.92 using single-driver and dual-driver configuration, the latter possibly allowing the simultaneous estimation of LS and SS) but the cut-off values were based on the retrospective patient cohort and the diagnosis of liver cirrhosis was based on clinical history and imaging findings. In addition, the cost and limited availability of MRE actually restrain its use for SS determination in individual patients in the daily clinical practice.
The prognosis of chronic liver diseases (CLD) of any aetiology is driven by the liver fibrosis stage, a reliable predictor of cirrhosis. Liver biopsy has traditionally been considered the reference method for evaluating and staging hepatic fibrosis but it is an expensive and invasive procedure that requires physicians and pathologists to be sufficiently trained in order to obtain adequate and representative results[56]. Recently, the determination of liver stiffness by different electrographic techniques (TE and ARFI techniques) has been shown to be a reliable non-invasive predictor of disease severity in chronic liver disease of different etiologies.
Owing to the known relationship between severe chronic liver disease and splenic involvement, in the recent years several studies investigated the role of SS in assessing liver fibrosis as an attractive alternative to liver stiffness[57,58].
Using TE several studies have showed significantly higher SS values in cirrhotic vs non-cirrhotic patients[20-22]. A study by our own group[20] was conducted on 132 patients with chronic liver disease, 48 patients being with hematological disease and 64 healthy controls. In the CLD patients SS showed very good diagnostic estimates in predicting fibrosis stage (Table 1). In particular for the diagnosis of liver cirrhosis using a cut-off value of 46 kPa the sensitivity was 89%, the specificity 78%, with corresponding LR+ 4.5 and LR- 0.1 and AUROC 0.84. In the study by Bota et al[28] using pSWE the overall diagnostic accuracy of SS in diagnosing the presence of cirrhosis showed an AUROC of 0.91. In Grgurevic et al[39]’s study the ratio between LS and SS is used as an additional indicator of cirrhosis because with liver fibrosis progressing the difference between LS and SS decreased. The study by Cabassa et al[30] showed that if spleen and liver ARFI were combined in a sequential modality even higher accuracy would be achieved. Similarly, to LS the diagnostic accuracy of SS is higher for severe liver fibrosis (F3/F4) than from early fibrosis[29,30]. All these data suggest that SS can be used as an alternative (especially in patients whose liver stiffness determination is not obtainable or is unreliable) or adjunctive diagnostic approach to stage liver fibrosis. However, as of SS, differently to LS, neither large cohort studies nor meta-analyses are available to confirm and validate this technique. Further studies are needed to confirm these interesting data.
| F≥2 (66%) | F = 4 (23%) | EV (10%) | ||||
| L-TE | S-TE | L-TE | S-TE | L-TE | S-TE | |
| Cut-off (kPa) | 8 | 36 | 12 | 46 | 19 | 65 |
| Sensitivity | 83 (54-76) | 76 (61-81) | 92 (80-100) | 89 (72-99) | 73 (59-93) | 91 (81-100) |
| Specificity | 65 (76-98) | 80 (65-93) | 80 (72-89) | 78 (82-96) | 47 (34-68) | 80 (65-94) |
| LR+ | 2.4 | 3.9 | 4.6 | 4.5 | 1.4 | 4.5 |
| LR- | 0.2 | 0.3 | 0.09 | 0.1 | 0.5 | 0.1 |
| AUROC | 0.85 (0.78-0.92) | 0.80 (0.65-0.85) | 0.93 (0.88-0.96) | 0.84 (0.76-0.93) | 0.62 (0.41-0.89) | 0.90 (0.79-100) |
The diagnostic estimates for SS determination reported in the primary studies are summarized in Table 2.
| Ref. | Technique | Cut-off | Patients | Sens | Spec | LR+ | LR- | PPV | NPV | AUROC |
| Chen et al[29], 2012 (prospective) | ARFI | 3.32 | 163 | 80.0 | 88.4 | 6.9 | 0.23 | 55.5 | 96.0 | 0.93 (0.89-0.97) |
| Fraquelli et al[20], 2013 (prospective) | TE | 46.00 | 110 | 89.0 | 78.0 | 4.5 | 0.10 | 54.7 | 95.0 | 0.84 (0.76-0.92) |
| Cabassa et al[30], 2015 (prospective) | ARFI | 3.05 | 51 | 73.0 | 84.0 | 4.5 | 0.32 | 91.0 | 77.0 | 0.80 (0.68-0.93) |
| Bota et al[28], 2010 (prospective) | ARFI | 2.51 | 67 | 85.2 | 91.7 | 10.2 | 0.16 | 73.3 | 87.1 | 0.910 |
| Grgurevic et al[39], 2015 (prospective) | RT-2D SWE | 24.00 | 66 | 66.7 | 86.7 | 5.01 | 0.38 | 75.0 | 81.3 | 0.821 |
PH is a typical condition of advanced chronic liver disease leading to the formation of EV and other severe complications, such as ascites, portosystemic encephalopathy and sepsis[59,60]. Therefore, this condition is one of the most important causes of morbidity and mortality in patients with liver cirrhosis[61]. Nowadays the reference standard methods to diagnose the presence and grade of PH and EV are HVPG and upper endoscopy, respectively: however, both techniques are invasive, expensive and perceived as unpleasant by patients. Therefore, in the last years several non-invasive methods have been proposed to predict the degree of PH and the presence of esophageal varices. In this regard, several serum biomarkers and ultrasound Doppler signs have been studied. Regarding biomarkers, when used as a single test, their diagnostic accuracy was not optimal in clinical practice[62]. Platelet count combined with spleen diameter, known as Giannini’s score, showed good accuracy in excluding the presence of EV[63] but not many studies have evaluated the relationship between Giannini’ s score and the degree of PH. The study by Colecchia et al[64] found that its AUROC was inferior compared with other parameters, such as spleen stiffness (AUROC 0.857 vs 0.941). Regarding ultrasound signs they were highly specific to the diagnosis of cirrhosis and the presence of PH but their sensitivity was quite low especially leading to a high rate of false negative results especially in compensated cirrhosis patients[65]. In addition, several studies have showed a relevant inter-observer and inter-equipment variability among different diagnostic centers[66-68].
More recently, there has been considerable interest in the potential use of LS and SS measurements in the detection of significant PH and the prediction of any presence of esophageal varices. One of the determinants of PH is liver fibrosis and LS has proved to correlate strongly with portal pressure as measured by HVPG[69,70] and it may predict clinical decompensation in compensated cirrhotic patients[71]. However, when HVPG values exceed 10-12 mmHg, which are the threshold of clinically significant PH and the development of varices, portal pressure becomes largely independent from liver fibrosis. Accordingly, the ability of liver stiffness to predict the presence and grade of EV is not optimal[70].
Indeed, on considering the physiopathology of PH, in its earlier phases PH depends on the accumulation of fibrillar extracellular matrix, whereas in the later phases the dynamic component related to hyperdinamic circulation and splanchnic vasodilatation become predominant. Splenomegaly plays an important role in the pathophysiology of PH even if it is not clear whether splenomegaly occurs only because of spleen congestion caused by the PH or, according to more recent theories, because of an increase of splanchnic inflow. Indeed, a proliferation of fibrotic hyperplastic components, a hyperactivation of the splenic lymphoid tissue and an increased angiogenesis have been found in the enlarged spleen of rat models with PH induced by portal vein ligation[72].
According to these physiopathogenetic considerations and the anatomic changes that occur at the level of the spleen in cirrhotic patients, spleen stiffness has recently received considerable attention as a potential indicator of PH.
In an animal model Nedredal et al[73] performed MRE to assess SS and direct HVPG measurements and showed a positive correlation between SS and direct portal vein pressure gradient (r2 = 0.86, P < 0.01). In the study by Hirooka et al[74] 60 patients with chronic liver disease of mixed etiology underwent LS and SS measurements by real-time tissue elastography, HVPG determination and upper endoscopy. Among the parameters associated with HVPG, the correlation was closer with SS (r = 0.854) than with LS (r = 0.51) (P < 0.0001). At multivariate analysis SS was the only independent predictor of HVPG ≥ 12 mmHg (OR = 17.7; 95%CI: 2.6-765; P = 0.040)[74]. Similarly, Stefanescu et al[75] found a good correlation between SS and EV using a SS cut-off value of 52 kPa (AUROC 0.74) and showed that using both LS and SS the presence of EV is correctly predicted with an overall diagnostic accuracy of about 90%.
In the study by Colecchia et al[64], 100 patients with HCV-related cirrhosis were consecutively investigated by transient elastography and HVPG: LS and SS significantly correlated with HVPG values and accurately predicted both the degree of PH assessed by HVPG (r2 = 0.85) and the presence of esophageal varices. Moreover, LS and SS at multivariate analysis resulted as the only independent predictors of esophageal varices. Using a SS cut-off value of 41.3 kPa the LR- was 0.029, showing that the test accurately ruled out the presence of esophageal varices and therefore can be efficiently used in a screening strategy.
In a study carried out by our own group we have confirmed these data[20]. In our study SS used as a single test or in combination with LS, accurately has ruled out the presence of esophageal varices (NPV of 100%) using a SS cut-off value < 48 kPa. Also the study by Sharma et al[76], using TE, found that HVPG was significantly correlated with SS (r = 0.433, P = 0.001) but not with LS (r = 0.178, P = 0.20) and using a cut-off value of 40.8 kPa the technique showed a sensitivity of 94%, a specificity of 76%, LR+ and LR- and PPV of 91%, NPV of 84% and a diagnostic accuracy of 89%: Furthermore, the authors found that SS was significantly higher in patients who had large varices (P = 0.001) and variceal bleeding (P = 0.001).
Later on, in order to better predict the degree of PH, Calvaruso et al[21] used a modified software version for TE, with a range between 1.5 and 150 kPa. As expected, patients with values of 75 kPa by standard TE had mean values of modified spleen TE of 117 kPa (range 81.7-149.5 kPa); linear regression revealed a significant correlation between modified spleen TE and esophageal varix size (r = 0.501; β: 0.763; SE 0.1444; P < 0.001). At multivariate analysis only modified SS and AST/ALT ratio resulted independently associated with grade 2/grade 3 EV and, compared to other non-invasive tools in diagnosis of the presence of large EV, modified SS performed better. The best cut-off value of modified SS for predicting the presence of large EV, identified on the ROC curve as the point that maximizes sensitivity and specificity, was 54 kPa (AUROC 0.82; NPV of 90%)[21]. Using modified spleen stiffness measurements, Stefanescu et al[22] reported similar results: a cut-off value of 75 kPa predicted the presence of large EV with an AUROC 0.90; NPV 100%. Despite their interesting results the authors stressed the concept that the correction of SS values using a calculation algorithm in the post-processing of each elastogram improves the SS measurement but better results would have to be obtained using a dedicated, modified probe and software installed on the Fibroscan device.
According to the results of the above mentioned studies, the diagnostic accuracy of spleen stiffness measurement to rule out the presence of PH was higher than that of liver stiffness and other non-invasive ultrasound signs. In particular in their study Colecchia et al[64] observed a better diagnostic accuracy of SS in diagnosing the presence of EV (AUROC 0.94) as compared to PSR (platelet spleen diameter ratio) (AUROC 0.86) (P = 0.05). For the detection of PH again SS showed a better accuracy in diagnosing HVPG >10 mmHg [AUROC 0.96 vs LSPS (LS × spleen diameter/platelet count) = 0.91 (P = 0.05) and vs PSR = 0.84 (P = 0.007)] and in diagnosing a HVPG > 12 mmHg [AUROC 0.96 vs LSPS = 0.90 (P = 0.048) and vs PSR = 0.82 (P = 0.003)]. Greater performance of SS compared to PSR and LSPS emerged also in the study by Sharma et al[76], especially in differentiating patients with large vs small varices. No US Doppler signs were considered in these studies among the non-invasive tests probably because they are highly specific to the diagnosis of cirrhosis and PH, but their sensitivity is relatively low, especially in compensated cirrhotic patients[65].
Singh et al[77] recently carried out a meta-analysis that evaluated the diagnostic accuracy of SS in predicting EV and included a total of 12 studies including 1497 patients with chronic liver disease. The analysis found a moderate SS accuracy in detecting the presence of EV (sensitivity 78%, specificity 76%; LR+ 3.4, LR- 0.2, AUROC = 0.86). Similarly, the diagnostic performance of SS in detecting the presence of clinically significant EV was moderate (sensitivity 81%, specificity 66%) even if the rate of false positive and negative results was far from optimal. The heterogeneity of the results among the studies can be related to either differences in the elastographic techniques or geographical differences among the studies (e.g., Asian and European populations). No significant differences were observed in the diagnostic accuracy of SS measurements according to the etiology of chronic liver disease but the different techniques used prevent any possible conclusion.
Table 3 summarizes the main studies that assessed SS measurement as non-invasive test to predict the presence of PH and/or EV.
| Ref. | Technique | Cut-off | n | Prevalence | Sens | Spec | LR+ | LR- | PPV | NPV | AUROC |
| Stefanescu et al[75], 2011 (prospective) | TE | 46.4 | 174 | 85% | 83.5 | 71.0 | 2.90 | 0.23 | 93.8 | 45.5 | 0.780 |
| Hirooka et al[74], 2011 (prospective) | RT-SWE | 8.24 | 60 | 43% | 96.0 | 85.0 | 6.40 | 0.04 | 83.0 | 97.0 | 0.91 (0.873-0.99) |
| Bota et al[85], 2012 (prospective) | ARFI | 2.55 | 140 | 43% | 96.7 | 21.0 | 18.00 | 0.15 | 47.6 | 89.4 | 0.578 |
| Colecchia et al[64], 2012 (prospective) | TE | 55.00 | 100 | 53% | 71.7 | 96.0 | 17.00 | 0.29 | 95.3 | 75.2 | 0.941 (0.90-0.98) |
| Vermehren et al[86], 2012 (prospective) | ARFI | 4.13 | 166 | 36% | 35.0 | 83.0 | 2.06 | 0.78 | 54.0 | 69.0 | 0.58 (0.49-0.67) |
| Calvaruso et al[21], 2013 (prospective) | TE | 50.00 | 96 | 56% | 65.0 | 61.0 | 1.70 | 0.60 | 69.0 | 57.0 | 0.701 |
| Fraquelli et al[20], 2013 (prospective) | TE | 48.00 | 26 | 42% | 100.0 | 60.0 | 2.50 | 0.01 | 33.5 | 98.7 | 0.90 (0.79-1.00) |
| Sharma et al[76], 2013 (prospective) | TE | 40.80 | 200 | 71% | 94.0 | 76.0 | 3.90 | 0.08 | 91.0 | 84.0 | 0.89 (0.84-0.95) |
| Takuma et al[87], 2013 (prospective) | ARFI | 3.18 | 340 | 38.8% | 98.5 | 60.1 | 2.46 | 0.02 | 61.0 | 98.4 | 0.93 (0.90-0.96) |
| Takuma et al[88], 2016 (prospective) | ARFI | 3.36 | 60 | 40% | 95.4 | 77.8 | 4.31 | 0.05 | 74.2 | 96.6 | 0.93 (0.84-0.98) |
Finally, limited data is available to date about the reproducibility of the technique and the definition of its methodological standards thus further evidence is needed in this field.
In conclusion, the current techniques for SS determination, even if very promising, are still sub-optimal to replace upper endoscopy as the screening modality of choice to detect any presence of EV.
Recently the Faculty of Baveno VI recommended the determination of LS by TE plus platelet count to identify among patients with compensated advanced chronic liver disease those who can safely avoid screening endoscopy. According to this suggestion, the patients with a LS < 20 kPa and with a platelet count > 150000 have a very low risk of having varices requiring treatment and can avoid endoscopy (level of evidence 1b; A)[78]. Several studies are presently on going with the aim of corroborating these data.
Considering the recent finding, SS too should probably be included in these recommendations, especially in the presence of possible confounders of LS determination, when LS measurement is inaccurate or a complementary test. Again further evidence is needed in this area.
Furthermore, it is reasonable to think that composite scores or diagnostic algorithms involving both LS and SS can potentially improve diagnostic accuracy in the prediction of PH and consequentially can avoid the use of other invasive and more expensive examinations.
A few preliminary studies evaluated the correlation of SS and portal pressure before and after the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Gao et al[79] using pSWE showed a statistically significant difference in mean SS values pre and post TIPS placement (P < 0.001) while mean LS post-TIPS value did not significantly differ from those measured pre TIPS placement. Other studies obtained similar results[80,81] suggesting a potential role of spleen elastography to confirm shunt effectiveness and indicate successful portal vein pressure reduction after TIPS placement. While interesting, all these studies had a very small sample size (10-12 patients), SS was measured only over a short period (< 10 d) and inter and intra-observer variability were not taken into account. Furthermore, in a recent study Novelli et al[82] evidenced a mean SS values reduction in only 58% of the patients who underwent TIPS even if all patients had a reduction in the portosystemic gradient to less than 12 mmHg. In addition, the analysis showed no measurable correlation between SS and portal venous pressure before and after TIPS placement. Thus, further studies are warranted to determine the feasibility and the role of SS in the surveillance of TIPS function.
Finally, one can anticipate that interesting preliminary results would occur in the use of SS to assess changes in portal pressure after liver transplantation reflecting the resolution PH[83].
Considering the recent data supporting the correlation between SS measurements and HVPG levels and considering that HVPG measurement represents the best predictor of clinical decompensation in cirrhotic patients, Colecchia et al[84] prospectively enrolled 92 compensated HCV-related cirrhosis, investigated with laboratory tests, spleen diameter, LS and SS (by TE), HVPG and EGD and followed up for 2 years. Patients on antiviral, beta blocker or diuretic treatment with previous clinical decompensation were excluded. At multivariate analysis the only two independent predictors of complications were SS and Model for End-Stage Liver Disease score (MELD): P = 0.0001, P = 0.014. The authors elaborated predictive models to detect patients with low risk of decompensation involving SS and MELD or only SS. Considering the simplified model including only SS, the patients with SS values lower than 54 kPa are at low risk (< 3%) of events at 2 years (Se 97%, LR- 0.05, NPV 97%). Also in their study the predictive accuracy of SS (alone or with MELD) measured by the c index does not seem to be higher than to that of HVPG but the presence of varices or other PH non-invasive tests and even HVPG could not provide any adjunctive information: 53.3% of patients had already small varices at their enrollment but the effect of SS on the primary outcome was independent from the presence of EV (interaction SS and EV P = 0.232).
Similar findings were observed also in the study by Radu et al[23] who used modified SS (by analysis of each elastogram to obtain an increment of the scale up to 150 kPa) performed on fifty-two compensated cirrhotic patients followed-up for above 13 mo. In their study the authors also calculated a decompensation prediction score involving modified SS, albumin and bilirubin, and found the best cut-off value for predicting liver decompensation (AUROC = 0.70, 95%CI: 0.53-0.85). In that paper a SS cut-off value was not clearly expressed and patient characteristics (diuretic, beta-blocker, antiviral treatment) were not adequately detailed.
Despite these interesting results, those studies presented some limitations, for example a small sample size in Radu’s study and the short follow-up period in both studies.
In the last few years, among several non-invasive tests proposed to better evaluate liver fibrosis and PH, elastographic techniques have gained an important role and have been firstly applied on the liver.
Currently, liver stiffness is an accepted and validated method to assess liver fibrosis stages and is a complementary test to exclude clinical PH. More recently, spleen stiffness has become particularly attractive as compared to liver stiffness: it appears to better represent the dynamic changes occurring in the advanced stages of liver cirrhosis and also its diagnostic performance in detecting esophageal varices is higher than that of liver stiffness.
For the assessment of liver fibrosis SS showed a good diagnostic accuracy especially when determined by TE and pSWE (AUROC 0.84-0.91). Up to now no meta-analysis is available to confirm these data but it would seem reasonable to use SS when LS is not reliable or its measurement is flawed.
Regarding the detection of PH many single studies have showed an optimal diagnostic accuracy of SS, particularly to rule out the presence of esophageal varices. Only one meta-analysis has been carried out to date, showing an adequate accuracy of SS measurement in detecting the presence of EV. Similarly, the diagnostic performance of SS for the detection of the presence of clinically significant EV (F3) is good enough even if it is not yet clear if this non-invasive tool can replace upper endoscopy to detect the presence of EV. Most importantly, there is a great heterogeneity among the current studies, such heterogeneity being owed to differences in the elastographic technique used and to geographical differences in the study setting.
Recent studies have showed promising yet preliminary results regarding the role of SS measurement in the prediction of cirrhosis-related complications, TIPS function and in the prediction of PH resolution after liver transplantation. These interesting findings should be confirmed by further larger prospective studies.
In conclusion, SS seems to be a very promising tool to be used in the work-up and follow-up of cirrhotic patients, In fact, in addition to the staging of PH, SS determination, alone or in association with other non-invasive markers, can predict the clinical outcome of liver cirrhosis and become a useful tool to stratify patients according to the different level of risk of disease progression and to select those patients requiring further investigations.
However, further studies are needed to better define the quality criteria and the diagnostic performance of spleen elastography techniques in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Abenavoli L, Cabibi D, Ferraioli G S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-199, 1970-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | de Lédinghen V, Vergniol J, Barthe C, Foucher J, Chermak F, Le Bail B, Merrouche W, Bernard PH. Non-invasive tests for fibrosis and liver stiffness predict 5-year survival of patients chronically infected with hepatitis B virus. Aliment Pharmacol Ther. 2013;37:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Vergniol J, Boursier J, Coutzac C, Bertrais S, Foucher J, Angel C, Chermak F, Hubert IF, Merrouche W, Oberti F. Evolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis C. Hepatology. 2014;60:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 507] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 5. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 6. | Viganò M, Massironi S, Lampertico P, Iavarone M, Paggi S, Pozzi R, Conte D, Colombo M. Transient elastography assessment of the liver stiffness dynamics during acute hepatitis B. Eur J Gastroenterol Hepatol. 2010;22:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, Seitz HK, Mueller S. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 8. | Lupşor M, Badea R, Stefănescu H, Grigorescu M, Sparchez Z, Serban A, Branda H, Iancu S, Maniu A. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C. Results from a cohort of 324 patients. J Gastrointestin Liver Dis. 2008;17:155-163. [PubMed] |
| 9. | Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, Rumi M, Lampertico P, Colombo M. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol. 2011;54:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Castéra L, García-Tsao G. When the spleen gets tough, the varices get going. Gastroenterology. 2013;144:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 12. | Vizzutti F, Arena U, Marra F, Pinzani M. Elastography for the non-invasive assessment of liver disease: limitations and future developments. Gut. 2009;58:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1095] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 14. | Abenavoli L, Beaugrand M. Transient elastography in non-alcoholic fatty liver disease. Ann Hepatol. 2012;11:172-178. [PubMed] |
| 15. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 16. | Chon YE, Choi EH, Song KJ, Park JY, Kim DY, Han KH, Chon CY, Ahn SH, Kim SU. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One. 2012;7:e44930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 18. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 19. | Stebbing J, Farouk L, Panos G, Anderson M, Jiao LR, Mandalia S, Bower M, Gazzard B, Nelson M. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroenterol. 2010;44:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Fraquelli M, Giunta M, Pozzi R, Rigamonti C, Della Valle S, Massironi S, Conti CB, Aghemo A, Ronchi G, Iurlo A. Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis. J Viral Hepat. 2014;21:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat. 2013;20:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Stefanescu H, Procopet B, Platon Lupsor M. Modified spleen stiffness measurement: a step forward, but still not the solution to all problems in the noninvasive assessment of cirrhotic patients. J Viral Hepat. 2014;21:e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Radu C, Stefanescu H, Procopet B, Lupsor Platon M, Tantau M, Grigorescu M. Is spleen stiffness a predictor of clinical decompensation in cirrhotic patients? J Gastrointestin Liver Dis. 2014;23:223-224. [PubMed] |
| 24. | Kuroda H, Kakisaka K, Tatemichi Y, Sawara K, Miyamoto Y, Oikawa K, Miyasaka A, Takikawa Y, Masuda T, Suzuki K. Non-invasive evaluation of liver fibrosis using acoustic radiation force impulse imaging in chronic hepatitis patients with hepatitis C virus infection. Hepatogastroenterology. 2010;57:1203-1207. [PubMed] |
| 25. | Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 28. | Bota S, Sporea I, Sirli R, Popescu A, Dănilă M, Sendroiu M, Focşa M. Spleen assessment by Acoustic Radiation Force Impulse Elastography (ARFI) for prediction of liver cirrhosis and portal hypertension. Med Ultrason. 2010;12:213-217. [PubMed] |
| 29. | Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Noninvasive assessment of liver fibrosis via spleen stiffness measurement using acoustic radiation force impulse sonoelastography in patients with chronic hepatitis B or C. J Viral Hepat. 2012;19:654-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Cabassa P, Ravanelli M, Rossini A, Contessi G, Almajdalawi R, Maroldi R. Acoustic radiation force impulse quantification of spleen elasticity for assessing liver fibrosis. Abdom Imaging. 2015;40:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Hudson JM, Milot L, Parry C, Williams R, Burns PN. Inter- and intra-operator reliability and repeatability of shear wave elastography in the liver: a study in healthy volunteers. Ultrasound Med Biol. 2013;39:950-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Bernuzzi S, Salvaneschi L, Filice C. Ultrasound point shear wave elastography assessment of liver and spleen stiffness: effect of training on repeatability of measurements. Eur Radiol. 2014;24:1283-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 292] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 34. | Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 506] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 35. | Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, Fedchuk L, Sattonnet F, Pais R, Lebray P. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013;58:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, Chu SH, Chan AW, Choi PC, Ahuja AT. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 37. | Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, Plessier A, Durand F, Valla D, Lebrec D. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 38. | Cassinotto C, Charrie A, Mouries A, Lapuyade B, Hiriart JB, Vergniol J, Gaye D, Hocquelet A, Charbonnier M, Foucher J. Liver and spleen elastography using supersonic shear imaging for the non-invasive diagnosis of cirrhosis severity and oesophageal varices. Dig Liver Dis. 2015;47:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Grgurevic I, Puljiz Z, Brnic D, Bokun T, Heinzl R, Lukic A, Luksic B, Kujundzic M, Brkljacic B. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur Radiol. 2015;25:3214-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Bensamoun SF, Wang L, Robert L, Charleux F, Latrive JP, Ho Ba Tho MC. Measurement of liver stiffness with two imaging techniques: magnetic resonance elastography and ultrasound elastometry. J Magn Reson Imaging. 2008;28:1287-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Hines CD, Bley TA, Lindstrom MJ, Reeder SB. Repeatability of magnetic resonance elastography for quantification of hepatic stiffness. J Magn Reson Imaging. 2010;31:725-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 43. | Lee DH, Lee JM, Han JK, Choi BI. MR elastography of healthy liver parenchyma: Normal value and reliability of the liver stiffness value measurement. J Magn Reson Imaging. 2013;38:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Lee Yj, Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, Yu MH, Baek JH, Shin CI, Han JK, Choi BI. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014;39:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Runge JH, Bohte AE, Verheij J, Terpstra V, Nederveen AJ, van Nieuwkerk KM, de Knegt RJ, Baak BC, Jansen PL, Sinkus R. Comparison of interobserver agreement of magnetic resonance elastography with histopathological staging of liver fibrosis. Abdom Imaging. 2014;39:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Talwalkar JA, Yin M, Fidler JL, Sanderson SO, Kamath PS, Ehman RL. Magnetic resonance imaging of hepatic fibrosis: emerging clinical applications. Hepatology. 2008;47:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Trout AT, Serai S, Mahley AD, Wang H, Zhang Y, Zhang B, Dillman JR. Liver Stiffness Measurements with MR Elastography: Agreement and Repeatability across Imaging Systems, Field Strengths, and Pulse Sequences. Radiology. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 48. | Shire NJ, Yin M, Chen J, Railkar RA, Fox-Bosetti S, Johnson SM, Beals CR, Dardzinski BJ, Sanderson SO, Talwalkar JA. Test-retest repeatability of MR elastography for noninvasive liver fibrosis assessment in hepatitis C. J Magn Reson Imaging. 2011;34:947-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 538] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 50. | Ichikawa S, Motosugi U, Ichikawa T, Sano K, Morisaka H, Enomoto N, Matsuda M, Fujii H, Araki T. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magn Reson Med Sci. 2012;11:291-297. [PubMed] |
| 51. | Talwalkar JA, Yin M, Venkatesh S, Rossman PJ, Grimm RC, Manduca A, Romano A, Kamath PS, Ehman RL. Feasibility of in vivo MR elastographic splenic stiffness measurements in the assessment of portal hypertension. AJR Am J Roentgenol. 2009;193:122-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Morisaka H, Motosugi U, Ichikawa S, Sano K, Ichikawa T, Enomoto N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging. 2015;41:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, Glaser KJ, Ehman RL. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology. 2014;272:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 54. | Yin M, Kolipaka A, Woodrum DA, Glaser KJ, Romano AJ, Manduca A, Talwalkar JA, Araoz PA, McGee KP, Anavekar NS. Hepatic and splenic stiffness augmentation assessed with MR elastography in an in vivo porcine portal hypertension model. J Magn Reson Imaging. 2013;38:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Dyvorne HA, Jajamovich GH, Besa C, Cooper N, Taouli B. Simultaneous measurement of hepatic and splenic stiffness using MR elastography: preliminary experience. Abdom Imaging. 2015;40:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1734] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 57. | Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212-e219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 58. | Ferraioli G, Lissandrin R, Filice C. Real-time tissue elastography in the assessment of liver stiffness. Hepatology. 2013;58:834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 60. | Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, Attili AF, Riggio O. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-272. [PubMed] |
| 61. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2124] [Article Influence: 111.8] [Reference Citation Analysis (2)] |
| 62. | Colecchia A, Marasco G, Taddia M, Montrone L, Eusebi LH, Mandolesi D, Schiumerini R, Di Biase AR, Festi D. Liver and spleen stiffness and other noninvasive methods to assess portal hypertension in cirrhotic patients: a review of the literature. Eur J Gastroenterol Hepatol. 2015;27:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [PubMed] |
| 64. | Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, Schiumerini R, Turco L, Di Biase AR, Mazzella G. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 371] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 65. | Berzigotti A, Piscaglia F. Ultrasound in portal hypertension--part 1. Ultraschall Med. 2011;32:548-568; quiz 569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Sabbà C, Merkel C, Zoli M, Ferraioli G, Gaiani S, Sacerdoti D, Bolondi L. Interobserver and interequipment variability of echo-Doppler examination of the portal vein: effect of a cooperative training program. Hepatology. 1995;21:428-433. [PubMed] |
| 67. | Sacerdoti D, Gaiani S, Buonamico P, Merkel C, Zoli M, Bolondi L, Sabbà C. Interobserver and interequipment variability of hepatic, splenic, and renal arterial Doppler resistance indices in normal subjects and patients with cirrhosis. J Hepatol. 1997;27:986-992. [PubMed] |
| 68. | Zoli M, Merkel C, Sabbà C, Sacerdoti D, Gaiani S, Ferraioli G, Bolondi L. Interobserver and inter-equipment variability of echo-Doppler sonographic evaluation of the superior mesenteric artery. J Ultrasound Med. 1996;15:99-106. [PubMed] |
| 69. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 525] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 70. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 71. | Robic MA, Procopet B, Métivier S, Péron JM, Selves J, Vinel JP, Bureau C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 72. | Mejias M, Garcia-Pras E, Gallego J, Mendez R, Bosch J, Fernandez M. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol. 2010;52:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Nedredal GI, Yin M, McKenzie T, Lillegard J, Luebke-Wheeler J, Talwalkar J, Ehman R, Nyberg SL. Portal hypertension correlates with splenic stiffness as measured with MR elastography. J Magn Reson Imaging. 2011;34:79-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Hirooka M, Ochi H, Koizumi Y, Kisaka Y, Abe M, Ikeda Y, Matsuura B, Hiasa Y, Onji M. Splenic elasticity measured with real-time tissue elastography is a marker of portal hypertension. Radiology. 2011;261:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol. 2011;26:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 76. | Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, Arora A. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol. 2013;108:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 77. | Singh S, Eaton JE, Murad MH, Tanaka H, Iijima H, Talwalkar JA. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:935-45.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 78. | de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2280] [Article Influence: 228.0] [Reference Citation Analysis (3)] |
| 79. | Gao J, Ran HT, Ye XP, Zheng YY, Zhang DZ, Wang ZG. The stiffness of the liver and spleen on ARFI Imaging pre and post TIPS placement: a preliminary observation. Clin Imaging. 2012;36:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Ran HT, Ye XP, Zheng YY, Zhang DZ, Wang ZG, Chen J, Madoff D, Gao J. Spleen stiffness and splenoportal venous flow: assessment before and after transjugular intrahepatic portosystemic shunt placement. J Ultrasound Med. 2013;32:221-228. [PubMed] |
| 81. | Guo J, Büning C, Schott E, Kröncke T, Braun J, Sack I, Althoff C. In vivo abdominal magnetic resonance elastography for the assessment of portal hypertension before and after transjugular intrahepatic portosystemic shunt implantation. Invest Radiol. 2015;50:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Novelli PM, Cho K, Rubin JM. Sonographic assessment of spleen stiffness before and after transjugular intrahepatic portosystemic shunt placement with or without concurrent embolization of portal systemic collateral veins in patients with cirrhosis and portal hypertension: a feasibility study. J Ultrasound Med. 2015;34:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Chin JL, Chan G, Ryan JD, McCormick PA. Spleen stiffness can non-invasively assess resolution of portal hypertension after liver transplantation. Liver Int. 2015;35:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Colecchia A, Colli A, Casazza G, Mandolesi D, Schiumerini R, Reggiani LB, Marasco G, Taddia M, Lisotti A, Mazzella G. Spleen stiffness measurement can predict clinical complications in compensated HCV-related cirrhosis: a prospective study. J Hepatol. 2014;60:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Bota S, Sporea I, Sirli R, Focsa M, Popescu A, Danila M, Strain M. Can ARFI elastography predict the presence of significant esophageal varices in newly diagnosed cirrhotic patients? Ann Hepatol. 2012;11:519-525. [PubMed] |
| 86. | Vermehren J, Polta A, Zimmermann O, Herrmann E, Poynard T, Hofmann WP, Bojunga J, Sarrazin C, Zeuzem S, Friedrich-Rust M. Comparison of acoustic radiation force impulse imaging with transient elastography for the detection of complications in patients with cirrhosis. Liver Int. 2012;32:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 87. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, Takabatake H, Shimomura H, Doi A, Sakakibara I. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144:92-101.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 88. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |