Copyright
©The Author(s) 2016.
World J Gastroenterol. Aug 7, 2016; 22(29): 6690-6705
Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6690
Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6690
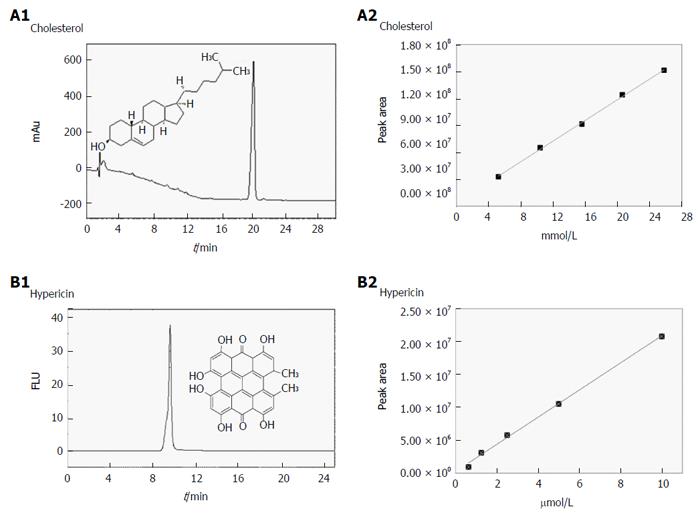
Figure 3 Validation of the chromatographic methods for cholesterol and hypericin quantification in gallstones.
A1-B2: Validation data of the analysis by reverse phase-high performance liquid chromatography on hypericin pre-incubated human gallstones with UV (204 nm) and fluorescence detection (excitation/emission wavelengths: 470/600 nm). A typical UV chromatogram of 5.2 × 10-3 M cholesterol with a retention time (RT) of 19 ± 0.22 min (A1) and the HPLC-generated calibration curve (A2) based on four replicate measurements of five working solutions of cholesterol are presented. A representative fluorescence chromatogram of 1.3 × 10-6 M hypericin with an RT of 9.68 ± 0.06 min (B1) along with corresponding HPLC-generated calibration curve of hypericin (B2) is shown. FLU: Fluorescence units; mAu: Milli-absorbance units.
- Citation: Miranda Cona M, Liu YW, Hubert A, Yin T, Feng YB, de Witte P, Waelkens E, Jiang YS, Zhang J, Mulier S, Xia Q, Huang G, Oyen R, Ni YC. Differential diagnosis of gallstones by using hypericin as a fluorescent optical imaging agent. World J Gastroenterol 2016; 22(29): 6690-6705
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6690.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6690