Published online Jul 21, 2016. doi: 10.3748/wjg.v22.i27.6318
Peer-review started: April 18, 2016
First decision: May 12, 2016
Revised: May 25, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 21, 2016
AIM: To investigate the efficacy of exercise interventions on hepatic fat mobilization in non-alcoholic fatty liver disease (NAFLD) patients.
METHODS: Ovid-Medline, PubMed, EMBASE and Cochrane database were searched for randomized trials and prospective cohort studies in adults aged ≥ 18 which investigated the effects of at least 8 wk of exercise only or combination with diet on NAFLD from 2010 to 2016. The search terms used to identify articles, in which exercise was clearly described by type, duration, intensity and frequency were: “NASH”, “NAFLD”, “non-alcoholic steatohepatitis”, “non-alcoholic fatty liver disease”, “fat”, “steatosis”, “diet”, “exercise”, “MR spectroscopy” and “liver biopsy”. NAFLD diagnosis, as well as the outcome measures, was confirmed by either hydrogen-magnetic resonance spectroscopy (H-MRS) or biopsy. Trials that included dietary interventions along with exercise were accepted if they met all criteria.
RESULTS: Eight studies met selection criteria (6 with exercise only, 2 with diet and exercise with a total of 433 adult participants). Training interventions ranged between 8 and 48 wk in duration with a prescribed exercise frequency of 3 to 7 d per week, at intensities between 45% and 75% of VO2 peak. The most commonly used imaging modality was H-MRS and one study utilized biopsy. The effect of intervention on fat mobilization was 30.2% in the exercise only group and 49.8% in diet and exercise group. There was no difference between aerobic and resistance exercise intervention, although only one study compared the two interventions. The beneficial effects of exercise on intrahepatic triglyceride (IHTG) were seen even in the absence of significant weight loss. Although combining an exercise program with dietary interventions augmented the reduction in IHTG, as well as improved measures of glucose control and/or insulin sensitivity, exercise only significantly decreased hepatic lipid contents.
CONCLUSION: Prescribed exercise in subjects with NAFLD reduces IHTG independent of dietary intervention. Diet and exercise was more effective than exercise alone in reducing IHTG.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is among the leading causes of chronic liver disease with an increasing prevalence worldwide. Diet and exercise are the mainstay of therapy for patients with NAFLD. This systematic review revealed that both aerobic and resistance exercise, independent of any other intervention, are successful in increasing hepatic fat mobilization. This effect is augmented by combining exercise with dietary interventions. The findings of this systematic review support that exercise interventions are effective in reducing intrahepatic triglyceride in patients with NAFLD independent of weight loss or dietary manipulation.
- Citation: Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J Gastroenterol 2016; 22(27): 6318-6327
- URL: https://www.wjgnet.com/1007-9327/full/v22/i27/6318.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i27.6318
Non-alcoholic fatty liver disease (NAFLD) is an important cause of chronic liver disease worldwide and represents a spectrum of liver diseases ranging from hepatic steatosis to non-alcoholic steatohepatitis (NASH)[1,2]. Recent studies clearly showed that the global prevalence of NAFLD is approximately 25%[3,4]. Although NAFLD or simple steatosis is not likely to progress to advanced stages of liver disease, it is associated with cardiovascular disease[5]. In contrast, NASH may progress to hepatic fibrosis, cirrhosis and hepatocellular carcinoma[6-8]. The histopathology of NAFLD is characterized by accumulation of liver fat, which exceeds 5% of liver weight in the absence of excessive amount of alcohol consumption, viral infection or other hepatic etiology. NAFLD is strongly associated with obesity, insulin resistance, and dyslipidemia and is known as the hepatic manifestation of metabolic syndrome[1,2,9,10].
Lifestyle modification is currently accepted as the first line of treatment for the management of NAFLD and weight loss is the only confirmed effective therapy for the treatment of NAFLD[1]. Lifestyle modification is a general term whose components often differ. It may be non-specific and is a clinical recommendation rather than a prescription. When health care providers suggest that patients follow recommendations for lifestyle changes, additional specificity is important to provide. The components of lifestyle changes usually include diet and exercise, as well as recommendations about smoking cessation, moderate use of alcohol, attention to sleep and stress reduction[11,12]. A good example of such recommendations is the possible beneficial effect of a Mediterranean diet. Previous studies pointed out that Mediterranean diet is associated with lower incidence of cardiovascular disease and metabolic disorders[13].
Exercise is different from activity. Activity refers to any movement requiring energy, that is, not resting. In fact, exercise is not synonymous with physical activity; it is a subcategory of it, a planned, structured, repetitive and purposive subcategory with a specific intensity, frequency and duration[14]. For most health outcomes, additional benefits occur as the amount of physical activity increases through higher intensity, greater frequency, and/or longer duration. Exercise has been documented to be an effective intervention for reducing intrahepatic fat by reducing hepatic lipogenesis[15]. In fact, three types of exercise have been reported to be effective. One type is walking and jogging, which are examples of aerobic exercise. This type of exercise is “any activity that uses large muscle groups, can be maintained continuously and is rhythmic in nature”[16]. The second type of exercise is muscle strengthening, this requires muscles to do a greater amount of work than usual. This is muscle overload and utilizes anerobic metabolism. Muscle strengthening, also known as resistance exercise increases strength, tone, muscle mass, and/or muscle endurance. Flexibility exercise is the activity such as stretching, designed to increase joint range of motion and extensibility of muscle[17,18]. The American Gastroenterological Association, the American Association for the Study of Liver Diseases and American College of Gastroenterology, all recommend aerobic exercise as a treatment for NAFLD[19].
The aim of this study was to conduct a systematic review of the pooled data from adult human trials to investigate the efficacy of exercise (aerobic, resistance or combined) interventions with or without dietary interventions on fat mobilization from liver in patients with NAFLD.
Ovid MEDLINE, PubMed, EMBASE and Cochrane database were searched from 2010 to 2016. Two of the authors (PG and MB) performed literature search. The last search of all databases was done on February 26, 2016. In case of a disagreement of eligibility of a study, the authors discussed the issue with a third author. The database searches were performed using the keywords: (“NAFLD”, “non-alcoholic fatty liver disease”, “NASH”“non-alcoholic steatohepatitis”, “fat”, “fatty liver”, “steatosis”) and (“exercise”, “aerobic training”, “resistance training”, “diet”) and (“fat mobilization”, “intrahepatic lipids”, “intrahepatic triglyceride”, “MRI”, “MR spectroscopy”, “H-MRS”, “liver biopsy”).
The search terms listed above were used to identify articles for consideration. Studies examining the association between exercise and fat mobilization, with duration of at least 8 wk, with participants older than 18 years of age, of any sex or ethnic origin with NAFLD/NASH and diagnosed on the basis of radiological/histological evidence of fatty liver were included. Furthermore, studies that clearly prescribed their intervention by type, duration, intensity and frequency, and provided adherence to study protocol were eligible for inclusion. Randomized trials were included. Trials that included dietary interventions were accepted if they met all criteria. Studies were excluded if they didn’t specify specific exercise prescriptions, outcome measures demonstrating an exercise effect (i.e., measures of fitness and/or strength), and quantitative measures of intrahepatic fat. Also, studies or study arms for which dietary supplements, herbal preparations, nutraceutical were the intervention to the study were not included.
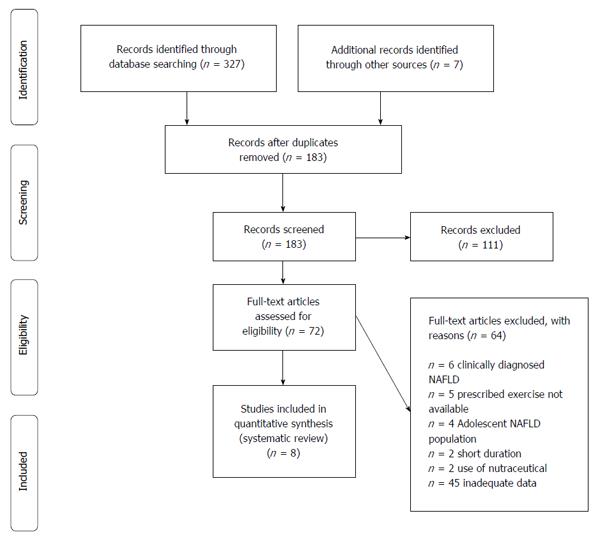
Titles and abstracts of studies retrieved were evaluated against eligibility criteria. Each manuscript was assessed for pertinence to the issue of prescribed exercise, quantitative measurement of fat in patients with NAFLD. Studies appearing eligible based on their abstract were read in full. Reference lists from all identified studies were searched for relevant studies. The material used was written in English (Figure 1).
Calculations of change with respect to percent liver fat were performed on all studies selected. The mean fat reduction from all studies was calculated by determining the mean reduction in fat reported for each study, determining the number of subjects in each study and what percent of the total patient group from all studies it constituted and totaled the findings. In this fashion we compared percent fat reduction in the group receiving exercise and those receiving diet plus exercise.
The primary outcome assessed was a decrease in IHTG as determined by histology or H-MRS.
Liver Biopsy: For the definitive diagnosis and grading of NAFLD, histological examination by liver biopsy is still the gold standard. However, it is being used less frequently and has some well-known limitations, such as the risk of complications, potential sampling errors and variability of pathologic interpretation[6]. Also, a typical liver biopsy samples only 1/50000 of all liver tissue.
Proton magnetic resonance spectroscopy: Previous studies validated the use of H-MRS for assessing intrahepatic lipid content[20-23]. The assessment of IHTG by H-MRS is highly reliable as this technique samples a much larger liver volume than can be obtained through routine liver biopsy, minimizing the likelihood of sampling error[21,24]. Indeed, H-MRS is the most direct MR based method to separate the liver signal into its water and fat components and calculate a signal fraction[25].
Exercise was classified according to the American College of Sports Medicine guidelines that define exercise intensity according to the maximum oxygen consumption (VO2max) that is reached during exercise and categorize intensity into 5 groups as follows: very light (< 37% VO2max), light (37%-45% VO2max), moderate (46%-64% VO2max), vigorous (64%-91% VO2max) and near maximal to maximal (> 91% VO2max). Exercise duration is divided into two groups; high duration includes exercising daily and at least for 60 min, whereas low duration includes exercising below this threshold[16,26]. Resistance exercise was defined in terms of number of repetitions per amount of weight lifted. Mean drop in IHTG was calculated after normalizing the contribution of fat reduction in each study. For each study, the ratio of controls to those receiving intervention and the total sample was calculated. This percent of the total was multiplied by the percent fat reduction (or increase) for each study.
There were 364 studies of patients NAFLD for whom exercise was prescribed and there were required outcome measures. An additional 7 studies were identified from review of references. After screening for studies published from 2010-2016, 183 studies were identified. Priority was given to well-powered randomized trials. One hundred and eleven were excluded after review of the abstract because they did not include people with NAFLD, or they did not assess the association between NAFLD, exercise and intrahepatic fat mobilization. Only eight studies met all criteria and were included in this review.
All of these studies were randomized trials. One study compared the efficacy of aerobic and resistance exercise in patients with NAFLD. One study utilized biopsy to measure the effects on hepatic histology. The most commonly employed imaging modality to determine change in hepatic steatosis was H-MRS.
This analysis combined 8 studies involving a total of 433 adult participants, of which all were randomized trials. In all studies, either aerobic or resistance exercise was prescribed for participants and in only two of them were there dietary intervention. Exercise prescription in studies varied in session duration, intensity, volume (exercise dose) and modality. Dropout rates ranged between 6%-45%. In our analysis, training interventions ranged between 8 and 48 wk in duration with a prescribed exercise frequency of 3 to 7 d per week, at intensities between 45% and 75% of VO2 peak. Adherence to exercise was monitored using objective measures such as heart rate monitor, blood pressure measurements, pedometer and accelerometers (Table 1).
| Ref. | Number of subjects with NAFLD | Age (mean) | Sex (male, %) | BMI (mean) | Primary measure | Exercise intervention | Dietary intervention | Program length | Session frequency (wk) | Exercise session duration (min) |
| Hallsworth et al[27], 2011 | 21 | AE: 52 | NR | AE: 32.3 | H-MRS | RE | No | 8 wk | 3 | 45-60 |
| C: 62 | C: 32.3 | |||||||||
| Sullivan et al[28], 2012 | 33 | E: 49 | AE: 33 | AE: 37.1 | H-MRS | AE | No | 16 wk | 5 | 30-60 |
| C: 48 | C: 17 | C: 40 | ||||||||
| Bacchi et al[29], 2013 | 40 | AE: 56 | AE: 71 | AE: 30.5 | H-MRS | AE and RE | No | 4 mo | 3 | 60 |
| RE: 56 | RE: 71 | RE: 28.8 | ||||||||
| Eckard et al[33], 2013 | 56 | LFDE: 44 | LFDE: 50 | LFDE: 32.7 | Liver biopsy | AE | Yes | 6 mo | 4-7 | 20-60 |
| MFDE: 55 | MFDE: 67 | MFDE: 40.3 | ||||||||
| ME: 52 | ME:67 | ME: 31.3 | ||||||||
| C: 51 | C: 64 | C: 34.7 | ||||||||
| Wong et al[12], 2013 | 154 | AE: 51 | AE: 52 | AE: 51 | H-MRS, | AE | Yes | 12 mo | 3-5 | 30 |
| C: 26 | C: 41 | C: 25.3 | Fibroscan | |||||||
| Pugh et al[30], 2014 | 31 | AE: 48 | AE: 54 | AE: 31 | H-MRS | AE | No | 16 wk | 3 | 30-45 |
| C: 47 | C: 50 | C: 30 | ||||||||
| Cuthbertson et al[31], 2016 | 69 | AE: 50 | AE: 77 | AE: 30.6 | H-MRS | AE | No | 16 wk | 3-5 | 30-45 |
| C: 52 | C: 80 | C: 29.7 | ||||||||
| Hallsworth et al[32], 2015 | 29 | AE: 54 | NR | AE: 31 | H-MRS | AE | No | 12 wk | 3 | 30-40 |
| C: 52 | C: 31 |
A total of 350 patients completed the studies. There were 184 included in the exercise only studies (64 controls and 120 received interventions). There were 177 who were treated with diet and exercise (82 controls and 95 received interventions). In the studies that assessed the effect of exercise only, mean drop in %fat was 30.2% in the intervention group and 5.6% in the control group; whereas, in studies with diet and exercise, mean %fat drop was 49.8% for the intervention group and 15.8% in the control group (Tables 2 and 3).
| Ref. | Number of subjects with NAFLD | Number of subjects who completed the study | Exercise group | Control group | Percentage of fat reduction in exercise group | Percentage of fat reduction in control group |
| Hallsworth et al[27], 2011 | 21 | 19 | 11 | 8 | 13% | 3% |
| Sullivan et al[28], 2012 | 33 | 18 | 12 | 6 | 10% | -8%3 |
| Bacchi et al[29], 2013 (Aerobic)1 | 40 | 31 | 14 | - | 33% | - |
| Bacchi et al[29], 2013 (Resistance)1 | 40 | 31 | 17 | - | 26% | - |
| Eckard et al[33], 20132 | 56 | 41 | 9 | 11 | 21% | 8% |
| Pugh et al[30],2014 | 31 | 21 | 13 | 8 | 33% | 16% |
| Cuthbertson et al[31], 2016 | 69 | 50 | 30 | 20 | 48% | 9% |
| Hallsworth et al[32], 2015 | 29 | 25 | 14 | 11 | 26% | -1%3 |
| Ref. | Number of subjects with NAFLD | Number of subjects who completed the study | Diet and exercise group | Control group | Percentage of fat reduction in exercise group | Percentage of fat reduction in control group |
| Eckard et al[33], 20131 | 56 | 41 | 9 | 11 | 27 | 8 |
| Eckard et al[33], 20131 | 56 | 41 | 12 | 11 | 35 | 8 |
| Wong et al[12], 2013 | 154 | 145 | 74 | 71 | 55 | 17 |
In their study among 21 patients with NAFLD, Hallsworth et al[27] analyzed the effects of resistance exercise on IHTG content in the absence of weight loss. In this study, participants performed a moderate intensity/low duration exercise. The intervention group exercised for 3 sessions per week (45-60 min each) for 8 wk. It was found that independent of any change in body weight, resistance exercise reduced IHTG in 8 wk quantified by H-MRS. This study revealed that although no significant changes in blood lipids or ALT were observed, glycemic control, insulin resistance and HOMA scores were improved and there was a 13% relative reduction in hepatic fat in exercise group.
In another study by Sullivan et al[28] the effects of aerobic exercise on IHTG content were assessed. The participants in the exercise group were prescribed a program of 5 sessions per week (30-60 min each) for 16 wk. This study found that without any change in body weight or % body fat, aerobic exercise had a small but beneficial effect on IHTG. It was reported that a 10.3% ± 4.6% relative decrease in IHTG content was seen in the exercise group and a 12.8% ± 3.1% decrease in ALT levels. The authors concluded that as hepatic VLDL-TG secretion rate and VLDL-apoB-100 secretion rate did not exhibit any change, hepatic lipoprotein kinetics remain unchanged.
A randomized trial was conducted in Italy in 2013 to compare aerobic and resistance exercise in type 2 diabetic patients with NAFLD[29]. In this male dominant study (22 males vs 9 females), mean ages of both exercise groups were similar (55.6 ± 2 vs 56 ± 2). The aerobic exercise group performed moderate to vigorous intensity exercise for 60 min, 3 sessions per week for 16 wk while resistance exercise group performed 3 series of 10 repetitions at 70%-80% VO2max, with 1 min of recovery between series for 60 min, 3 sessions per week for 16 wk. In H-MRS quantifications at the end of study period, resistance exercise was found to be equally effective as aerobic exercise in reducing hepatic fat mass. Both groups exhibited significant reductions in IHTG content (32.8% in aerobic vs 25.9% in resistance) and showed improvements in HbA1c, HDL, TG levels and insulin sensitivity.
In another randomized controlled trial in United Kingdom, Pugh et al[30] investigated the associations between hepatic fat and endothelial dysfunction among obese NAFLD patients. Participants in the intervention group performed a supervised aerobic exercise, which was moderate intensity and low duration, and was gradually increased during the study period of 16 wk. It was found that there was a 33.3% change in IHTG by H-MRS in the exercise group and 16.8% in the control group, as well as improvements in the flow mediated dilatation on brachial arteries. They concluded that moderate intensity exercise can improve endothelial dysfunction and reduce the risk of cardiovascular disease.
Another study was conducted among 69 patients with NAFLD to determine if there was dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homeostasis in NAFLD[31]. In this randomized controlled trial, patients were randomly assigned to either 16 wk of supervised exercise or conventional counselling. Intensity of the exercise started from 3 times a week, for 30 min per session, reaching 30% of heart rate reserve and increased to 5 times a week, for 45 min per session and reaching to 60% of heart rate reserve. After 16 wk, IHTG content significantly decreased from 19.4% to 10.1% in the exercise group, but not in the control group (from 16% to 14.6%). There was a significant difference with the amount of weight reduction between two groups (mean change -2.5 Kg in exercise group and 0.2 in control group). Although liver function tests decreased in both groups, it was not statistically significant. In the exercise group, peripheral insulin resistance improved as opposed to hepatic insulin resistance.
In another randomized controlled study by Hallsworth et al[32], the effect of high-intensity interval training on liver fat, cardiac function and metabolic control in patients with NAFLD was assessed. Twelve weeks of cycle ergometry three times per week resulted in reduction in H-MRS measured IHTG of 27%. Exercise also resulted in reduction in fat mass (mean 1.8 kg), plasma ALT, AST and improvement in cardiac diastolic function, but with limited impact on glucose control.
We identified two eligible articles that studied 177 participants in which both diet and exercise were prescribed[12,33]. In the study by Wong et al[12], the intervention involved a community-based lifestyle modification was for 12 mo, moderate in intensity and low in duration. Seventy-four patients were in the diet and exercise group and 71 patients were in the control. The two groups were well-matched in demographic characteristics, clinical and laboratory data, IHTG, and liver stiffness measurements. In this study 64% of patients achieved remission of NAFLD in the intervention group and 20% in the control group. Patients in the intervention group had greater reduction in body weight (5.6 kg), BMI and waist circumference, total cholesterol, LDL, ALT and liver stiffness. Also, in intervention group IHTG component reduced by 6.6% as compared to 2.1% in control group (P < 0.001).
The second study conducted with diet and exercise included 56 biopsy proven NAFLD patients who were divided into four groups as follows: low fat diet and moderate exercise, moderate fat diet and moderate exercise, moderate exercise, and control[33]. Exercise prescribed was moderate in intensity and low in duration, for a total of 6 mo. The effects of the interventions were evaluated at the end of study with a repeat liver biopsy. It was found that low or moderate fat diet and moderate exercise significantly decreased the mean NAFLD activity scores (NAS) in 6 mo. By comparison, the change in NAS in the exercise only and control groups was not significant. It was also stated that weight loss was not necessary for improvement in liver histology. The data are summarized in Table 4.
| Ref. | Intervention | Changes in fat | Physiologic changes | Clinical outcome | Exercise outcome |
| Hallsworth et al[27], 2011 | RE | 13% relative decrease in IHTG in exercise group | No significant change in blood lipids or ALT | No effect on body weight, visceral adipose tissue volume or whole body fat | RE without weight change is effective in reducing IHTG in people with NAFLD |
| Approximately 12% increase in insulin sensitivity and increased fat oxidation | |||||
| Sullivan et al[28], 2012 | AE | 10.3% ± 4.6% relative decrease in IHTG in exercise group | Plasma ALT decreased 12.8% + 3.1 in exercise group | Body weight, body fat mass remained same | Small decrease in IHTG content |
| Bacchi et al[29], 2013 | AE and RE | Reduction in IHTG by 35.8% in AE vs 25.9% in RE | HbA1c, HDL, TG, insulin sensitivity improved | BMI, total body fat mass, VAT, SAT were reduced | Absolute and relative reduction in IHTG in both exercise groups |
| Eckard et al[33], 2013 | Diet and AE | Significant change was found in pre to post NAFLD activity score | Significant decrease in Brunt grade, ALT, AST | No subgroup achieved a significant weight loss of > 5% | Lifestyle modification improved liver histology after 6 mo intervention |
| Changes in % body fat were minimal | Weight loss is not the key to improving liver histology | ||||
| Wong et al[12], 2013 | Diet and AE | 6.7% decrease in IHTG in intervention group | Decrease in Total cholesterol, LDL, ALT and liver stiffness | Reduction in body weight 5.6 kg, in BMI and waist circumference | 64% of patients achieved remission of NAFLD in exercise group |
| Pugh et al[30], 2014 | AE | IHTG decreased by 33% in exercise group SAT decreased no significant difference in VAT, total abdominal fat and muscle fat | Fasting glucose decreased No difference in HOMA score, insulin, liver enzymes, lipid profile, adiponectin, and leptin | No weight change Cardiorespiratory fitness improved Waist circumference decreased | improved endothelial dysfunction in the absence of change in liver fat and visceral fat content exercise training can reduce intrinsic CVD risk in NAFLD |
| Cuthbertson et al[31], 2016 | AE | IHTG Significantly decreased (19.4%→10.1% in AE, 16%→14.6% in control) | No significant change in HOMA, plasma insulin, fetuin, irisin, adiponectin | Cardiorespiratory fitness improved in exercise group | Improvement in peripheral IR but not in hepatic IR |
| Hallsworth et al[32], 2015 | AE | 27% reduction in IHTG in exercise group | Decrease in ALT and AST Improvement in diastolic function | No weight change Mean 1.8 kg reduction in fat mass and body fat percentage | Significant reduction in IHTG, liver enzymes and body fat |
This systematic review assessed the published literature to determine the efficacy of exercise interventions in modifying the amount of IHTG in adults. The results suggest that regardless of type, exercise reduces the amount of IHTG in patients with NAFLD. In fact, the beneficial effects of exercise on intrahepatic lipids are seen even in the absence of significant weight loss. Although combining exercise program with dietary interventions augments the reduction in IHTG, as well as improves measures of glucose control and/or insulin sensitivity, exercise only can also significantly decreases hepatic lipid contents. Also, it is emphasized in the recent guidelines that for patients with NAFLD, the choice of training should be tailored based on patients’ preferences to be maintained in the long term[34]. It can be suggested that exercise 3-4 times a week, at 20-40 min per session with achieving 70% VO2max is ideal for mobilizing fat from liver among NAFLD patients. This is considered a moderate level.
Exercise is considered to be one of the most effective, non-pharmacological interventions in the treatment of nonalcoholic fatty liver disease[35,36]. Although the protective effects of exercise on metabolic disease was demonstrated many decades ago, still relatively little is known about the underlying molecular mechanisms. The most striking hepatic adaptation to exercise is the decrease in hepatic lipid content, even when overall weight loss is not observed[37,38]. Our main findings are in agreement with previous systematic reviews and meta-analyses[39-41]. Keating et al[39], aimed to assess the efficacy of aerobic or resistance training on both hepatic fat and ALT levels and demonstrated that exercise alone was effective on fat mobilization from liver. On the other hand, the authors concluded that there was no significant difference in either ALT levels or total body weight between the exercise and control groups[39]. Another systematic review aimed to assess the efficacy of lifestyle interventions and included diet only interventions along with exercise only interventions and combined studies. It was noted that weight reductions of 4%-14% resulted in significant reductions in IHTG levels of 35%-81%. It was also stated that exercise could lead to decrements in IHTG and weight loss was not a prerequisite for this change[40].
In contrast to the findings of this review, in a study that did not meet our inclusion criteria (study population was not restricted to subjects with NAFLD), the investigators found that calorie restriction only was equal to calorie restriction with exercise in reducing liver fat[42]. The authors stated that there was no additive effect of exercise training. The two major caveats of this study were: CT was used to assess IHTG and some participants at the baseline did not have intrahepatic fat accumulation.
One of the limiting factors of this study is that there are relatively few studies that meet all criteria for inclusion and that many of the studies had a small number of participants (< 100 subjects). This is not uncommon for exercise intervention studies that require behavioral change to assure adherence for a relatively long period, because one often needs > 8 wk to see an increase in aerobic capacity or strength. However, the authors arbitrarily used very strict criteria for what was considered exercise and which outcomes would be acceptable for determining an exercise effect. The former required that studies list the frequency and intensity not only the term “exercise” or "activity", to qualify. The outcomes needed to include standard physiological assessments of exercise such as heart rate, or oxygen consumption. This assures that the exercise is actually performed. Secondly, it is often difficult to keep patients motivated to participate in this type of interventional study. We believe that the relative infrequency of studies performed for exercise-induced intrahepatic fat reduction is, in part due to these factors.
One of the most significant challenges we face in utilizing this effective treatment is adherence to exercise. Interpretation of the cumulative data from these reviews suggests that one strategy to increase adherence might be to target exercise and not substantially limit dietary intake or change the ratios of macronutrients. Additionally, selecting an exercise program that targets 50%-70% of heart rate maximum is likely to be well tolerated and not to be experienced as too challenging. In other words, this level of intensity is not likely to require the exercise to be done in anaerobic range, thereby minimizing discomfort.
In conclusion, this systematic review permits a pooling of studies that met strict criteria for measures of intrahepatic fat and a prescribed exercise intervention. An exercise intervention of moderate intensity is effective for the mobilization of IHTG. The findings support the view that exercise is effective in reducing IHTG in patients with NAFLD independent of weight loss or dietary manipulation. Combining exercise with dietary interventions augments the reduction in IHTG.
The authors would like to thank all the staff at Inova Fairfax Hospital Library for their great support and conscientious work during the literature search of this study.
Non-alcoholic fatty liver disease (NAFLD) has become a significant healthcare problem around the world. Lifestyle modifications are the cornerstone of treatment for the management of NAFLD. NAFLD can be reversed by reducing intrahepatic fat content which may decrease the undesired hepatic and metabolic effects. This is why the authors recommend for regular exercise healthy eating plan for these patients.
Multiple studies have tried to assess the effect of exercise for hepatic fat mobilization with or without combination dietary interventions. Studies have focused on both aerobic and resistance type of exercise, based on the duration, intensity and frequency of exercise. Most of the studies used moderate intensity/low duration exercise as an intervention. The details of dietary interventions include the amount of carbohydrates and fat in diet.
Both aerobic and resistance exercise interventions have been shown to be effective in reducing intrahepatic triglyceride content. The addition of dietary interventions augments hepatic fat reduction of exercise. Exercise interventions are successful in mobilizing fat from liver tissue, independent of weight loss.
The studies selected for this systematic review support the benefits of lifestyle modifications. An exercise intervention of moderate intensity is effective for the mobilization of intrahepatic triglycerides. Combining exercise with dietary intervention augments the success of lifestyle modification for hepatic fat reduction.
In most studies, in order to assess the change in intrahepatic fat content, hydrogen-magnetic resonance spectroscopy (H-MRS) is utilized. H-MRS is a non-invasive magnetic resonance imaging based imaging technique which is highly reliable for assessing hepatic parenchyma. It works by separating liver signal into water and fat components from which one is able to calculate a signal fraction.
In this systematic review, the authors aimed to evaluate the effect of exercise on hepatic fat content via conducting a broad literature search with strict inclusion criteria. The approach was performed in a careful, systematic way in order to determine the level of evidence for exercise as an effective mode for mobilizing fat from the liver.
Manuscript source: Invited manuscript
P- Reviewer: Abenavoli L, Pimenta NM, Trovato GM S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1226] [Cited by in F6Publishing: 1253] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 2. | Montecucco F, Mach F. Does non-alcoholic fatty liver disease (NAFLD) increase cardiovascular risk? Endocr Metab Immune Disord Drug Targets. 2008;8:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5322] [Cited by in F6Publishing: 6281] [Article Influence: 785.1] [Reference Citation Analysis (0)] |
| 4. | Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126-133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Mishra A, Younossi ZM. Epidemiology and Natural History of Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Golabi P, Sayiner M, Fazel Y, Koenig A, Henry L, Younossi ZM. Current complications and challenges in nonalcoholic steatohepatitis screening and diagnosis. Expert Rev Gastroenterol Hepatol. 2016;10:63-71. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, McCullough A, Goodman Z, Younossi ZM. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci. 2013;58:3017-3023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Attar BM, Van Thiel DH. Current concepts and management approaches in nonalcoholic fatty liver disease. ScientificWorldJournal. 2013;2013:481893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 452] [Cited by in F6Publishing: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 10. | Sdiri W, Romdhane H, Mbarek D, Ben Abdallah H, Longo S, Abdelli MN, Boujnah MR. Non alcoholic fatty liver disease: a new risk factor for cardiovascular disease? Tunis Med. 2013;91:171-174. [PubMed] [Cited in This Article: ] |
| 11. | Baran B, Akyüz F. Non-alcoholic fatty liver disease: what has changed in the treatment since the beginning? World J Gastroenterol. 2014;20:14219-14229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 13. | Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol. 2014;20:16831-16840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 72] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 14. | Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126-131. [PubMed] [Cited in This Article: ] |
| 15. | Johnson NA, Keating SE, George J. Exercise and the liver: implications for therapy in fatty liver disorders. Semin Liver Dis. 2012;32:65-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | American College of Sports Medicine. US Department of Health and Human Services, 2008 Physical Activity Guidelines for Americans. 2008. Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf. [Cited in This Article: ] |
| 17. | Guo R, Liong EC, So KF, Fung ML, Tipoe GL. Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2015;14:139-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Miyakoshi N. Therapeutic exercise. Clin Calcium. 2008;18:1611-1615. [PubMed] [Cited in This Article: ] |
| 19. | American Gastroenterological Association. American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702-1704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | McPherson S, Jonsson JR, Cowin GJ, O’Rourke P, Clouston AD, Volp A, Horsfall L, Jothimani D, Fawcett J, Galloway GJ. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Fabbrini E, Conte C, Magkos F. Methods for assessing intrahepatic fat content and steatosis. Curr Opin Clin Nutr Metab Care. 2009;12:474-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Banerjee R, Pavlides M, Tunnicliffe EM, Piechnik SK, Sarania N, Philips R, Collier JD, Booth JC, Schneider JE, Wang LM. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol. 2014;60:69-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 23. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1113] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 24. | Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34:spcone. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 290] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334-1359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5130] [Cited by in F6Publishing: 5260] [Article Influence: 404.6] [Reference Citation Analysis (0)] |
| 27. | Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278-1283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 311] [Article Influence: 23.9] [Reference Citation Analysis (2)] |
| 28. | Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738-1745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58:1287-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 30. | Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Cable NT, Jones H, Cuthbertson DJ. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307:H1298-H1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, Kemp GJ, Barrett M, Jackson NC, Thomas EL. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2016;130:93-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 32. | Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond). 2015;129:1097-1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 33. | Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6:249-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 100] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 34. | European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016;9:65-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 35. | Federico A, Zulli C, de Sio I, Del Prete A, Dallio M, Masarone M, Loguercio C. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:16841-16857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Ordonez R, Carbajo-Pescador S, Mauriz JL, Gonzalez-Gallego J. Understanding nutritional interventions and physical exercise in non-alcoholic fatty liver disease. Curr Mol Med. 2015;15:3-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Königsrainer A, Königsrainer I, Kröber S, Niess A. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 38. | Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105-1112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 418] [Cited by in F6Publishing: 407] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 39. | Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 40. | Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol. 2012;56:255-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 352] [Cited by in F6Publishing: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 41. | Whitsett M, VanWagner LB. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J Hepatol. 2015;7:2041-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Yoshimura E, Kumahara H, Tobina T, Matsuda T, Ayabe M, Kiyonaga A, Anzai K, Higaki Y, Tanaka H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes. 2014;2014:197216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |