Published online Apr 21, 2016. doi: 10.3748/wjg.v22.i15.3962
Peer-review started: November 30, 2015
First decision: December 16, 2015
Revised: December 30, 2015
Accepted: January 17, 2016
Article in press: January 18, 2016
Published online: April 21, 2016
AIM: To study the therapeutic effect of norcantharidin (NCTD) combined with ABT-737 on hepatocellular carcinoma cells and the molecular mechanism.
METHODS: Two hepatocellular carcinoma (HCC) cell lines, HepG2 and SMMC-7721, were selected. ABT-737 and NCTD were allocated into groups to be used alone or in combination. HepG2 and SMMC-7721 cells were cultured in vitro. Liver cancer cells in the logarithmic phase of growth were vaccinated and cultured to the cell wall stage; these cells were treated for 48 h with different concentrations of NCTD, or ABT-737, or NCTD combined with ABT-737. The cell proliferation inhibition rate was detected by methyl thiazolyl tetrazolium. The expression of Mcl in HCC cells was detected by Western Blotting, and the cells in each group after treatment had apoptosis detected by flow cytometry. The proliferation inhibition rate, the expression of Mcl-1 in cells and the apoptosis inducing effect of treatment were observed in each group, and the effect of NCTD on ABT-737 in the treatment of HCC and its mechanism of action were analyzed.
RESULTS: As the concentration of NCTD increased, the cell proliferation inhibition rate gradually decreased; and the treatment effect of ABT-737 1-3 μm combined with NCTD on cell proliferation inhibition was stronger than that of ABT-737 alone. The difference was statistically significant (P < 0.05). In observing the expression of Mcl-1 in cells after the treatment of different concentrations of NCTD, this was partially inhibited after treatment with NCTD 15 μm, and the expression of Mcl-1 was almost undetectable after treatment with NCTD 30 μm and 60 μm. The effect on inducing apoptosis with the treatment of ABT-737 or NCTD alone for 48 h was lower than that of the control group. The difference was not statistically significant (P > 0.05). The effect on inducing apoptosis in HepG2 and SMMC-7721 cells with the treatment of ABT-737 combined with NCTD for 48 h was greater than that of ABT-737 or NCTD alone. The difference was statistically significant (P < 0.05).
CONCLUSION: NCTD combined with ABT-737 has a positive role in the treatment of HCC, and it has great value in clinical research.
Core tip: The effects of ABT-737 and norcantharidin (NCTD) alone or in combination on HepG2 and SMMC-7721 cells were tested by methyl thiazolyl tetrazolium, Western blot and flow cytometry. We found that as the concentration of NCTD increased, the cell proliferation inhibition rate gradually decreased; and the treatment effect of ABT-737 1-3 μm combined with NCTD on cell proliferation inhibition was stronger than that of ABT-737 alone (P < 0.05). The effect on inducing apoptosis in HepG2 and SMMC-7721 cells with the treatment of ABT-737 combined with NCTD for 48 h was greater than that of ABT-737 or NCTD alone (P < 0.05). NCTD combined with ABT-737 has a positive role in the treatment of HCC.
- Citation: Ren J, Li G, Zhao W, Lin L, Ye T. Norcantharidin combined with ABT-737 for hepatocellular carcinoma: Therapeutic effects and molecular mechanisms. World J Gastroenterol 2016; 22(15): 3962-3968
- URL: https://www.wjgnet.com/1007-9327/full/v22/i15/3962.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i15.3962
Myeloid cell leukemin-1 (Mcl-1) is a special B-cell lymphoma 2 (Bcl-2) family protein. It can not only control cell survival and death, but also plays an important role in regulating apoptosis signaling[1-3]. Several studies have shown that Mcl-1 generally has a high expression in hepatocellular carcinoma (HCC) and other malignant tumors[4-6], and this has become a cancer research focus of molecular targeted therapy. ABT-737 is a novel cancer therapeutic agent that has good prospects for clinical application[7]. However, ABT-737-mediated apoptosis is limited when there is high expression of Mcl-1 in liver cancer and other solid tumors[8-10], and this has become a major obstacle point in clinical application. Research has shown the treatment sensitivity of tumor cells to ABT-737 can be enhanced by its combination with other chemotherapy drugs[11-14].
Norcantharidin (NCTD) is a derivative of the Chinese medicine cantharidin, which has good anti-tumor effects[15-17]. Studies have reported that the anti-tumor effect of NCTD may be related to the role of Bcl-2 family members[18], which can inhibit Mcl-l expression in HCC cells[19]. Therefore, this study aims to investigate the therapeutic effects of NCTD combined with ABT-737 on HCC cells, and to preliminarily analyze its mechanism of action for the future development of anticancer drugs, aiming to provide theoretical guidance for clinical applications.
HCC cell lines: HepG2, SMMC-7721 (purchased from Cell Bank of Beijing Concord Technical Institute). Reagents and equipment details are displayed in Table 1.
| Primary reagents | Source |
| ABT-737 | Biochempartner |
| NCTD | Nanjing Zelang Medical Technology Co., Ltd. |
| DMSO | Hyclone |
| 96-well and 6-well cell culture plates | Costar, United States |
| Methyl thiazolyl tetrazolium (MTT) | Sigma, United States |
| Trypsin | Hangzhou Gino Biomedical Technology Co., Ltd. |
| CO2 Incubator | Thermo Scientific, United States |
| Multiskan MK3 microplate reader | Thermo Scientific, United States |
| Flow cytometer | BD Biosciences |
| TUNEL Assay Kit for Apoptosis Detection | Nanjing KeyGEN BioTECH |
Cultured cell lines: Hepatoma cell lines HepG2 and SMMC-7721 were cultured in vitro, placed in RPMI-1640 medium containing 10% fetal bovine serum, and placed in an incubator with 5% CO2 at 37 °C.
Cell proliferation inhibition detection by methyl thiazolyl tetrazolium assay: HepG2 and SMMC-7721 hepatoma cells in the logarithmic growth phase were seeded into 96-well plates and cultured. Cells were divided into the following groups when they attached to the wall: ABT-737 monotherapy group, NCTD monotherapy group, ABT-737 combined with NCTD group, control group, and apoptosis group; and each group had 3 parallel wells. After treatment, culture was continued for 48 h. Then, 20 μL of methyl thiazolyl tetrazolium (MTT) solution was added into each well, and incubated for 4 h in an incubator. The supernatant was discarded, 150 μL of DMSO was added into each well, and they were placed in the incubator for 10 min. Optical density (OD) value was measured with an enzyme mark instrument. Measurements were repeated three times, and the average value was obtained. Proliferation inhibitory rates of the drug-treated groups were calculated as follows: inhibition rate (%) = [1 - (average OD value of drug-treated groups - average OD value of the apoptosis group)/(average OD value of the control group - average OD value of the apoptosis group)] × 100%.
Western blot detection of Mcl expression in hepatoma cells: Before initiation of the experiment, 4 × 105 hepatoma cells were seeded in 6-well plates. After cell adhesion, they were treated with NCTD alone, ABT-737 alone, and NCTD combined with ABT-737, and placed in an incubator for 24 h. After drug-treated cells were trypsinized, cells were collected by centrifugation, total protein was extracted, then protein was quantified by Bradford assay. Western blot detection was carried out as follows: (1) loading volume: the sample injection volume per well was 20 μg, boiled in water for 5 min, centrifuged 5 min, and the supernatant sample was obtained; (2) SDS-PAGE electrophoresis, electrophoresis procedure: 100 V for 15 min and 180 V for 45 min; (3) electricity facing: 45 V for 35 min, 100 V for 10 min and blocked, then the membrane was washed for 5 min twice; (4) the primary antibody was added, incubated at 4 °C overnight and membrane was washed for 5 min three times, the secondary antibody was added, the membrane was washed twice; and (5) development and fixing: the membrane was fixed, chemiluminescent was added, wrapped in plastic wrap after drying, washed after exposure for 1-3 min, then scanned and protein bands analyzed.
Apoptosis detection by flow cytometry: After treatment, cells in the NCTD group, ABT-737 group, and NCTD combined with ABT-737 group were trypsinized, collected, centrifuged, washed, and resuspended. Then, flow cytometry detection and analysis were performed according to the TUNEL apoptosis kit manufacturer’s instructions.
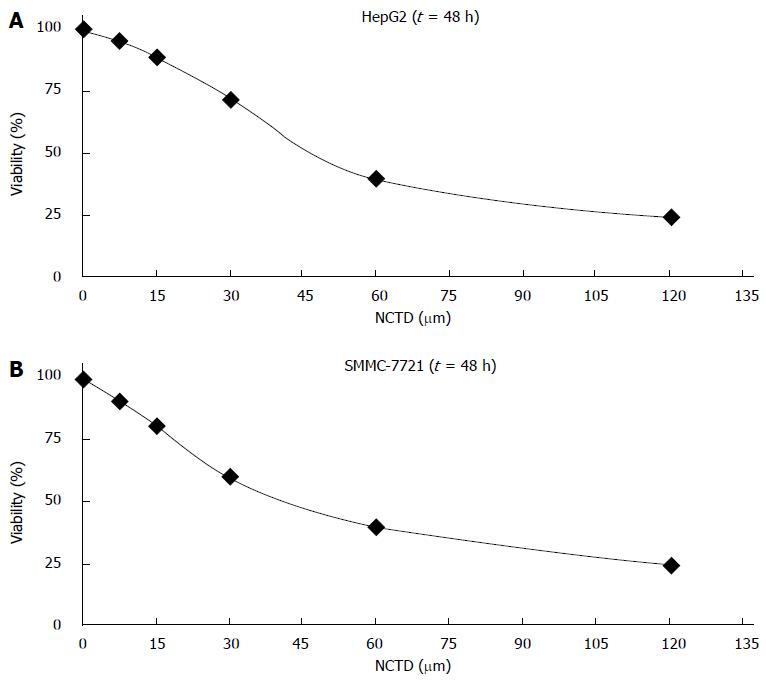
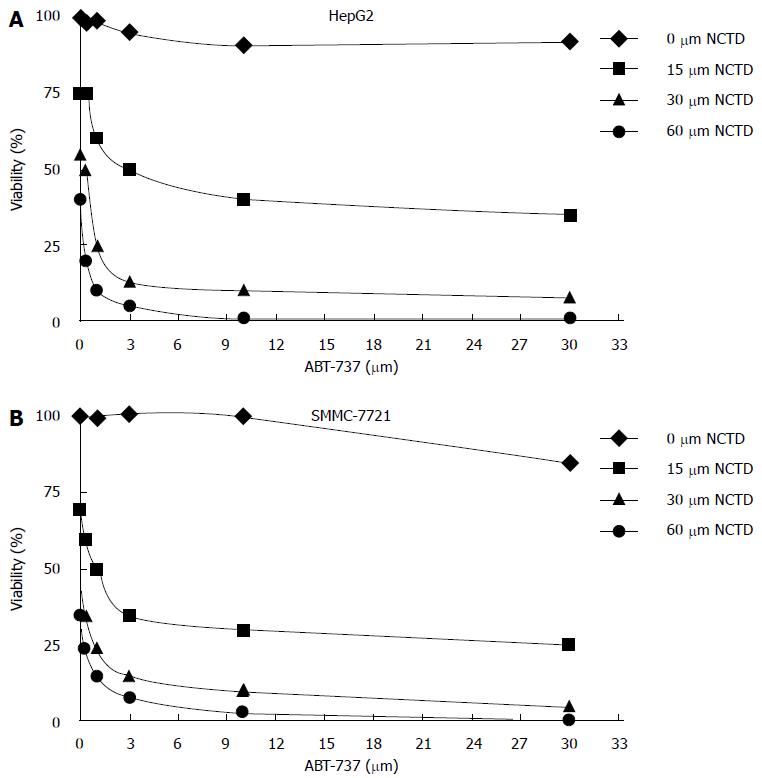
After treatment of HepG2 and SMMC-7721 cells with different concentrations of NCTD for 48 h, cell proliferation inhibition rates detected by MTT were as follows: when concentrations of NCTD were increased, the cell proliferation inhibition rate became smaller (Figure 1A and B); meanwhile, the effect of ABT-737 1-3 μm and NCTD combined treatment on cell proliferation inhibition was stronger than ABT-737 alone. The difference was statistically significant (P < 0.05) (Figure 2A and B).
After HepG2 and SMMC-7721 cells were treated with NCTD 15 μm, the expression of Mcl-1 was partially inhibited; and when the concentration of NCTD was 30 and 60 μm, the expression of Mcl-1 was almost undetectable (Figure 3).
Results showed that the expression of cytochrome C was not detected in cells in the control group or the ABT-737 monotherapy group, and that a low expression of cytochrome C was detected in cells in the NCTD monotherapy group. Cytochrome C was highly expressed in cells in the ABT-737 combined with NCTD group (Figure 4A and B).
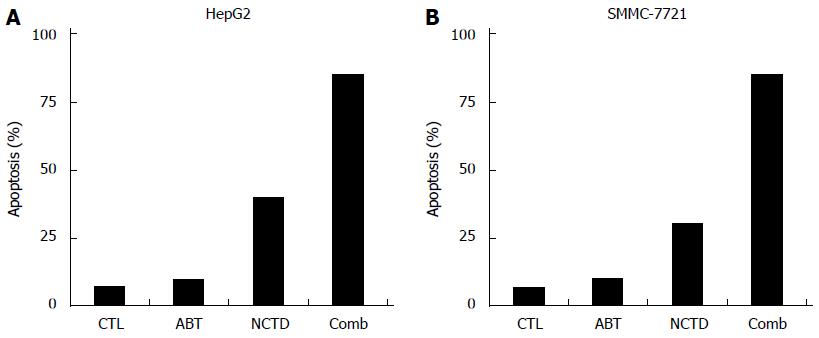
In the control group, ABT-737 3 μm monotherapy group, NCTD 30 μm monotherapy group, or ABT-737 combined with NCTD group, after the 48 h treatment of HepG2 and SMMC-7721 cells, cell apoptosis detection by flow cytometry showed the following: in the monotherapy groups, cells showed an small increase in apoptosis induction compared with the control group, and the difference was not statistically significant (P > 0.05); while after the combination treatment for 48 h, HepG2 and SMMC-7721 cells had a greater amount of apoptosis compared with ABT-737 and NCTD monotherapy, and the difference was statistically significant (P < 0.05) (Figure 5A and B).
ABT-737 is an antagonist of small molecule Bcl-2[20], and a novel anti-cancer drug that induces tumor cell apoptosis without causing damage to normal cells[21-23]; it has broad prospects for development. However, ABT-737 is inhibited in the induction process of apoptosis in hepatocellular carcinoma and some solid tumors that have high expression of Mcl-1[24,25]. Therefore, determining how to reduce the expression of Mcl-1 in cells to increase the efficiency of the therapeutic effect of ABT-737 for liver cancer would be a breakthrough. Studies have reported norcantharidin treatment for cancer can inhibit the expression of Mcl-1[26-28]. Therefore, norcantharidin combined with ABT-737 was used in this study to analyze its effect in the treatment of liver cancer, and to explore its mechanism.
The results are as follows: when HepG2 and SMMC-7721 cells are treated for 48 h with different concentrations of NCTD, it is apparent that NCTD has a good inhibitory effect on cell proliferation; and comparing 1-3 μm of ABT-737 alone with ABT-737 combined with NCTD, results show that the ABT-737 combined with NCTD treatment has a stronger inhibition of cell proliferation compared with ABT-737 alone[29,30]. In order to verify this, we further detected its apoptotic effect by flow cytometry. The results showed that ABT-737 combined with NCTD treatment for 48 h applied to HepG2 and SMMC-7721 cells had a stronger apoptosis-inducing effect than ABT-737 and NCTD monotherapy; and thus, we confirm the rationality of these results. These results also show that NCTD enhances ABT-737 in inhibiting cell proliferation by inducing apoptosis.
Regarding detection of Mcl-1 expression in cells, results showed that after treatment of HepG2 cells and SMMC-7721 cells with NCTD 15 μm, the expression of Mcl-1 was partially inhibited, while the expression of Mcl-1 was almost undetectable when NCTD concentration was 30 and 60 μm. As expected for NCTD, there were better inhibitory effects on Mcl-1 expression in cells at higher doses. To study its mechanism, cytochrome C was further detected in the cytoplasm and the mitochondrial membrane.
In comparing HepG2 cells and SMMC-7721 cells treated in the control group, ABT-737 monotherapy group, NCTD monotherapy group, and ABT-737 combined with NCTD group, the following expressions of cytochrome C were found: in the control group and ABT-737 monotherapy group, the expression of cytochrome C in cells was not detected, while a low expression of cytochrome C was detected in cells in the NCTD monotherapy group; and cytochrome C showed a high expression in cells in the ABT-737 combined with NCTD group. This result prompts us to conclude that this two-drug combination can enhance the expression of cytochrome C, and it also proves that NCTD enhances the release of cytochrome C induced by ABT-737. These results are due to cytochrome C release from the mitochondria into the cytosol in cells, and this is an important symbol of the Bcl-2 family proteins in the regulation of apoptosis. Therefore, we can speculate that NCTD inhibits Mcl-1 enabling ABT-737 to release cytochrome C in cells.
Studies for ABT-737 drugs are promising. Although we have a number of significant results, there are still many issues that need to be explored in in-depth studies, such as: the inhibition by NCTD of the expression of Mcl-1 to enhance ABT-737 in the treatment of hepatocellular carcinoma drug resistance; the release of cytochrome C induced by ABT-737, and whether there is an impact on other factors; and to determine whether ABT-737 combined with other anti-cancer chemotherapy drugs will show improvements. In our study, these problems are not investigated, and the role of its mechanism needs to be further explored through in-depth studies.
In summary, NCTD and ABT-737 combined can solve the ABT-737 drug resistance problem for the treatment of liver cancer. NCTD can inhibit the expression of Mcl-1 to enhance the release of cytochrome C induced by ABT-737. NCTD has a role of inducing apoptosis to enhance ABT-737 in its inhibition of cell proliferation; thus, enhancing ABT-737 induces hepatocellular carcinoma cell apoptosis. Therefore, NCTD combined with ABT-737 has a positive impact on the treatment of hepatocellular carcinoma cells; clinical research in this field has great value, and it deserves further investigation.
Myeloid cell leukemia-1 (Mcl-1) is a special B-cell lymphoma 2 (Bcl-2) family protein. It cannot only control cell survival and death, but also plays an important role in regulating apoptosis signaling. Several studies have shown that Mcl-1 generally has high expression in hepatocellular carcinoma (HCC) and other malignant tumors; and this has become a cancer research focus of molecular targeted therapy. ABT-737 is a novel cancer therapeutic agent that has good prospects for clinical application. Norcantharidin (NCTD) is a derivative of the Chinese medicine cantharidin, which has good anti-tumor effects.
The ABT-737-mediated apoptosis signal is limited when there is high expression of Mcl-1 in liver cancer and other solid tumors; and this has become a major obstacle point in clinical application. Research has shown that the treatment sensitivity of tumor cells to ABT-737 can be enhanced when it is in combination with other chemotherapy drugs. Studies have reported that the anti-tumor effect of NCTD may be related to the role of Bcl-2 family members, which can inhibit Mcl-l expression in HCC cells.
Research has shown the treatment sensitivity of tumor cells to ABT-737 can be enhanced by combination with other chemotherapy drugs. Therefore, this study aims to investigate the therapeutic effects of NCTD combined with ABT-737 on HCC cells, and to preliminarily analyze its mechanism of action for the future development of anticancer drugs and to provide theoretical guidance for clinical applications.
This study demonstrated that combining NCTD and ABT-737 can solve the ABT-737 drug resistance problem for the treatment of liver cancer. It shows that the effect on inducing apoptosis in HepG2 and SMMC-7721 cells with the treatment of ABT-737 combined with NCTD for 48 h was greater than that of ABT-737 or NCTD alone.
HepG2 and SMMC-7721 hepatocellular carcinoma cell lines were tested.
This study demonstrated that NCTD can inhibit the expression of Mcl-1 to enhance the release of cytochrome C induced by ABT-737. NCTD has a role of inducing apoptosis to enhance ABT-737 for inhibiting cell proliferation; thus, enhancing ABT-737 induces hepatocellular carcinoma cell apoptosis. Therefore, NCTD combined with ABT-737 has a positive impact for the treatment of hepatocellular carcinoma cells; the clinical research has great value, and it deserves further investigation.
P- Reviewer: Abd el Moety HA, Campanale M, Syngal S S- Editor: Ma YJ L- Editor: Logan S E- Editor: Ma S
| 1. | Thomas S, Quinn BA, Das SK, Dash R, Emdad L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M. Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther Targets. 2013;17:61-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Song T, Li X, Chang X, Liang X, Zhao Y, Wu G, Xie S, Su P, Wu Z, Feng Y. 3-Thiomorpholin-8-oxo-8H-acenaphtho [1,2-b] pyrrole-9-carbonitrile (S1) derivatives as pan-Bcl-2-inhibitors of Bcl-2, Bcl-xL and Mcl-1. Bioorg Med Chem. 2013;21:11-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Zhang SD, Shan L, Li W, Li HL, Zhang WD. Isochamaejasmin induces apoptosis in leukemia cells through inhibiting Bcl-2 family proteins. Chin J Nat Med. 2015;13:660-666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Casanelles E, Gozzelino R, Marqués-Fernández F, Iglesias-Guimarais V, Garcia-Belinchón M, Sánchez-Osuna M, Solé C, Moubarak RS, Comella JX, Yuste VJ. NF-κB activation fails to protect cells to TNFα-induced apoptosis in the absence of Bcl-xL, but not Mcl-1, Bcl-2 or Bcl-w. Biochim Biophys Acta. 2013;1833:1085-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Eichhorn JM, Alford SE, Sakurikar N, Chambers TC. Molecular analysis of functional redundancy among anti-apoptotic Bcl-2 proteins and its role in cancer cell survival. Exp Cell Res. 2014;322:415-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Anilkumar U, Weisová P, Düssmann H, Concannon CG, König HG, Prehn JH. AMP-activated protein kinase (AMPK)-induced preconditioning in primary cortical neurons involves activation of MCL-1. J Neurochem. 2013;124:721-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kelly PN, Grabow S, Delbridge AR, Adams JM, Strasser A. Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ. 2013;20:57-63. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, Deb S, Ritchie ME, Takano E, Ward T. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci USA. 2012;109:2766-2771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Balakrishnan K, Gandhi V. Bcl-2 antagonists: a proof of concept for CLL therapy. Invest New Drugs. 2013;31:1384-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782-791. [PubMed] [Cited in This Article: ] |
| 11. | Allaman-Pillet N, Oberson A, Munier F, Schorderet DF. The Bcl-2/Bcl-XL inhibitor ABT-737 promotes death of retinoblastoma cancer cells. Ophthalmic Genet. 2013;34:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lieber J, Ellerkamp V, Vogt F, Wenz J, Warmann SW, Fuchs J, Armeanu-Ebinger S. BH3-mimetic drugs prevent tumour onset in an orthotopic mouse model of hepatoblastoma. Exp Cell Res. 2014;322:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Beurlet S, Omidvar N, Gorombei P, Krief P, Le Pogam C, Setterblad N, de la Grange P, Leboeuf C, Janin A, Noguera ME. BCL-2 inhibition with ABT-737 prolongs survival in an NRAS/BCL-2 mouse model of AML by targeting primitive LSK and progenitor cells. Blood. 2013;122:2864-2876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Mattoo AR, FitzGerald DJ. Combination treatments with ABT-263 and an immunotoxin produce synergistic killing of ABT-263-resistant small cell lung cancer cell lines. Int J Cancer. 2013;132:978-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Beverly LJ, Howell LA, Hernandez-Corbacho M, Casson L, Chipuk JE, Siskind LJ. BAK activation is necessary and sufficient to drive ceramide synthase-dependent ceramide accumulation following inhibition of BCL2-like proteins. Biochem J. 2013;452:111-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Tarleton M, Gilbert J, Sakoff JA, McCluskey A. Synthesis and anticancer activity of a series of norcantharidin analogues. Eur J Med Chem. 2012;54:573-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Shimizu T, Iizuka M, Matsukura H, Hashizume D, Sodeoka M. Synthesis of optically pure norcantharidin analogue NCA-01, a highly selective protein phosphatase 2B inhibitor, and its derivatives. Chem Asian J. 2012;7:1221-1230. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lu S, Gao Y, Huang X, Wang X. Cantharidin exerts anti-hepatocellular carcinoma by miR-214 modulating macrophage polarization. Int J Biol Sci. 2014;10:415-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Zhang S, Li G, Ma X, Wang Y, Liu G, Feng L, Zhao Y, Zhang G, Wu Y, Ye X. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of Mcl-1. Cell Signal. 2012;24:1803-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Varadarajan S, Vogler M, Butterworth M, Dinsdale D, Walensky LD, Cohen GM. Evaluation and critical assessment of putative MCL-1 inhibitors. Cell Death Differ. 2013;20:1475-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Billard C. BH3 mimetics: status of the field and new developments. Mol Cancer Ther. 2013;12:1691-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Boiani M, Daniel C, Liu X, Hogarty MD, Marnett LJ. The stress protein BAG3 stabilizes Mcl-1 protein and promotes survival of cancer cells and resistance to antagonist ABT-737. J Biol Chem. 2013;288:6980-6990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Yao H, Mi S, Gong W, Lin J, Xu N, Perrett S, Xia B, Wang J, Feng Y. Anti-apoptosis proteins Mcl-1 and Bcl-xL have different p53-binding profiles. Biochemistry. 2013;52:6324-6334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Simonin K, N’Diaye M, Lheureux S, Loussouarn C, Dutoit S, Briand M, Giffard F, Brotin E, Blanc-Fournier C, Poulain L. Platinum compounds sensitize ovarian carcinoma cells to ABT-737 by modulation of the Mcl-1/Noxa axis. Apoptosis. 2013;18:492-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Stefaniková A, Kliková K, Hatok J, Račay P. ABT-737 accelerates butyrate-induced death of HL-60 cells. Involvement of mitochondrial apoptosis pathway. Gen Physiol Biophys. 2013;32:505-516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Cippà PE, Kamarashev J, Chen J, Kraus AK, Segerer S, Feldmeyer L, Fehr T. Synergistic Bcl-2 inhibition by ABT-737 and cyclosporine A. Apoptosis. 2013;18:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Dong X, Li JC, Jiang YY, Xia MY, Tashiro S, Onodera S, Ikejima T. p38-NF-κB-promoted mitochondria-associated apoptosis and G2/M cell cycle arrest in norcantharidin-treated HeLa cells. J Asian Nat Prod Res. 2012;14:1008-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Karlsson H, Lindqvist AC, Fransson M, Paul-Wetterberg G, Nilsson B, Essand M, Nilsson K, Frisk P, Jernberg-Wiklund H, Loskog A. Combining CAR T cells and the Bcl-2 family apoptosis inhibitor ABT-737 for treating B-cell malignancy. Cancer Gene Ther. 2013;20:386-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Bianchi J, Cabral-de-Mello DC, Marin-Morales MA. Toxicogenetic effects of low concentrations of the pesticides imidacloprid and sulfentrazone individually and in combination in in vitro tests with HepG2 cells and Salmonella typhimurium. Ecotoxicol Environ Saf. 2015;120:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Abdul Rahman Sazli F, Jubri Z, Abdul Rahman M, Karsani SA, Md Top AG, Wan Ngah WZ. Gamma-tocotrienol treatment increased peroxiredoxin-4 expression in HepG2 liver cancer cell line. BMC Complement Altern Med. 2015;15:64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |