Published online Mar 28, 2016. doi: 10.3748/wjg.v22.i12.3355
Peer-review started: August 1, 2015
First decision: September 9, 2015
Revised: September 25, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: March 28, 2016
AIM: To present our initial experience regarding the feasibility of ultrasound virtual endoscopy (USVE) and its measurement reliability for polyp detection in an in vitro study using pig intestine specimens.
METHODS: Six porcine intestine specimens containing 30 synthetic polyps underwent USVE, computed tomography colonography (CTC) and optical colonoscopy (OC) for polyp detection. The polyp measurement defined as the maximum polyp diameter on two-dimensional (2D) multiplanar reformatted (MPR) planes was obtained by USVE, and the absolute measurement error was analyzed using the direct measurement as the reference standard.
RESULTS: USVE detected 29 (96.7%) of 30 polyps, remaining a 7-mm one missed. There was one false-positive finding. Twenty-six (89.7%) of 29 reconstructed images were clearly depicted, while 29 (96.7%) of 30 polyps were displayed on CTC with one false-negative finding. In OC, all the polyps were detected. The intraclass correlation coefficient was 0.876 (95%CI: 0.745-0.940) for measurements obtained with USVE. The pooled absolute measurement errors ± the standard deviations of the depicted polyps with actual sizes ≤ 5 mm, 6-9 mm, and ≥ 10 mm were 1.9 ± 0.8 mm, 0.9 ± 1.2 mm, and 1.0 ± 1.4 mm, respectively.
CONCLUSION: USVE is reliable for polyp detection and measurement in in vitro study.
Core tip: We present our initial experience regarding the feasibility of ultrasound virtual endoscopy (USVE) and its measurement reliability for polyp detection in an in vitro study using pig intestine specimens. USVE is a new technique that simulates views of computed tomography colonography (CTC). We found that USVE is an accurate screening method for simulated polyp detection and compares favorably to CTC and optical colonoscopy. As a dynamic, non-invasive, radiation-free, cost-effective method, USVE shows great promise for the screening and surveillance of colorectal cancers.
- Citation: Liu JY, Chen LD, Cai HS, Liang JY, Xu M, Huang Y, Li W, Feng ST, Xie XY, Lu MD, Wang W. Ultrasound virtual endoscopy: Polyp detection and reliability of measurement in an in vitro study with pig intestine specimens. World J Gastroenterol 2016; 22(12): 3355-3362
- URL: https://www.wjgnet.com/1007-9327/full/v22/i12/3355.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i12.3355
Colorectal cancer (CRC), which generally develops from benign adenomatous polyps, is a major cause of morbidity and mortality worldwide[1,2]. Evidence-based guidelines recommend CRC screening[3,4] because the early detection and removal of polyps has been shown to reduce both the incidence and the mortality of CRC[5,6]. Optical colonoscopy (OC) is the primary method employed for CRC screening and the removal of polyps. As a primary imaging test, computed tomography colonography (CTC) is performed in average-risk individuals, particularly when endoscopy is contraindicated or incomplete[4,7]. It is highly sensitive for CRC screening with a sensitivity of 96.1% according to a meta-analysis[8]. Unfortunately, these tests have some drawbacks, such as OC related invasiveness, procedure-related discomfort, the risk of bowel perforation, and CTC related ionizing radiation[9]. Ultrasound virtual endoscopy (USVE) is a new technique that simulates views of CTC. It allows for the reconstruction of inner bowel-surface structures from the dynamic three-dimensional (3D) ultrasound data sets. Based on a surface reconstruction algorithm by interactive settings of threshold values and surface displays, endoscopic views can be obtained within seconds. Similar ultrasound virtual endoscopy imaging has been used for detection of carotid atherosclerosis and portal vein thrombus[10-12]. But, USVE has never been used for polyp detection. It has several potential advantages for CRC screening over other modalities. USVE is dynamic, non-invasive, radiation-free, and cost-effective. Therefore, this new method shows great promise for the screening and surveillance of CRC.
The likelihood that a given polyp develops into malignancy or demonstrates high-grade dysplasia is directly related to its size[13-15]. This risk is estimated to be less than 1% for lesions 5-mm or smaller, whereas the risk increases to approximately 10%-25% for lesions 10 mm or larger. Polyps of 6-9 mm are also almost always benign. Advanced adenomas, defined as lesions 10 mm or larger, are the target of CRC screening and should be referred for polypectomy[16]. However, radiologists suggested 6-mm as the minimum size for reporting polyp lesions[17]. Hence, understanding of the polyp size measurement error by USVE is very important for accurate polyp matching in the diagnostic performance.
Currently, there is no published report regarding the role of USVE in polyp detection, and its sensitivity and specificity for the detection of polyps are unknown. In this ideal in vitro study, we investigated the detection rate of simulated polyps using USVE compared with CTC and OC. We estimated the conspicuity of the reconstructed endoscopic images. Additionally, we evaluated the reliability of the two-dimensional (2D) optimized polyp measurements of USVE using the direct measurement as the reference standard. The goal of this study was to report our initial experience regarding the feasibility of USVE and its measurement reliability in an in vitro study using porcine intestine specimens.
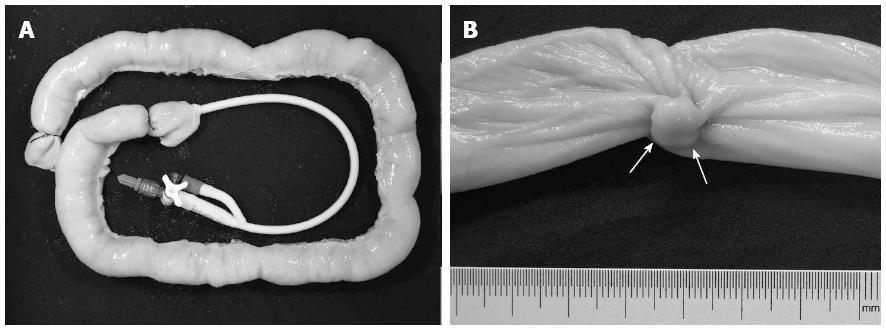
Six porcine small intestine specimens, each approximately 50 cm long, were acquired from fresh pig intestines that were commercially available at an abattoir. Each specimen was cleansed to remove fecal matter, and no polyploid structures were found on the mucosal surface. Thirty simulated sessile polyps with maximum diameters of 4-13 mm were created from pork wrapped by other intestinal mucosa with sutures. The maximum diameter of the polyp was confirmed by means of physical measurement with a caliper and a millimeter marked ruler. Then, the polyps were sutured to the mucosa of the inverted specimens and randomly placed with different distances between adjacent polyps. Either the number or the size of the polyps was randomized in each specimen. A detailed polyp map of the specimen was recorded. Next, each specimen was re-inverted, and the distal end was double-tied with sutures. An 18-F urinary catheter was inserted into the open proximal end, which was then closed with a double-tied suture (Figure 1). A 50-mL syringe with a 3-way stopcock was attached to the urinary catheter.
A 0.9% saline solution was introduced through the syringe, and the specimen was maximally distended to a diameter of approximately 3.5 cm. Then, it was placed in a plastic container containing water. The wall and the bottom of the container were covered with 2-mm-thick black sponges that helped absorb the ultrasound and reduce echo reflection.
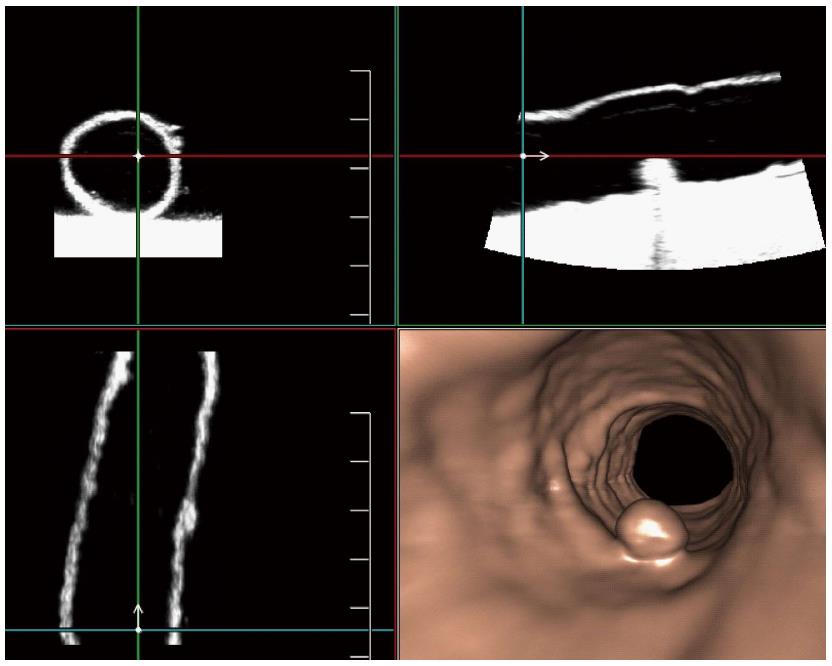
The USVE was performed with an ultrasound scanner (Aplio 500, Toshiba, Otawa, Japan) equipped with a volume transducer (7-14 MHz) (PLT-1204MV, Toshiba Medical Systems). Imaging was performed by one radiologist (Wang W) who was unaware of the locations, numbers and sizes of the polyps in each specimen. After optimal 2D images were obtained, 3D scan mode was initiated, and volume data were acquired, including the following parameters: longitudinal scanning orientation (running along the longitudinal axis of the specimen); 3D frequency, 8 MHz under the difference mode; 3D gain, 70%-80%; dynamic range, 40-50 dB; depth (distance between the probe and the proximal colon wall), approximately 30 mm; focus, placed in the middle of the lumen; 3D Aplipure, on; and 3D scanning angle, 600. Each specimen was scanned from the distal to the proximal end, and 3D volume data were obtained once approximately every 5 cm because of the sweeping length of the PLT-1204MV volume transducer. Therefore, one specimen was artificially separated into several approximately 5-cm-long segmentations. Then, the data from the scanning were loaded to the Fly Thru workstation (Fly Thru 3.0, Toshiba Medical Systems), which was capable of producing 2D multiplanar reformatted (MPR) images and 3D endoluminal surface renderings (Figure 2, Video 1). Virtual endoscopic images were shown by setting optimal threshold values based on a surface reconstruction algorithm. To prevent holes in the intestinal wall and intraluminal artifact, a threshold value (0-150) was selected for all reconstructions with other defined settings as follows: transparency, 20; and filter, 3. All machine parameters remained unchanged between the examinations and were verified before the imaging.
Two independent readers (Chen LD and Xu M), who did not know the size ranges and numbers of polyps, reviewed all the ultrasound examinations and recorded the numbers, positions (distance between adjacent polyps) and sizes of the simulated polyps. To achieve a valid match between virtual endoscopy and specimen, a polyp had to appear with the same segment, same position and similar diameters.
Lesion size is best defined as the single largest diameter of the polyp head, excluding the stalk[17]. With electronic calipers, the two readers independently measured the polyp size on an optimized 2D MPR plane, on which the maximum polyp diameter was viewed. The measurements were determined to the nearest millimeter. For the same polyp, the mean measured size was the average of measurements from the two readers. For the analysis of measurement accuracy, the absolute measurement error referred to the difference between the actual size and the mean measured size. The standardized polyp size was defined as the mean measured optimized 2D diameter divided by the reference diameter, multiplied by 100%.
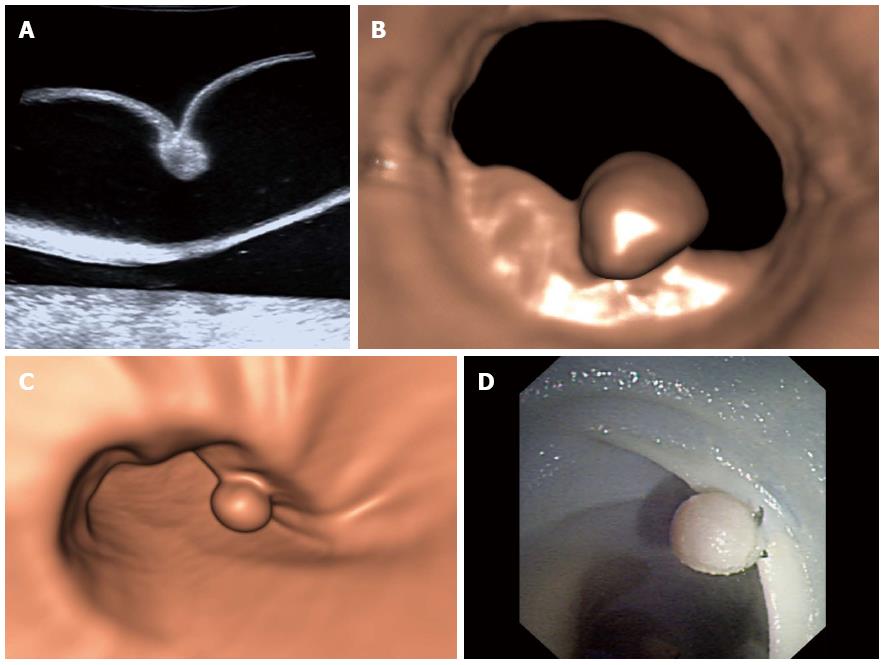
An overall ranking of image quality of the USVE protocol was performed. Both radiologists graded conspicuity and evaluated the images together to resolve any disagreements. The image conspicuity of each polyp was graded on a three-point scale[18,19]: grade 1 = not visible or very poorly depicted; grade 2 = poorly depicted, with some artifacts that but do not affect diagnosis; and grade 3 = clearly depicted, without artifacts.
After US examination, the six specimens were transferred to the CT suite. They were placed in a plastic container and then manually distended with room air using a syringe. Five liters of 93.7% soybean oil was poured into the container until the specimen was completely submerged[20]. To avoid flotation, the specimen was fastened to the bottom of the container by adhesion of plastic tape, before the oil was poured into the container.
Blinded to the locations, sizes and numbers of polyps, two independent radiologists (Feng ST and Cai HS), performed the CT examinations with a 64-detector row CT scanner (Aquillion 64, Toshiba, Japan). The images were obtained with these parameters: 120 kV; 200-250 mA; section thickness, 0.5 mm; beam collimation, 64 mm × 0.5 mm; reconstruction interval, 0.5 mm; reconstructed section thickness, 0.5 mm; beam pitch, 0.828; gantry rotation time, 0.4 second; and field of view, 5 cm. The scanning matrix was 512 × 512. Then, the data were loaded to a workstation (HP workstation XW8200, Vitrea 2, Version 3.7). The reconstructed virtual endoscopic images were read by the above radiologists, who synchronously recorded the locations, numbers and sizes of the simulated polyps.
The OC of the six specimens was performed with a colonoscope (CF-H260AI, CV-260, Olympus, Tokyo, Japan) by two independent endoscopists (Liang JY and Xie XY) immediately after CT scanning. The oil mixture was removed. The gastroenterologists were unaware of the locations, numbers and sizes of the polyps in each specimen, but were aware of the presence of polyps. The colonoscope was introduced through the open proximal end after the urinary catheter was removed. The examination was started, and endoscopy views of the entire specimen were acquired. The same endoscopists recorded the locations, numbers and sizes of the simulated polyps.
The relationship between conspicuity grades and polyp sizes was examined using Pearson’s Chi-square test (SPSS 18, IBM Corporation, New York, NY, United States). A P value of less than 0.05 was considered to indicate a statistically significant difference. The intraclass correlation coefficient (MedCalc version 10.2.0.0, Medalc Software, Mariakerke, Belgium) was applied to analyze the inter-observer agreement for polyp measurement.
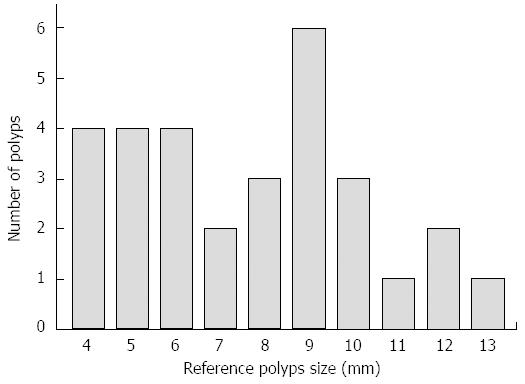
The reference polyp size and the number of the 30 simulated polyps for evaluating the detectability are shown in Figure 3. The polyps were classified into three groups according to size (≤ 5 mm, 6-9 mm, and ≥ 10 mm)[21,22]. The detection rates for each group of the three modalities are shown in Table 1. With USVE, 29 (96.7%) of the 30 polyps were detected; only a 7-mm polyp was missed. There was one false-positive finding (identified as a 10-mm polyp). An initial analysis of the polyp conspicuity data revealed that no observation was assigned a grade of 1. Twenty-six (89.7%) of 29 polyps were clearly depicted (grade 3) (Figure 4), whereas the remaining 3 (10.3%) polyps were poorly depicted (grade 2). There was no significant interaction between polyp conspicuity grade and polyp size (P = 0.627) (Table 2).
| Actual polyp size, mm | Referenced No. of polyps | OC | CTC | USVE | |||
| No. of detected polyps | Sensitivity | No. of detected polyps | Sensitivity | No. of detected polyps | Sensitivity | ||
| ≤ 5 | 9 | 9 | 100% | 8 | 88.9% | 9 | 100% |
| 6-9 | 15 | 15 | 100% | 15 | 100% | 14 | 93.3% |
| ≥ 10 | 6 | 6 | 100% | 6 | 100% | 6 | 100% |
| All | 30 | 30 | 100% | 29 | 96.7% | 29 | 96.7% |
| Actual polyp size, mm | Conspicuity grade 2 | Conspicuity grade 3 | P value |
| ≤ 5 (n = 9) | 1 (11.1) | 8 (88.9) | 0.627 |
| 6-9 (n = 14) | 2 (14.3) | 12 (85.7) | |
| ≥ 10 (n = 6) | 0 (0) | 6 (100) | |
| All (n = 29) | 3 (10.3) | 26 (89.7) |
Twenty-nine (96.7%) of the 30 polyps were depicted on the CTC. All of the polyps 6 mm or larger were detected. One 5-mm polyp, which was located behind a fold, was not prospectively detected by the observer on any of the images but was depicted in a retrospective review. There was one false-positive finding (identified as a 6-mm lesion) as well. All of the polyps were clearly detected by OC.
The intraclass correlation coefficient was 0.876 (95% confidence interval: 0.745, 0.940) for measurements obtained with USVE. The pooled absolute measurement errors ± standard deviations of the depicted polyps with actual sizes of 5-mm or smaller, 6-9-mm, and 10-mm or larger were 1.9 ± 0.8 mm, 0.9 ± 1.2 mm, and 1.0 ± 1.4 mm, respectively. The pooled standardized polyp size of the depicted polyps with actual sizes of 5-mm or smaller, 6-9-mm, and 10-mm or larger were 142.5% ± 18.7%, 111.2% ± 16.7%, and 109.2% ± 12.2%, respectively (Table 3).
| Actual polyp size, mm | Mean measurementerror ± SD, mm | Pooled standardizedpolyp size ± SD, % |
| ≤ 5 (n = 9) | 1.9 ± 0.8 | 142.5 ± 18.7 |
| 6-9 (n = 14) | 0.9 ± 1.2 | 111.2 ± 16.7 |
| ≥ 10 (n = 6) | 1.0 ± 1.4 | 109.2 ± 12.2 |
| ≥ 6 (n = 20) | 0.9 ± 1.3 | 110.5 ± 14.6 |
This study demonstrates, for the first time, that USVE is technically feasible in an in vitro intestinal specimen. We found that 96.7% (29/30) of the simulated polyps with different sizes ranging from 4 to 13 mm in diameter were depicted using USVE. Thus, USVE is an accurate screening method and compares favorably with CTC and OC for polyp detection. With USVE, the pooled error ± standard deviation and the pooled standardized polyp size of the optimized 2D MPR measurements were 0.9 ± 1.3 mm and 110.5% ± 14.6% for polyps ≥ 6 mm, respectively. These results indicate that USVE is reliable for polyp measurement.
Gastrointestinal tract ultrasonography is challenging. Gas and fecal residue within the colonic lumen make visibility difficult. The value of abdominal ultrasound in the diagnosis of CRC has previously studied[23-26]. Following the standard bowel preparation, including colonic cleansing and oral administration of a solution for adequate luminal distention[19], transabdominal ultrasound is capable of acquiring 3D data of the colon which then generates virtual endoscopic imaging. Additionally, transabdominal ultrasound can observe the presence of lymphadenopathy, the extracolonic extension of a mass, and the presence of distant metastases.
In 2001, a study reported on ultrasound virtual endoscopic imaging[27], using a curved array probe or a linear array probe with a position-sensing sweeper device. A series of 2D images were manually scanned and then stored in a graphics workstation. In our study, however, we used a new 3D image processing system, called the Fly Thru workstation, and a 3D volume probe. The new method is simpler, more convenient, and has higher spatial resolution. In this ideal pig intestine specimen, USVE was capable of efficiently depicting polyps as small as 4-mm in diameter. In total, 89.7% of the reconstructed images were clearly depicted (Grade 3), which was approximately equal to the polyp conspicuity of CTC[18]. Furthermore, the detection rate of polyps ≥ 6-mm on USVE approached 100% with only sporadic failures, which was similar to the detection rate of in vitro CTC[28]. With the high resolution and high sensitivity of detecting polyps ≥ 6-mm, USVE is expected to be a new colon neoplasm screening and surveillance modality.
Neither of the observers found one of the four 7-mm polyps during the retrospective reviews of USVE. In contrast, a 10-mm polyp, which was located at the division of two adjacent scan areas, was depicted twice and was regarded as a false-positive finding by both observers. These missing depiction and false-positive findings resulted from the limited sweeping range of the 3D volume transducer. When performing USVE, this transducer scanned approximately 5-cm of the intestine specimen for each acquisition. Thus, as one specimen was artificially separated into several segmentations, the polyps located at the division of adjacent scan areas could be repeatedly depicted or even missed. Based on our preliminary experience, we conclude that the limited scanning range is a major limitation of USVE.
The achievement of precise polyp matching is critical for the sensitivity of USVE. According to previous in vitro studies of CTC, the optimized 2D MPR measurement was as accurate as the 3D endoluminal measurement[20,29]. In our study, we adapted the optimized 2D MPR measurement as the standard method of measurement. The mean absolute optimized 2D measurement errors and the mean standardized polyp sizes in the ≥ 6-mm group were generally consistent with values of CTC[20,29]. This important finding demonstrates that the optimized 2D MPR measurement of USVE is reliable for clinical practice.
This study has some limitations. First, we assessed the technical accuracy of USVE performed under ideal conditions, which includes the absence of patient motion or peristaltic bowel activity, the clean, debris-free mucosal surface without folds, and maximal lumen distention. The study was not able to reflect an in vivo examination on human. The polyps, either their morphologic features or locations, may show more variability in patients. Also, polyp detection and measurement can be affected by colonic curvature, haustral folds, and even different degrees of colonic distention. Additionally, we did not attempt to create flat adenomas in our specimens. Second, polyp size was not measured on the 3D view because the system is currently not able to obtain 3D measurements. Typically, 3D polyp measurements are closer to the “truth” than 2D measurements as the maximum diameter of a polyp is straightforward with the former measurement. Although 2D measurements would optimize polyp diameter by comparing measurements on MPR planes, even using nonstandard oblique planes, this complex procedure would prolong the measurement[29].
In summary, USVE enables the reliable detection and measurement of small simulated mucosal polyps in our in vitro model. It is a good potential alternative for colorectal polyp detection. Further in vivo studies are required to determine its sensitivity and specificity for polyp detection in patients.
We are grateful to Chuan Peng, Xiao-Er Zhang, and Xiao-Wen Huang, Department of Medical Ultrasonics, Institute of Diagnostic and Interventional Ultrasound, The First Affiliated Hospital of Sun Yat-Sen University, for their valuable contributions to the specimen preparation for this study.
Most colorectal cancers (CRC) arise from adenomatous polyps, and the early detection and removal of polyps resulted in reducing both the incidence and mortality of CRC. Optical colonoscopy (OC) is widely accepted as the best available method for CRC screening. Computed tomography colonography (CTC) has yielded promising results. However, these tests have some drawbacks such as invasiveness or ionizing radiation. Ultrasound virtual endoscopy (USVE), a novel non-invasive, radiation-free modality, shows great promise for the screening of CRC. Currently, the role of USVE in polyp detection has never been investigated, and its sensitivity and specificity are unknown.
Virtual colonoscopy is an attractive alternative for CRC screening. According to some guidelines, CTC has been regarded as the leading imaging technique for CRC screening and the preferred test following incomplete OC.
This in vitro study has revealed, for the first time, the feasibility of USVE and its measurement reliability in pig intestine specimens.
USVE enables reliable detection and measurement of small simulated mucosal polyps in the in vitro specimens. It is a good alternative for colorectal polyp screening.
USVE is a novel trans-abdominal ultrasonic technique that simulates views of CTC. It allows for the reconstruction of inner bowel-surface structures from dynamic three-dimensional ultrasound data sets.
This is an in vitro study that reports the feasibility of a new, non-invasive, radiation-free technique (USVE) in detecting and measuring polyps. The authors compared USVE with CTC and colonoscopy, as the reference standard, with interesting results. The authors performed a well-designed study. It is detailed, easy to read and the subject (with all of the important limitations of a preliminary approach) may be relevant in medicine in future. In vivo studies were encouraged.
P- Reviewer: Velayos B S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Wang CH
| 1. | Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290-1314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 865] [Cited by in F6Publishing: 862] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1848] [Cited by in F6Publishing: 2025] [Article Influence: 202.5] [Reference Citation Analysis (0)] |
| 3. | Burt RW, Cannon JA, David DS, Early DS, Ford JM, Giardiello FM, Halverson AL, Hamilton SR, Hampel H, Ismail MK, Jasperson K, Klapman JB, Lazenby AJ, Lynch PM, Mayer RJ, Ness RM, Provenzale D, Rao MS, Shike M, Steinbach G, Terdiman JP, Weinberg D, Dwyer M, Freedman-Cass D; National comprehensive cancer network. Colorectal cancer screening. J Natl Compr Canc Netw. 2013;11:1538-1575. [PubMed] [Cited in This Article: ] |
| 4. | Yee J, Kim DH, Rosen MP, Lalani T, Carucci LR, Cash BD, Feig BW, Fowler KJ, Katz DS, Smith MP. ACR Appropriateness Criteria colorectal cancer screening. J Am Coll Radiol. 2014;11:543-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Atkin WS, Cook CF, Cuzick J, Edwards R, Northover JM, Wardle J; UK Flexible Sigmoidoscopy Screening Trial Investigators. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 346] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 6. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2085] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 7. | Spada C, Stoker J, Alarcon O, Barbaro F, Bellini D, Bretthauer M, De Haan MC, Dumonceau JM, Ferlitsch M, Halligan S, Helbren E, Hellstrom M, Kuipers EJ, Lefere P, Mang T, Neri E, Petruzziello L, Plumb A, Regge D, Taylor SA, Hassan C, Laghi A; European Society of Gastrointestinal Endoscopy; European Society of Gastrointestinal and Abdominal Radiology. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Endoscopy. 2014;46:897-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, Ransohoff DF. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849-57, W152. [PubMed] [Cited in This Article: ] |
| 10. | He W, Zhang HQ, Shi CY, Chen J, Gao J. Fly through ultrasound imaging in assessment of carotid atherosclerosis: a pictorial essay. Clin Imaging. 2013;37:811-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kunte H, Rückert RI, Schmidt C, Harms L, Grigoryev M, Fischer T. Inverse fly-through technique in ultrasound imaging of carotid stenosis. Neurology. 2013;80:122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Wang W, Liu GJ, Chen LD, Wang Z, Zhou LY, Lu MD, Xie XY, Huang Y, Li W. Preliminary experience of a new perspective view technology for the detection of portal vein thrombus in hepatocellular carcinoma patients. Abdom Imaging. 2014;39:1145-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg. 1979;190:679-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 346] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Eide TJ. Risk of colorectal cancer in adenoma-bearing individuals within a defined population. Int J Cancer. 1986;38:173-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 156] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009-1013. [PubMed] [Cited in This Article: ] |
| 16. | Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:3053-3063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 261] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, Glick SN, Laghi A, Macari M, McFarland EG, Morrin MM, Pickhardt PJ, Soto J, Yee J; Working Group on Virtual Colonoscopy. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 18. | Slater A, Taylor SA, Burling D, Gartner L, Scarth J, Halligan S. Colonic polyps: effect of attenuation of tagged fluid and viewing window on conspicuity and measurement--in vitro experiment with porcine colonic specimen. Radiology. 2006;240:101-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Bakir B, Acunas B, Bugra D, Yamaner S, Asoglu O, Salmaslioglu A, Balik E. MR colonography after oral administration of polyethylene glycol-electrolyte solution. Radiology. 2009;251:901-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Park SH, Choi EK, Lee SS, Byeon JS, Jo JY, Kim YH, Lee KH, Ha HK, Han JK. Polyp measurement reliability, accuracy, and discrepancy: optical colonoscopy versus CT colonography with pig colonic specimens. Radiology. 2007;244:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1495] [Cited by in F6Publishing: 1257] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 22. | Cotton PB, Durkalski VL, Pineau BC, Palesch YY, Mauldin PD, Hoffman B, Vining DJ, Small WC, Affronti J, Rex D. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713-1719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 522] [Cited by in F6Publishing: 548] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 23. | Rutgeerts LJ, Verbanck JJ, Crape AW, Buyse BM, Ghillebert GL. Detection of colorectal cancer by routine ultrasound. J Belge Radiol. 1991;74:11-13. [PubMed] [Cited in This Article: ] |
| 24. | Richardson NG, Heriot AG, Kumar D, Joseph AE. Abdominal ultrasonography in the diagnosis of colonic cancer. Br J Surg. 1998;85:530-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Martínez-Ares D, Martín-Granizo Barrenechea I, Souto-Ruzo J, Yáñez López J, Pallarés Peral A, Vázquez-Iglesias JL. The value of abdominal ultrasound in the diagnosis of colon cancer. Rev Esp Enferm Dig. 2005;97:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Shirahama M, Koga T, Ishibashi H, Uchida S, Ohta Y. Sonographic features of colon carcinoma seen with high-frequency transabdominal ultrasound. J Clin Ultrasound. 1994;22:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Nakata N, Miyamoto Y, Tsujimoto F, Harada J, Tada S, Fukuda K. Ultrasound virtual endoscopic imaging. Semin Ultrasound CT MR. 2001;22:78-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Taylor SA, Halligan S, Bartram CI, Morgan PR, Talbot IC, Fry N, Saunders BP, Khosraviani K, Atkin W. Multi-detector row CT colonography: effect of collimation, pitch, and orientation on polyp detection in a human colectomy specimen. Radiology. 2003;229:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology. 2005;236:872-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |